- *Corresponding Author:
- Bihua Xia
Department of Cardiovascular Medicine,
The Second Affiliated Hospital of Guizhou Medical University,
Kaili, Guizhou 556000,
China
E-mail: 798810260@qq.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3)Spl Issue “71-75” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the efficacy and safety of rivaroxaban in the antithrombotic treatment of patients with coronary heart disease and atrial fibrillation after percutaneous coronary intervention is the objective of the study. A total of 124 patients with coronary heart disease and atrial fibrillation admitted and undergoing percutaneous coronary intervention in our hospital from August 2019 to August 2021 were selected and randomly divided into an experimental group and a control group, with 62 cases in each group. After the operation, both groups were treated with triple antithrombotic therapy for 6 mo, then switched to dual antithrombotic therapy for six more months, in which the experimental group was treated with rivaroxaban and the control group was treated with warfarin. The coagulation function of the two groups of patients before and after percutaneous coronary intervention was compared and the occurrence of thromboembolic events and bleeding events were observed at the same time. Compared with the situation before treatment, the activated partial thromboplastin time and prothrombin time in the two groups were significantly increased after treatment, with statistically significant differences (p<0.05); compared with the experimental group, the increase of activated partial thromboplastin time and prothrombin time in the control group was more significant (p<0.05). There was no significant difference in the incidence of thromboembolic events between the two groups (p>0.05). The incidence of bleeding events in the experimental group was significantly lower than that in the control group and the difference was statistically significant (p<0.05). Compared with warfarin, rivaroxaban had comparable efficacy, but a lower bleeding incidence and a safer effect in the antithrombotic treatment of patients with coronary heart disease and atrial fibrillation after percutaneous coronary intervention.
Keywords
Rivaroxaban, coronary heart disease, atrial fibrillation, percutaneous coronary intervention, antithrombotic therapy
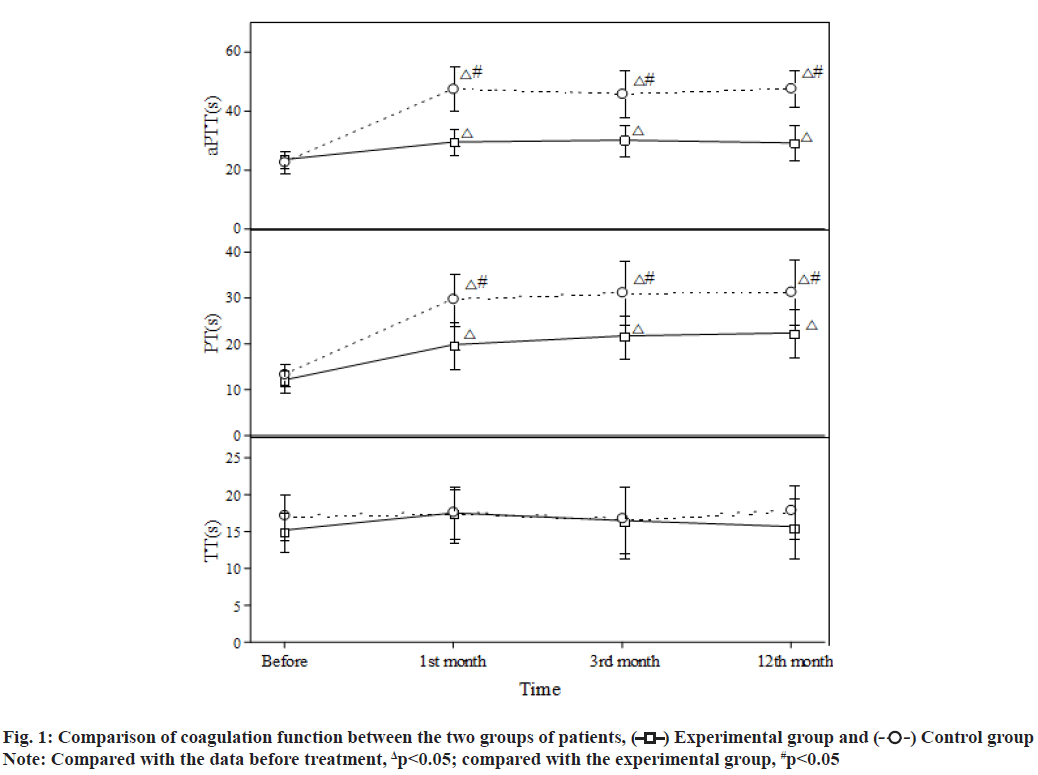
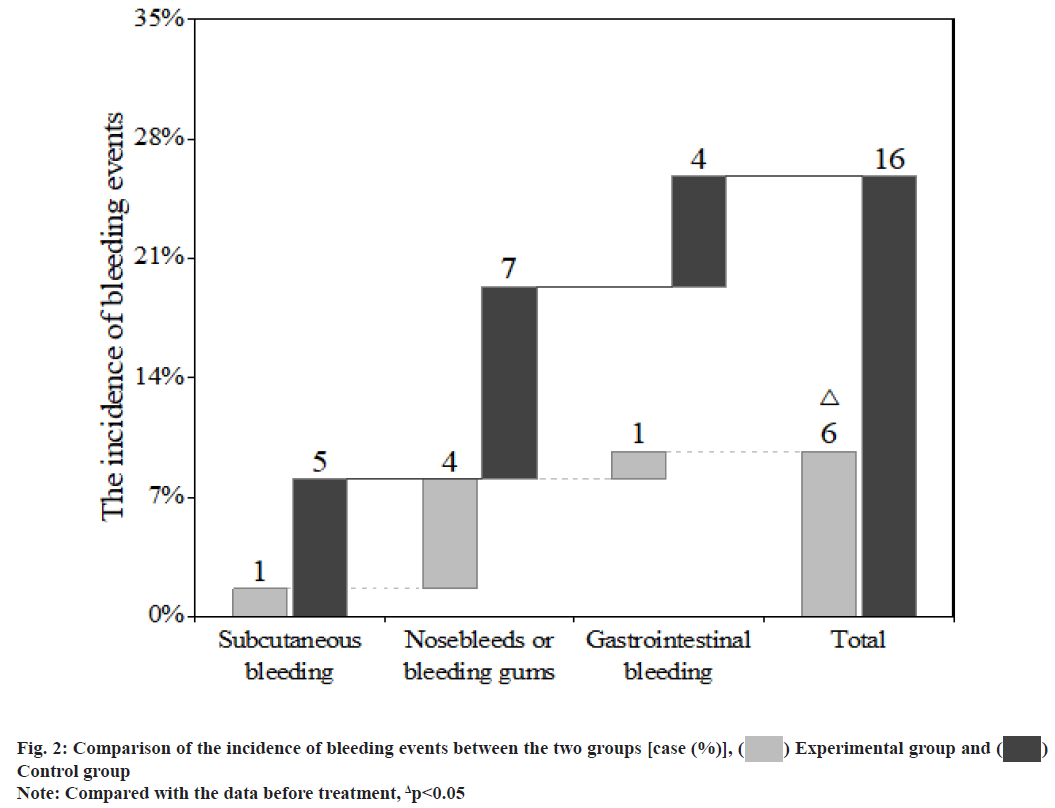
