- *Corresponding Author:
- R. AldahashDepartment of General Medicine, Dr. D.Y. Patil Medical College, Hospital & Research Centre, Dr. D.Y. Patil Vidyapeeth, India E-mail: alhjlah@su.edu.sa
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “109-116” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Our retrospective study aimed to evaluate the effectiveness of monoclonal antibodies (casirivimab and imdevimab) on mild cases of coronavirus disease 2019 patients admitted to the tertiary care center. A total of 161 patients were evaluated of which the test group consisted of 79 and the control group of 82. In the test group the patients had been administered with diluted 250 ml of 0.9 % sodium chloride along with co-formulated casirivimab (600 mg) and imdevimab (600 mg) solution intravenously and in the control group the patients were administered standard coronavirus disease 2019 treatment protocol. The monitoring of patients in both groups was done at least 1 h after drug infusion in the designated room. Post-treatment designed interviews were taken to evaluate the effectiveness of treatment. This retrospective analysis discovered a significant association of symptoms with the group at 48 h for injected and non-injected patients and 1 mo from the chi-square test after injecting monoclonal antibodies. There is no significant association of symptoms with the groups at 3 mo. A significant difference in the symptom distribution through different time points in the injected group and not injected group was observed. From the pairwise McNemar’s test, a significant difference in the symptoms between each time in 48 h, the difference was p=0.0075 and after 1 mo, p<0.001 points in both groups. The combination of casirivimab and imdevimab could be considered a treatment of choice for vaccinated, non-vaccinated and mild to highrisk coronavirus disease 2019 patients.
Keywords
Monoclonal antibody cocktail, casirivimab, imdevimab, coronavirus disease 2019
Coronavirus was first seen in late 2019 in China and it is a huge family from Nidovirales order that has various side effects from the usual cold to intense viral infections[1-3]. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), as a new member of this family, outbreak and subsequent coronavirus pandemic have negatively impacted the public health system, necessitating the development of effective therapeutic strategies[4,5]. Even though most Coronavirus Disease 2019 (COVID-19) cases are asymptomatic or having mild to moderate symptoms, up to 10 % of individuals might develop a severe disease that requires hospitalization[5], as far as, this illness leads to intense respiratory disease in infected patient[6]. The high pandemic mortality rate and morbidity encouraged clinicians to search appropriate adjunctive therapeutic methods to treat this disease[7]. Omicron, the novel COVID-19 strain, triggered the third pandemic wave[5]. According to recent World Health Organization (WHO) statistics, nearly 5 million people have died from the new strain of COVID-19 and the count is regularly increasing worldwide[8]. The population of the United States and those of other countries are on the verge of a catastrophic calamity. Despite the vaccination’s success in reducing COVID-19 outbreaks, the situation necessitates advanced and potential treatment strategies for eradicating the virus in the clusters of people infected. Monoclonal antibodies are effective as well as safe when treating viral infections, reducing the risk of viral infection. These antibodies directly bind with the neutralizing antigen and stimulate the antibody-mediated phagocytosis[9].
The United States Food and Drug Administration (USFDA), Central Drug Standard Control Organization (CDSCO) and the European Medical Agency (EMA) have issued Emergency Use Authorization (EUA) for Casirivimab (CAS) and Imdevimab (IMD), two Immunoglobulin G1 (IgG1) anti-SARS-CoV-2 monoclonal antibodies. These medicines prevent the virus from entering host cells by adhering to receptor-binding region of SARS-spike CoV-2's glycoprotein[10]. CAS and IMD combination is recommended for mild to high-risk patients with diabetes mellitus (Hemoglobin A1c (HbA1c)>10 %), chronic liver, respiratory diseases, kidney diseases (estimated glomerular filtration rate 60 ml/min), cardiovascular diseases, immunocompromising diseases, malignancies or those with a body mass index of 35 kg, aged 65 y and other indications approved by institutional medical boards. For those over 12 y of age and atleast 40 kg, the recommended dose for each medicine is 600 mg[11]. The diluted mixture must be given as a single intravenous infusion lasting for a minimum of 1 h or as a subcutaneous injection[10]. There is paucity of evidence of the combinations used in coronavirus, population differences are likely an issue and more research is needed. A recent trial on safety and efficacy of CAS and IMD in coronavirus is a double-blinded, two-part trial in the United States[12]. Another trial published in the same year, on CAS and IMD concluded to have lowered hospitalization or deaths by 70 % in non-hospitalized patients with coronavirus[13]. There is a death of research in this domain acknowledged in India. Even though this combination has gained worldwide attention and is being prescribed by most physicians globally, it emphasizes the importance of further research into the medications influence on the Indian people[14]. Hence, the current study aims to evaluate effectiveness of CAS and IMD on mild cases of COVID-19 patients admitted in the tertiary care center.
