- *Corresponding Author:
- Jinpeng Qiao
Department of Oncology, Shanghai Mengchao Cancer Hospital, Xuhui, Shanghai 201805, China
E-mail: qjp007007@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “242-247” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This research aims to comprehensively evaluate the safety and efficacy of anlotinib, and quantify its treatment effect in advanced metastatic colorectal cancer patients. 150 advanced metastatic colorectal cancer patients received third-line or more treatment in the oncology department of a tertiary hospital from January 2019 till January 2022. Their clinical data were collected and retrospectively analyzed. We evaluated the efficacy and adverse reactions of anlotinib. The objective remission rate of patients was 10 %, while the disease control rate was 64 %. The median progression-free survival time was 4.1 mo. It is revealed that liver metastasis and eastern cooperative oncology group score were related to progressionfree survival by univariate analysis. It is showed by multivariate analysis that liver metastasis was an independent factor that influencing the treatment of advanced metastatic colorectal cancer (p<0.05). Hypertension, fatigue, nausea and vomiting, albuminuria, and hypothyroidism were the relatively high incidences of complications caused by anlotinib. Anlotinib as the third line and above treatment is both effective and safe in treating advanced metastatic colorectal cancer.
Keywords
Anlotinib, efficacy, metastatic colorectal cancer, albuminuria, hypothyroidism, cytotoxicity
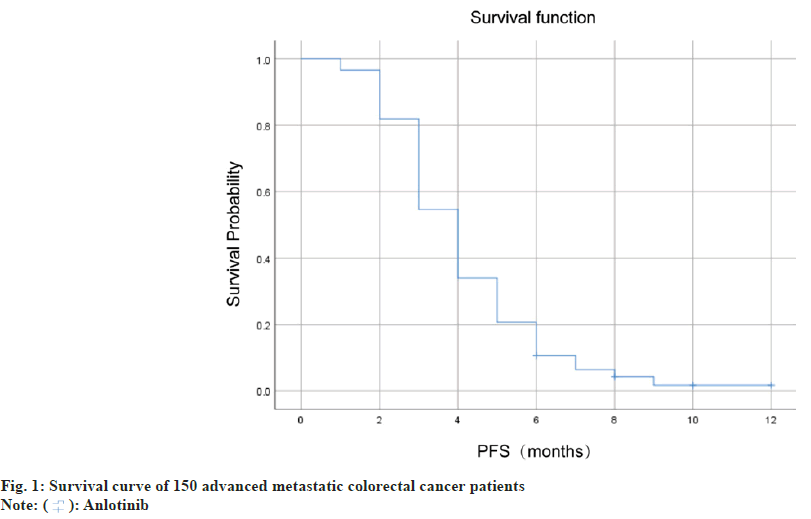
Colorectal cancer is one of the most frequent malignant tumors worldwide, and it is also a leading cause of death[1]. According to the statistics of International Agency for Research on Cancer, colorectal cancer is one of the third most common type of cancer, after lung and breast cancer[2]. Early distant metastasis of colorectal cancer is a manifestation of the final stage. At present, the most recognized treatment for metastatic colorectal cancer is a combination of cytotoxic and targeted agents, mainly oxaliplatin, irinotecan and fluorouracil[3]. Although the therapeutic effect for metastatic colorectal cancer has been significantly improved and has been widely implemented in recent years[4], drug resistance is a common problem encountered by tumor patients during the further evolution of cancer[5]. In recent years, a variety of new anticancer drugs have emerged with anlotinib being one of them. Regorafenib, a multi-target tyrosine kinase inhibitor, regorafenib has been approved by the Food and Drug Administration (FDA) as a third-line or beyond treatment for advanced colorectal cancer[6]. After platinum chemotherapy, fluorouracil chemotherapy and temozolomide, anlotinib is a third-line or beyond drug in treating advanced metastatic colorectal cancer[7,8]. Anlotinib inhibits capillary endothelial growth factor protein kinase, fibroblast growth factor protein kinase, and other targets to inhibit the transformation of the tumor to capillaries, thus achieving the purpose of inhibiting tumor cell proliferation[9]. Anlotinib, as a third-line or beyond drug, has been shown to be effective in patients with metastatic colorectal cancer who have failed all treatment options. Available data show that anlotinib has good tolerance and moderate efficacy, but the therapeutic effect is still limited. Through a more in depth and clear understanding of the efficacy and safety of the drug, we can provide better guidance for clinical treatment[10,11]. This research aims to comprehensively evaluate the safety and efficacy of anlotinib, and quantitatively evaluate its effect on advanced metastatic colorectal cancer above the third line. To achieve this, we recruited advanced metastatic colorectal cancer patients who had previously received second-line chemotherapy. All selected patients received more than three-line treatment of anlotinib, and relevant clinical data and biological samples were obtained before and after the operation. By comparing the results of clinical imaging and biological examination before and after treatment, we will evaluate the patient's response to treatment and changes in survival time, and record drug side effects. We hope to find the advantages and disadvantages of anlotinib in the treatment of advanced colorectal cancer through this study, so