Atrial Fibrillation (AF) is the most common arrhythmia in clinical practice. AF can reduce cardiac output by 20 % to 30 %, which is more likely to cause ischaemic stroke and peripheral arterial embolism. Coronary Heart Disease (CHD) is the main cause of non-valvular AF. Clinically, about 1/3 of AF patients also have CHD and 5 % to 7 % of them need Percutaneous Coronary Intervention (PCI)[1,2]. PCI is an invasive treatment method. Routine antithrombotic therapy after PCI is necessary and crucial, as it will significantly reduce postoperative thrombosis, improve the efficacy and safety of PCI and reduce the infarction recurrence and mortality of patients. Antithrombotic therapy mainly includes antiplatelet and anticoagulant therapies. Since patients with CHD and AF need Dual Antiplatelet Therapy (DAPT) to prevent stent thrombosis after PCI and Oral Anticoagulants (OACs) to prevent AF-induced thromboembolism[3,4] their bleeding risk is further increased. How to balance the risk of thromboembolism and bleeding has become a hot issue of clinical concern. This paper aims to explore the efficacy and safety of rivaroxaban in the antithrombotic treatment of patients with CHD and AF after PCI. The report is as follows. Research subjects included in this study are as follows. A total of 124 patients with CHD and AF admitted to our hospital from August 2019 to August 2021 were included in this study. Inclusion criteria include meeting the diagnosis of CHD complicated with nonvalvular AF and undergoing PCI; no recent significant active bleeding or other contraindications to the use of antiplatelets and anticoagulants; AF stroke risk score ≥2 points (Congestive Heart Failure, Hypertension, Age≥75 (doubled), Diabetes, Stroke (doubled), Vascular Disease, Age 65 to 74 and Sex Category (female) CHA2DS2-VASc)); AF anticoagulant-induced bleeding risk (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio (INR), Elderly, Drugs/Alcohol Concomitantly (HA-BLED)) score≤2 points. Exclusion criteria include severe hepatic and renal insufficiencies; allergy to warfarin or rivaroxaban; poor medication adherence, unable to regularly monitor the INR or self-adjust, replace and stop the therapeutic drugs; malignant tumours. This study has been approved by the hospital ethics committee and patients have signed the informed consent form. Grouping and processing methods is clearly explained in detail. The 124 patients were randomly divided into an experimental group and a control group, with 62 cases in each group. The perioperative drug therapies and surgical strategies of the two groups were conducted in accordance with the Chinese Guidelines for PCI[5]. After the PCI, both groups were treated with a triple antithrombotic therapy [enteric-coated aspirin tablets (manufacturer: Bayer, Germany; approval number: Guo Yao Zhun Zi J20171021) 100 mg/d+clopidogrel tablets (manufacturer: Shenzhen Salubris Pharmaceuticals Limited; approval number: Guo Yao Zhun Zi H20000542) 75 mg/d+an OAC] for the first 6 mo, followed by a dual antithrombotic therapy [clopidogrel tablets 75 mg/d+an OAC] for the next 6 mo. In terms of the OAC, the experimental group was given rivaroxaban (manufacturer: Bayer, Germany; approval