Materials and Methods
A retrospective comparative observational research was conducted on SARS-CoV-2 patients to assess the influence of the CAS and IMD antibody cocktail which was conducted in a general medical hospital in South India for 5 mo (duration) after receiving ethical approval from the institutional ethical committee.
Study procedure:
All in-patients and out-patients confirmed with COVID-19 by antigen test or Reverse Transcription-Polymerase Chain Reaction (RT-PCR) without hypoxia (oxygen saturation of 93 % or higher) were enrolled for this study. The research purpose was explained to patients in their native language (risk and benefits of both the drugs) and a written informed consent was taken. The patients between 18 y to 80 y old and had COVID-19 symptoms onset for less than 10 d, with one or more comorbidities elevating disease risk or severity were included. The study excluded patients with moderate to severe stages of disease during admission and those with a history of anaphylaxis reaction to the mentioned drugs. 161 patients were enrolled in the study and were divided into test and control groups. This research was divided into 2 phases.
Phase 1 data collection and standard COVID-19 treatment with an antibody cocktail: The patients were then divided into two groups for comparison. The test group (n=79) included patients treated with CAS and IMD antibody cocktail, while controls (n=82) consisted of standard anti-COVID-19 treatments. The clinical findings and demographics of patients were taken through the predesigned questionnaire that the general medical physician of the hospital approved. The questionnaire consists of patient demographics like patient identification number, age, gender, COVID positive date, admission date and length of hospital stay.
Patients enrolled in the test group were administered with diluted, 250 ml of 0.9 % sodium chloride and co-formulated CAS (600 mg) and IMD (600 mg) solution intravenously. However, the controls were administered with standard COVID-19 treatment protocol. Clinical monitoring of patients in the test group was done at least 1 h after drug infusion in the designated room. Any adverse effects of drugs were promptly noted, treated and documented.
Phase 2 feedback of patient on antibody cocktail study: Patients were recalled at regular intervals after 3 d, the 1st and the 3rd w and 3 mo to review and evaluate their post-COVID-19 treatment experiences. After consulting with the COVID-19 general medicine physicians at the hospital, well-designed interview questions were prepared. Patients in both groups were asked questions about their health. What is the current state of health following the COVID-19 therapy?, treatment satisfaction (Did you face any discomfort throughout the injection? (if yes, what is the reason?). Are you satisfied with the treatment regimen? (if yes/no, why), post-COVID-19 issues, adverse reactions and re-hospitalization. Were you readmitted to the hospital after being treated for COVID-19? What were the reasons for re-admission (apart from COVID-19)? The negative effect of the drug was noted and treatment requirements were also verified. Any long-term COVID symptoms were also taken into consideration. The patient’s information was retrieved in less than 5 min.
Statistical analysis:
R 4.1.2 software and excel were used to analyze the data. The frequency table was made to represent categorical data. Mean, Standard Deviation (SD)/Median (min, max) were used for continuous variables. For categorical variables, chi-square test was employed. Two sample t-test were employed to compare the means of variables over groups. Cochran’s Q test compares the distribution of symptoms between time points. Pairwise McNemar’s test was performed for post hoc analysis. A p-value less than or equal to 0.05 indicated statistical significance.