as to provide a better choice for clinical treatment. We retrospectively analyzed clinical data collected from 150 patients with advanced metastatic colorectal cancer who were treated in the oncology department of a tertiary hospital between January 2019 and January 2022. Inclusion criteria including the aged between 18 to 75 y; diagnosis of advanced metastatic colorectal cancer pathologically or in accordance with clinical criteria; completed surgical resection or without surgical treatment; received second-line standard chemotherapy and failed, and received anlotinib treatment after progression, and completed follow-up for at least 6 mo. Exclusion criteria including the suffering from other malignant tumors; combined with cellular immunotherapy; not completed chemotherapy or anlotinib treatment required; patients with severe cardiopulmonary diseases, and abnormal liver and kidney function; mental and intellectual abnormalities preventing data matching and follow-up; abnormal blood coagulation and poor blood pressure control in hypertension patients. All patients were treated with anlotinib (Zhengda Tianqing Pharmaceutical Group Co., Ltd). They were given 12 mg drug once every 21 d as a cycle. The medication would be taken for 14 d and stopped for 7 d. Evaluation of curative effect was done every 2 cycles. The dosage of anlotinib was reduced or stopped if severe side effects were experienced. All 150 patients were followed up through inpatient and outpatient medical record systems and telephone. The deadline for follow-up was December 23, 2022. Progression-Free Survival (PFS) refers to the cutoff time from anlotinib initiation to disease progression, death or follow-up. Short-term efficacy of anlotinib was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria after 2 cycles of treatment. Treatment outcomes were classified as Progression Disease (PD), Stability Disease (SD), Partial Response (PR) and Complete Response (CR). Disease Control Rate (DCR)=(CR+PR+SD)/(total cases)×100 %; Objective Response Rate (ORR)=(CR+PR)/(total number of cases)×100 %. Adverse reactions were classified into mild (≤grade 2), moderate (3-4) and severe (≥5) according to the National Cancer Institute General Toxicity Criteria (CTCAE) 4.0. Data were analyzed using Statistical Package for the Social Sciences (SPSS) 23.0 software, Kaplan-Meier method and Log-rank test for survival analysis. Cox proportional risk regression model was used for multivariate analysis. When the p<0.05, the difference was considered statistically significant. 150 patients were included in this research, with 88 male and 62 female participants between the ages of 45 and 74. Of these patients, 81 were over 60 y old, while the remaining 69 were below 60. In terms of tumor location, 73 cases were located in the left colon and 77 cases in the right colon. The cohort comprised of 78 cases with liver metastasis, 29 cases with lung metastasis, and 61 patients with a history of operation. In addition, 52 cases had an Eastern Cooperative Oncology Group (ECOG) score of 0-1, while 98 cases scored 2 points as shown in Table 1. After 2 treatment cycles, the short-term efficacy was determined by RECIST 1.1 standard, including 0 cases of CR, 15 cases of PR, 81 cases of SD and 54 cases of PD. ORR was 10 % and DCR was 64 % as shown in Table 2. In this research, the median PFS of 150 advanced metastatic colorectal cancer patients was 4.1 mo, and the survival curve was shown in fig. 1. Univariate analysis showed that liver metastases and ECOG scores were associated with PFS, but not with age, sex, tumor location, lung metastases, surgical history or Rat Sarcoma (RAS) mutations. The results of multivariate analysis showed that liver metastasis was an independent factor affecting the treatment of advanced metastatic colorectal cancer (p<0.05). The results of univariate analysis of all included factors are shown in Table 3. 