number: Guo Yao Zhun Zi J20180075) 15 mg/d and the control group was given warfarin (manufacturer: Qilu Pharmaceutical Co., Ltd.; approval number: Guo Yao Zhun Zi H37021314) once a day and the dose was adjusted to maintain the INR between 2.0-3.0. According to the patient’s conditions, both groups were given other standardized medications. Both groups were followed up for 12 mo at the same time. Observational measures used in this study are as follows. The blood coagulation measures, including activated Partial Thromboplastin Time (aPTT), Prothrombin Time (PT) and Thrombin Time (TT), in the two groups before the PCI and 1, 3 and 12 mo after the PCI were recorded; the incidence of thromboembolic events and bleeding events in the two groups was recorded. The Statistical Package for the Social Sciences (SPSS) 20.0 software system was used for statistical analysis. The measurement data were expressed as mean±standard deviation (x?±s) and analysed with the t-test; the count data were expressed by rate (%) and analysed with the chi-square (χ2) test. Statistical significance could be attained when the p-value was less than 0.05. There was no statistically significant difference between the two groups of patients in general clinical data such as age, sex, underlying diseases complicated and severity of illness (p>0.05) (Table 1). After treatment, both groups showed significantly higher aPTT and PT than those before treatment, with statistically significant differences (p<0.05) and compared with the experimental group, the aPTT and PT in the control group were significantly increased (p<0.05); for the comparison within the groups, there were no significant differences in the aPTT and PT in different time periods after treatment (p>0.05). After treatment, the INR in the control group increased significantly and the difference was statistically significant (p<0.05); for the comparison within the groups, there were no statistically significant differences in the INR in different time periods (p>0.05). There was no significant difference in the TT between the two groups before and after treatment (p>0.05) (fig. 1). After 12 mo of follow-up, there was no significant difference in the incidence of thromboembolic events between the two groups (p>0.05) (Table 2). After 12 mo of follow-up, compared with the control group, the incidence of bleeding events in the experimental group was significantly reduced and the difference was statistically significant (p<0.05) (fig. 2). CHD and AF are common cardiovascular diseases. In recent years, with the development of the global economy and the formation of an aging society, the prevalence of the diseases has been increasing year by year worldwide. CHD complicated with AF has also become a common clinical situation. They share common risk factors (such