Results and Discussion
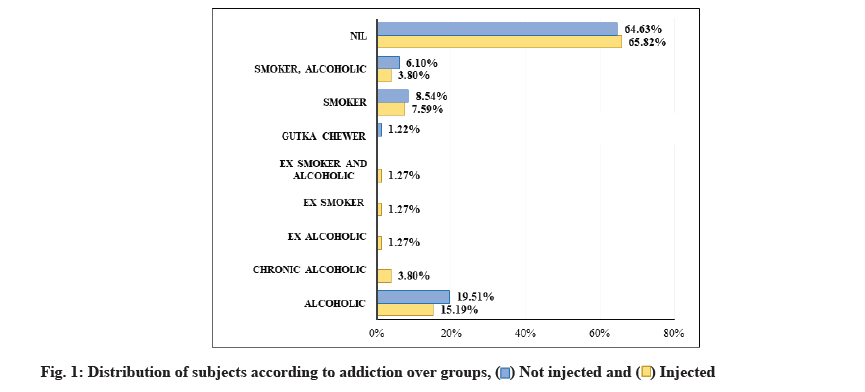
The study comprised of 161 patients, 79 patients in test and 82 patients in control groups. The median age of the patients included in this study was 51.8±14.38 y, amongst which 59.63 % were male and 40.37 % were female (Table 1). Monte Carlo simulation test showed no statistical difference in the age group of patients. At the same time, the chi-square test reported statistical significance while comparing adverse outcomes over addictions (fig. 1). However, no statistical significance was noted amongst gender, clinical onset and vaccination status over the groups when compared using the chi-square test. Table 1 represents a comparison of the groups.
| Variables | Sub category | Group | Total | p-value | |
|---|---|---|---|---|---|
| Injected | Not injected | ||||
| Age (y) | 20-39 | 18 (22.78 %) | 14 (17.07 %) | 32 (19.88 %) | 0.3658 MC |
| 40-59 | 40 (50.63 %) | 40 (48.78 %) | 80 (49.69 %) | ||
| 60-79 | 20 (25.32 %) | 23 (28.05 %) | 43 (26.71 %) | ||
| ≥80 | 1 (1.27 %) | 5 (6.1 %) | 6 (3.73 %) | ||
| Mean±SD Median (min, max) |
50.18±13.14 52 (24, 86) |
53.37±15.4 53.5 (20, 83) |
51.8±14.38 52 (20, 86) |
0.1601 t | |
| Gender | Female | 32 (40.51 %) | 33 (40.24 %) | 65 (40.37 %) | 0.9729 C |
| Male | 47 (59.49 %) | 49 (59.76 %) | 96 (59.63 %) | ||
| Addictions | Alcoholic | 12 (15.19 %) | 16 (19.51 %) | 28 (17.39 %) | <0.001 MC* |
| Chronic alcoholic | 3 (3.8 %) | 0 | 3 (1.86 %) | ||
| Ex-alcoholic | 1 (1.27 %) | 0 | 1 (0.62 %) | ||
| Ex-smoker | 1 (1.27 %) | 0 | 1 (0.62 %) | ||
| Ex-smoker and alcoholic | 1 (1.27 %) | 0 | 1 (0.62 %) | ||
| Gutka chewer | 0 | 1 (1.22 %) | 1 (0.62 %) | ||
| Smoker | 6 (7.59 %) | 7 (8.54 %) | 13 (8.07 %) | ||
| Smoker, alcoholic | 3 (3.8 %) | 5 (6.1 %) | 8 (4.97 %) | ||
| Nil | 52 (65.82 %) | 53 (64.63 %) | 105 (65.22 %) | ||
| Dose | 1st dose | 23 (29.11 %) | 40 (48.78 %) | 63 (39.13 %) | 0.0381 C |
| 2nd dose | 28 (35.44 %) | 21 (25.61 %) | 49 (30.43 %) | ||
| Unvaccinated | 28 (35.44 %) | 21 (25.61 %) | 49 (30.43 %) | ||
| Type vaccine | COVAXIN | 18 (22.78 %) | 26 (31.71 %) | 44 (27.33 %) | 0.2925 C |
| COVISHIELD | 33 (41.77 %) | 35 (42.68 %) | 68 (42.24 %) | ||
| Unvaccinated | 28 (35.44 %) | 21 (25.61 %) | 49 (30.43 %) | ||
| Clinical worsening | No | 77 (97.47 %) | 63 (76.83 %) | 140 (86.96 %) | <0.001 MC* |
| Stage 1 to 2 | 2 (2.53 %) | 19 (23.17 %) | 21 (13.04 %) | ||
Note: C: Chi-square test; MC: Monte Carlo simulation; t: Two-sample t-test and *indicates statistical significance.
Table 1: Comparison of different variables over groups.