150 patients were involved in this research, and there was no death caused by adverse reactions. Among them, the relatively high incidence of complications are hypertension, fatigue, nausea, vomiting, albuminuria and hypothyroidism, as shown in Table 4. As one of the most common malignant tumors in the world, colorectal cancer is a major threat to human health[12]. Compared to early colorectal cancer, advanced metastatic colorectal cancer is harder to treat with limited curative options[13,14]. Anlotinib is a molecular targeted drug which can inhibit the growth, differentiation and metastasis of cancer cell by targeting tumor cell receptor tyrosine kinases[6]. Nevertheless, the efficacy of Anlotinib in the treatment of advanced metastatic colorectal cancer remains controversial. Therefore, the purpose of this study was to investigate the efficacy of anlotinib and its influencing factors, and to provide a reference for further guiding the clinical application of anlotinib. The study revealed that the ORR of anlotinib in treating advanced metastatic colorectal cancer was 10 %, while DCR was 64 %. The median PFS was only 4.1 mo, indicating that the efficacy of anlotinib still has certain limitations. In a study on the treatment of advanced metastatic colorectal cancer with three or more line or beyond of regorafenib, it was found that the median PFS was 3.5 mo, which was comparable to that of anlotinib [15]. Similar to our findings, a domestic study reported a median PFS of 4.1 mo for patients treated with anlotinib, while the most frequent complication was hypertension[16]. Additionally, another retrospective study found the median PFS of patients in the anlotinib group to be 3.46 mo[17]. We further analyzed several factors affecting the treatment of advanced metastatic colorectal cancer with anlotinib in third line and above. The results of our univariate analysis show that sex, age, tumor location, lung metastasis, surgical history and RAS gene mutations were not associated with PFS. Hence, these factors may not be effective predictors of anlotinib treatment. However, liver metastasis and ECOG score were found to be related to the PFS. Our findings suggest that treatment options may need closer monitoring when colorectal cancer patients develop liver metastasis. Similar to our study, domestic research asserts that liver and lung metastasis are independent prognostic factors that affect the PFS of advanced metastatic colorectal cancer treated with anlotinib, which may be related to the tumor's load[10]. The results of multivariate analysis showed that liver metastasis was an independent factor affecting the treatment of advanced metastatic colorectal cancer (p<0.05). We recommend that clinicians be attentive to liver metastases during anlotinib treatment and carry out timely examinations and treatment to improve therapeutic effects. Regarding complications, anlotinib carries relatively high incidence rates of hypertension, fatigue, nausea and vomiting, albuminuria and hypothyroidism. The emergence of these complications can significantly affect the smooth progress of patient treatment, and therefore, timely diagnosis and treatment is necessary. Although this study has produced some meaningful results, it still has some limitations. One of these limitations is the relatively small sample size. While this study included 150 patients, the small sample size could potentially affect the accuracy of the statistical significance of some of the results. Additionally, there was a lack of molecular biological analysis of the therapeutic response in this study, which limits the promotion of molecular precision medicine research and treatment. Despite these limitations, our study reveals that anlotinib has some therapeutic effects for advanced metastatic colorectal cancer patients, even with some adverse reactions. It is essential for clinicians to consider patients' specific details, including their liver condition and other factors. They should also carry out targeted supervision and treatment when choosing this treatment plan. We also recommend that more research be conducted to better understand the longterm efficacy and adverse reactions of anlotinib in the future.
| Features | Cases (n) | Proportion (%) |
|---|---|---|
| Gender | ||
| Male | 88 | 58.7 |
| Female | 62 | 41.3 |
| Age | ||
| >60 | 81 | 54 |
| ≤60 | 69 | 46 |
| Tumor location | ||
| Left colon | 73 | 48.7 |
| Right colon | 77 | 51.3 |
| Liver metastases | ||
| No | 72 | 48 |
| Yes | 78 | 52 |
| Lung metastases | ||
| No | 121 | 80.7 |
| Yes | 29 | 19.3 |
| ECOG score | ||
| 0-1 point | 52 | 34.7 |
| 2 points | 98 | 65.3 |
| Surgical history | ||
| No | 89 | 59.3 |
| Yes | 61 | 40.7 |
| RAS gene mutation | ||
| Mutant type | 80 | 53.3 |
| Wild type | 70 | 46.7 |
Table 1: Clinical Data of Advanced Metastatic Colorectal Cancer Patients
| CR | PR | SD | PD | ORR | DCR | |