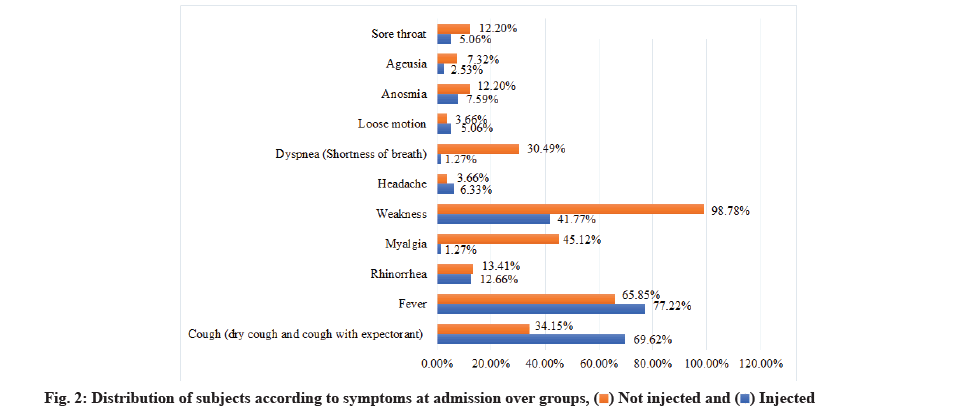
There was a significant association between cough, myalgia, weakness and dyspnea between the groups from the Chi-square test as shown in Table 2 and fig. 2.
| Symptoms at admission | Groups | Total | p-value | |
|---|---|---|---|---|
| Injected | Not injected | |||
| Cough (dry cough and cough with expectorant) | 55 (69.62 %) | 28 (34.15 %) | 83 (51.55 %) | <0.001 C* |
| Fever | 61 (77.22 %) | 54 (65.85 %) | 115 (71.43 %) | 0.1106 C |
| Rhinorrhea | 10 (12.66 %) | 11 (13.41 %) | 21 (13.04 %) | 0.8867 C |
| Myalgia | 1 (1.27 %) | 37 (45.12 %) | 38 (23.6 %) | <0.001 C* |
| Weakness | 33 (41.77 %) | 81 (98.78 %) | 114 (70.81 %) | <0.001 C* |
| Headache | 5 (6.33 %) | 3 (3.66 %) | 8 (4.97 %) | 0.5122 MC |
| Dyspnea (Shortness of breath) | 1 (1.27 %) | 25 (30.49 %) | 26 (16.15 %) | <0.001 MC* |
| Loose motion | 4 (5.06 %) | 3 (3.66 %) | 7 (4.35 %) | 0.7286 MC |
| Anosmia | 6 (7.59 %) | 10 (12.2 %) | 16 (9.94 %) | 0.3294 C |
| Ageusia | 2 (2.53 %) | 6 (7.32 %) | 8 (4.97 %) | 0.2954 MC |
| Sore throat | 4 (5.06 %) | 10 (12.2 %) | 14 (8.7 %) | 0.1084 C |
Note: C: Chi-square test; MC: Monte Carlo simulation and *indicates statistical significance.
Table 2: The following table gives the comparison of Symptoms at Admission over Groups.
There was a significant association of symptoms with the groups at 48 h for injected and non-injected patients (p=0.0075) and 1 mo (p<0.01) from the Chi-square test. There is no significant association of symptoms with the group at 3 mo (Table 3).
| Time points | Symptoms | Group | p-value | |
|---|---|---|---|---|
| Injected | Not injected | |||
| 24 h | Absent | 0 | 0 | - |
| Present | 79 (100 %) | 82 (100 %) | ||
| 48 h | Absent | 19 (24.05 %) | 7 (8.54 %) | 0.0075 C* |
| Present | 60 (75.95 %) | 75 (91.46 %) | ||
| 1 mo | Absent | 44 (55.7 %) | 24 (29.27 %) | <0.001 C* |
| Present | 35 (44.3 %) | 58 (70.73 %) | ||
| 3 mo | Absent | 71 (89.87 %) | 70 (85.37 %) | 0.386 C |
| Present | 8 (10.13 %) | 12 (14.63 %) | ||
| p-value | <0.001 CQ* | <0.001 CQ* | - | |
Note: CQ: Cochran’s Q test; C: Chi-square test and *indicates statistical significance.
Table 3: Comparison of Symptoms at different Time Point over Groups.
From Cochran’s Q test, a significant difference in the distribution of symptoms between time points in injected group (p<0.01) and not injected group (p<0.01) was observed. From the pairwise McNemar’s test, a significant difference in the symptoms between injected group and not injected group each time in 48 h was observed, the difference was p=0.0075 and after 1 mo the difference was p<0.001 point in both groups (Table 3).
This observational study evaluated the effectiveness of CAS and IMD on mild cases of COVID-19. The treatment of coronavirus with CAS and IMD (REGN-COV2) was on the basis of FDA and EUA for outpatient care[15]. To neutralize the pathogenic effects of coronavirus, these antibodies bind to a specific cell surface of the virus[15]. A study done by Deb et al. reported monoclonal antibodies to be safer and more efficient in targeting SARS-CoV-2 surface proteins than alternative techniques such as convalescent plasma[16]. REGN-COV2 is most effective at lowering infection rates in patients without antibodies at baseline[15].
Similarly, Weinreich et al. found that individuals who were not vulnerable to infection at baseline had a relatively high viral load on 7th d of the post-infusion[12]. Recent research shows that combining these two monoclonal antibodies is more beneficial than one monoclonal antibody in treating COVID-19 patients[12,17,18]. CAS and IMD target the spike protein on distinct epitopes, resulting in the REGN-COV2 effectiveness[17,18]. Baum et al. further stated that the cocktail’s combination in REGN-COV2 limits rapid mutational escape, an important factor that given the widespread emergence of mutant SARS-CoV-2 strains[19].
Due to the pandemics termination ambiguity, monoclonal antibodies were proposed to help patients cope with COVID-19. Because of its long half-life and single-dose schedule, Magleby et al. called for developing new antibody therapies[20]. However, the cost prevented patients in developing nations from receiving this comprehensive treatment. In India, the majority of the older population is economically destitute. They are obliged to proceed with additional COVID-19 treatments with significant adverse drug reactions[19,21]. This was the primary reason for the restricted number of samples (n=79) available for the CAS and IMD treatment groups. Furthermore, the Indian Ministry of Health recommended half of the doses indicated by the FDA and EMA (CAS 1200 mg and IMD 1200 mg) for mild COVID-19 outpatients[14,22,23].
The main objective of the current study was to discuss the clinical implications of CAS and IMD in coronavirus symptomatic individuals. According to a recent study by O'Brien et al., on monoclonal antibody cocktails, there was a significant reduction in PCR-positive coronavirus compared to a control group[9]. The viral infection in their study population was reported in less than a week and required medical attention, especially in patients with hypoxia[9]. However, the subjects in our study have shown no significant association in the duration of hospital stay within both groups. Similarly, in phase 1 to 3 clinical trials conducted in the United States of America, only a small number of patients given the antibody cocktail, required medical attention[10].
Furthermore, Ganesh et al. reported that a few of their patients required ventilator support; however, 2.83 % of their CAS and IMD subjects were hospitalized and 7.56 % required medical treatment only a few had been sent to the intensive care unit[16]. In the current study, few patients (2.53 %) injected with an antibody cocktail were readmitted during their post-COVID phase. This was substantially lower when patients on regular coronavirus therapy were considered (23.17 %). CAS and IMD, although, are not suggested for coronavirus patients with hypoxia[18]. Moreover, in the current study, we have excluded patients with hypoxia, as studies indicate the negative effect of monoclonal antibodies on patients with hypoxia[8,13].
The current study portrayed that most patients are immunized with the first dose of the vaccine. The government of India has also recommended COVID-19 antibody cocktail treatment for vaccinated patients. However, patients were instructed not to take any vaccine till 90 d after taking the antibody treatment regimen[24,25]. This study also reported a reduction in the need for mechanical ventilation and Intensive Care Unit (ICU) support following CAS and IMD, even for the non-immunized patients which could be owing to efficacy of the antibody cocktail’s reduced response to interfering with the vaccine-induced immune response[26]. A similar study was also in agreement with the current findings[27].
Our findings suggested a significant association between adverse habits such as alcohol (3.08 %), smokers (7.59 %) and treatment outcomes. Similarly, a systematic review by Siemieniuk et al. indicated a delay in response to monoclonal antibody treatment for the patient with alcohol addiction[28]. However, more studies are needed in various hospital settings to support these findings. Of 79 patients in the experimental group, 77 (89.97 %) patients reported no post COVID symptoms after 3 mo of receiving an antibody regimen. However, 71 patients recovered completely in the control group, while 12 patients reported some post-COVID symptoms. Consequently, previous studies performed to check the efficacy of antibody cocktails and the complete recovery of patients within 30-50 d[11,19,28]. However, the controls had a higher rate of adverse effects compared to the test group, which could be attributed to different treatment modalities. Both groups reported no hypersensitivity or adverse reaction at the injection site. Throughout the efficacy evaluation period, no emergency department visits or hospitalization were seen among the experimental group. After administering the antibody cocktail regimen, the concentration of antibodies in serum (according to the data) was recorded well above the target neutralization concentration as early as the 1st d of injection throughout the efficacy assessment period.