|---|---|---|---|---|---|---|
| Cases (n) | 0 | 15 | 81 | 54 | 15 | 96 |
| Proportion (%) | 0 | 10 | 54 | 36 | 10 | 64 |
Table 2: Comparison of the Short-Term Efficacy of Anlotinib in Treating Advanced Metastatic Colorectal Cancer Patients
| Features | Cases (n) | Median PFS (months) | c2 | p |
|---|---|---|---|---|
| Gender | 0.458 | 0.625 | ||
| Male | 88 | 3.8 | ||
| Female | 62 | 4.2 | ||
| Age | 0.532 | 0.367 | ||
| >60 | 81 | 3.9 | ||
| ≤60 | 69 | 4.2 | ||
| Tumor location | 4.362 | 0.086 | ||
| Left colon | 73 | 4.4 | ||
| Right colon | 77 | 3.8 | ||
| Liver metastases | 6.134 | 0.002 | ||
| No | 72 | 5.3 | ||
| Yes | 78 | 3.0 | ||
| Lung metastases | 0.023 | 0.862 | ||
| No | 121 | 4.2 | ||
| Yes | 29 | 3.6 | ||
| ECOG score | 4.278 | 0.015 | ||
| 0-1 point | 52 | 5.5 | ||
| 2 points | 98 | 3.4 | ||
| Surgical history | 0.152 | 0.214 | ||
| No | 89 | 4.0 | ||
| Yes | 61 | 4.3 | ||
| RAS gene mutation | 1.287 | 0.771 | ||
| Mutant type | 80 | 4.3 | ||
| Wild type | 70 | 3.9 |
Table 3: The Univariate Analysis of Included Factors
| Adverse reaction | n | Incidence rate 9 (%) | 3-4 grade (n) | Incidence rate (%) | Total cases (n) | Overall incidence rate (%) |
|---|---|---|---|---|---|---|
| Hand-foot syndrome | 15 | 10 | 3 | 2 | 18 | 12 |
| Fatigue | 27 | 18 | 4 | 2.7 | 31 | 20.7 |
| Hypertension | 39 | 26 | 8 | 5.3 | 47 | 31.3 |
| Skin rash | 10 | 6.7 | 0 | 0 | 10 | 6.7 |
| Thrombocytopenia | 4 | 2.7 | 1 | 0.7 | 5 | 3.4 |
| Nausea and vomiting | 21 | 14 | 2 | 1.3 | 23 | 15.3 |
| Leukopenia | 15 | 10 | 3 | 2 | 18 | 12 |
| Hypothyroidism | 18 | 12 | 1 | 0.7 | 19 | 12.7 |
| Abnormal liver function | 4 | 2.7 | 0 | 0 | 4 | 2.7 |
| Albuminuria | 17 | 11.3 | 3 | 2 | 20 | 13.3 |
Table 4: Adverse Reactions Comparison among Patients in Each Group
Conflict of interests:
The authors declared no conflict of interests.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7-34.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Jiang Z, Shen J. Meta-analysis of prophylactic enterostomy in laparoscopic anterior resection of rectal cancer. World's Latest Med Inform Abstracts 2019;14:2.
- Sandhu J, Lavingia V, Fakih M. Systemic treatment for metastatic colorectal cancer in the era of precision medicine. J Surg Oncol 2019;119(5):564-82.
[Crossref] [Google Scholar] [PubMed]
- Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol 2015;21(41):11767.
[Crossref] [Google Scholar] [PubMed]
- Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013;153(1):13-9.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Zhao X. Efficacy and safety of anlotinib in the treatment of elderly patients with advanced colorectal cancer. Chin Med J 2021;56(6):4.
- Syed YY. Anlotinib: First global approval. Drugs 2018;78(10):1057-62.
[Crossref] [Google Scholar] [PubMed]
- Si X, Zhang L, Wang H, Zhang X, Wang M, Han B, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer 2018;122:32-7.
[Crossref] [Google Scholar] [PubMed]
- Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11(1):120.
[Crossref] [Google Scholar] [PubMed]
- Qiu T, Zhou Y, Chen X, Gu Y, Sun Y. Clinical observation of anlotinib posterior line in the treatment of advanced metastatic colorectal cancer. J Clin Oncol 2020;25(10):4.
- Liu Y, Li Xi, Chang J. Analysis of the efficacy of anlotinib in the treatment of advanced colorectal cancer. Chin J Med 2020;18(10):8-10.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
[Crossref] [Google Scholar] [PubMed]
- Rahimi Pordanjani S, Baeradeh N, Lotfi MH, Pourmohammadi B. Epidemiology of colorectal cancer: Incidence, mortality, survival rates and risk factors. Razi J Med Sci 2016;23(144):41-50.
- Ren Y, Wang K, Huang L. Relationship between expression of KiSS-1 in colorectal cancer and clinicopathological features and prognosis of patients. Chin Med J 2019;54(5):4.
- Yan J. Observation on the efficacy and adverse reactions of regorafenib in the treatment of metastatic colorectal cancer. Appl Mod Med China 2022;15(24):171-3.
- Chi Y, Shu Y, Ba Y, Bai Y, Qin B, Wang X, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist 2021;26(10):e1693-703.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Du FC, Fang FQ, Duan ZJ, Lei W, Shi KG. Third-line treatment for metastatic colorectal cancer: Anlotinib is superior to chemotherapy and similar to fruquintinib or regorafenib. Neoplasma 2020;67(6):1384-90.
[Crossref] [Google Scholar] [PubMed]
 : Anlotinib
: Anlotinib