The above data supports the potential use of an antibody cocktail to prevent SARS-CoV-2 infection in patients requiring immediate medical attention to reduce disease transmission from mild to severe. Recent studies have evaluated the effect of antibody cocktails in maintaining the spread of coronavirus as well as lowering post-COVID side effects[19,25,28]. The current study also showed that the CAS and IMD cocktail administration was efficacious and provided an acceptable safety profile. This combination could serve as a better treatment alternative in mild coronavirus patients.
In the future, larger sample sizes and multi-centric studies are required to investigate the outcomes of this drug combination. Changes in laboratory measurements, particularly inflammatory markers in COVID-19, can also help to create a treatment protocol for mild COVID-19 patients. The results of our study supported the use of CAS and IMD in mild cases and have reported higher post-COVID-19 treatment satisfaction. However, more research is required in a different setting to support the present study’s findings.
CAS and IMD treated patients in this study reported a reduced need for mechanical ventilation and oxygen supply and no reported mortality. After assessing each patient’s post-COVID-19 infection status, most of those on the antibody cocktail were healthy and satisfied with the treatment. As a result, the CAS and IMD regimens are practical and can be suggested for COVID-19 individuals at high risk. In hospitalized mild cases and outpatient care, clinicians may consider using monoclonal antibody cocktail infusions for patients at risk of developing severe disease progression. However, further research is required with a larger sample size to support the findings of this study.
Author’s contributions:
Vini Mehta and Sharif Alhajlah contributed equally to this work. Conceptualization: Anu Gaikwad, Vini Mehta, Chirali Shah and Mansi Agrawal; methodology and resources: Riyaz Ahamed Shaik, Mashael B. Alharbi, Vini Mehta; software and validation: Chirali Shah, Vini Mehta and Mohammed Alrouji; formal analysis: Vini Mehta; investigation: Chirali Shah, Mansi Agrawal, Anu Gaikwad, Mohannad T Hemdi; writing, original draft preparation: Riyaz Ahamed Shaik, Mashael B. Alharbi, Mohammed Alrouji, Sharif Alhajlah; writing, review and editing: Othman Alomeir, Ritu Kumar Ahmad, Mohammad Shakil Ahmad, Faisal Holil AlAnazi; visualization: Abdulreman Alharbi, Abdullah Tawakul, Khalid K. Aldossari; supervision, University commission.; project administration: Anu Gaikwad, Sharif Alhajlah, Raed Aldahash, Khalid K. Aldossari. All authors have read and agreed to the published version of the manuscript.
Acknowledgements:
The author would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Conflict of interests:
The authors declare no conflict of interest.
References
- Jaan S, Waheed S, Bashir S, Javed MS, Amjad A, Nishan U, et al. Virtual screening and molecular docking of FDA approved antiviral drugs for the identification of potential inhibitors of SARS-CoV-2 RNA-MTase protein. Int J Adv Biol Biomed Res 2021;9:105-18.
- Sahraeai R, Hooshmand F, Ghaedi M, Shafa S, Eftekharian F, Farrokhi S. Vitamin D in mild to moderate COVID-19: A retrospective cross-sectional study. J Med Chem Sci 2023;6:55-61.
[Crossref]
- Bag HG, Kivrak M, Guldogan E, Colak C. Prediction of COVID-19 severity in SARS-COV-2 RNA-positive patients by different ensemble learning strategies. Acta Med Mediterr 2022;38(2):1085-91.
- Vafaei S, Razmi M, Mansoori M, Asadi-Lari M, Madjd Z. Spotlight of remdesivir in comparison with ribavirin, favipiravir, oseltamivir and umifenovir in coronavirus disease 2019 (COVID-19) pandemic. Lancet 2019:1-22.
- Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 2020;12(3):e7423.
[Crossref] [Google scholar] [PubMed]
- Sahraeai R, Sarikhani Y, Kalani N, Hatami N, Abiri AA, Eftekharian F. Prevalence of gastrointestinal symptoms in patients with COVID-19 in Jahrom County, Fras Province, Southwest of Iran. J Med Chem Sci 2022;5(4):483-90.
- Jonny J, Violetta L. Coupled plasma filtration adsorption as a potential therapy for critically III COVID-19 patients. J Med Chem Sci 2022:197-203.
- Wang Z, Deng H, Ou C, Liang J, Wang Y, Jiang M, et al. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine 2020;99(48):e23327.
[Crossref] [Google scholar] [PubMed]
- O'Brien MP, Neto EF, Chen KC, Isa F, Heirman I, Sarkar N, et al. Casirivimab with imdevimab antibody cocktail for COVID-19 prevention: Interim results. Top Antivir Med 2021:33-4.
- Jaworski JP. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed J 2021;44(1):7-17.
[Crossref] [Google scholar] [PubMed]
- Hur K, Price CP, Gray EL, Gulati RK, Maksimoski M, Racette SD, et al. <?COVID-19?> factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg 2020;163(1):170-8.
[Crossref] [Google scholar] [PubMed]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021;384(3):238-51.
[Crossref] [Google scholar] [PubMed]
- Razonable RR, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bierle DM, et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. eClinicalMedicine 2021;40:101102.
[Crossref] [Google scholar] [PubMed]
- Srivastava S, Chauhan S, Patel R. Socio-economic inequalities in the prevalence of poor self-rated health among older adults in India from 2004 to 2014: A decomposition analysis. Ageing Int 2021;46(2):182-99.
- Both L, Banyard AC, van Dolleweerd C, Wright E, Ma JK, Fooks AR. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine 2013;31(12):1553-9.
[Crossref] [Google scholar] [PubMed]
- Deb P, Molla MM, Saif-Ur-Rahman KM. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health 2021;3(2):87-91.
[Crossref] [Google scholar] [PubMed]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581(7809):465-9.
[Crossref] [Google scholar] [PubMed]
- di Domenico SL, Coen D, Bergamaschi M, Albertini V, Ghezzi L, Cazzaniga MM, et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: A single-center retrospective study. Intern Emerg Med 2021;16(4):1051-60.
[Crossref] [Google scholar] [PubMed]
- Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020;369(6506):1014-8.
[Crossref] [Google scholar] [PubMed]
- Magleby R, Westblade LF, Trzebucki A, Simon MS, Rajan M, Park J, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2021;73(11):e4197-205.
[Crossref] [Google scholar] [PubMed]
- Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID‐19: Implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol 2020;31(5):454-70.
[Crossref] [Google scholar] [PubMed]
- Ganesh R, Philpot LM, Bierle DM, Anderson RJ, Arndt LL, Arndt RF, et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis 2021;224(8):1278-86.
[Crossref] [Google scholar] [PubMed]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020.
[Crossref] [Google scholar] [PubMed]
- Schultz-Cherry S, McGargill MA, Thomas PG, Estepp JH, Gaur AH, Allen EK, et al. Cross-reactive antibody response to mRNA SARS-CoV-2 vaccine after recent COVID-19-specific monoclonal antibody therapy. Open Forum Infect Dis 2021;8(9):ofab420.
[Crossref] [Google scholar] [PubMed]
- Boggiano C, Eisinger RW, Lerner AM, Anderson JM, Woodcock J, Fauci AS, et al. Update on and future directions for use of anti–SARS-CoV-2 antibodies: National institutes of health summit on treatment and prevention of COVID-19. Ann Intern Med 2022;175(1):119-26.
[Crossref] [Google scholar] [PubMed]
- Joy AP, Augustine AT, Karattuthodi MS, Parambil JC, Chandrasekher D, Danisha P, et al. The impact of casirivimab-imdevimab antibody cocktail in patients amidst and post COVID 19 treatment: A retro-prospective comparative study in India. Clin Epidemiol Glob Health 2022;14:100967.
[Crossref] [Google scholar] [PubMed]
- Falcone M, Tiseo G, Valoriani B, Barbieri C, Occhineri S, Mazzetti P, et al. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther 2021;10(4):2479-88.
[Crossref] [Google scholar] [PubMed]
- Siemieniuk RA, Bartoszko JJ, Martinez JP, Kum E, Qasim A, Zeraatkar D, et al. Antibody and cellular therapies for treatment of COVID-19: A living systematic review and network meta-analysis. BMJ 2021;374.
[Crossref] [Google scholar] [PubMed]
 .
.
 .
.