as diabetes, hypertension), jointly promote the occurrence and development of ischaemic events, organ dysfunction and death and other end-point events, and have become threats to human health and quality of life[6,7]. Since its introduction in 1977 and after more than 30 y of rapid development, PCI has become one of the most commonly used, mature and promising techniques for the treatment of CHD. It is a treatment method that uses special catheters, guide wires, balloons, stents, etc. to dredge narrowed or blocked coronary arteries under the guidance of an angiography system. Improvements in operating equipment, especially the emergence of drug-eluting stents, have greatly improved the prognosis and quality of life of patients[8,9].
| Clinical features | Experimental group (62 cases) | Control group (62 cases) | p-value |
|---|---|---|---|
| Age (x?±s, year) | 65.25±8.57 | 64.58±9.15 | 0.137 |
| Male (n/%) | 37 (59.68) | 33 (53.22) | 0.541 |
| Inside diameter of the left atrium (x?±s, mm) | 46.36±3.17 | 43.69±3.28 | 0.362 |
| Left ventricular ejection fraction (x?±s, %) | 43.7±10.4 | 42.8±11.7 | 0.531 |
| Hypertension (n/%) | 31 (50.00) | 36 (58.06) | 0.426 |
| Diabetes mellitus (n/%) | 18 (29.03) | 16 (25.81) | 0.664 |
| Stable angina pectoris (n/%) | 8 (12.90) | 11 (17.74) | 0.397 |
| Unstable angina pectoris (n/%) | 29 (46.77) | 25 (40.32) | 0.421 |
| STEMI n (%) | 16 (25.81) | 18 (29.03) | 0.662 |
| NSTEMI n (%) | 10 (16.13) | 8 (12.90) | 0.774 |
Note: STEMI: ST-Segment Elevation Myocardial Infarction and NSTEMI: Non-ST-Segment Elevation Myocardial Infarction
Table 1: Comparison of General Clinical Data of the Two Groups of Patients
| Measure | Experimental group (62 cases) | Control group (62 cases) |
|---|---|---|
| Stent thrombosis (case) | 1 | 2 |
| Ischaemic stroke (case) | 3 | 4 |
| Systemic embolism (case) | 1 | 2 |
| Incidence of embolism [case (%)] | 5 (8.06)Δ | 8 (12.90) |
Note: Compared with the data before treatment, Δp<0.05
Table 2: Comparison of the Incidence of Thromboembolic Events between the Two Groups [Case (%)]
Current knowledge and management recommendations of AF-2015[10] pointed out that for AF patients after PCI, it is generally recommended that triple antithrombotic therapy should only be used for a short period of time, followed by the dual therapy of an antiplatelet+OAC and finally switching to the monotherapy of an OAC 1 y later. The 2016 European Society of Cardiology (ESC) guidelines[11] recommended that for patients with high risk of thrombosis, the plan of a triple antithrombotic therapy for 6 mo, followed by a dual antithrombotic therapy and finally switching to an OAC monotherapy 1 y later is necessary. For patients with a high risk of bleeding, the triple therapy is recommended for 1 mo, followed by a dual therapy for the next 11 mo. It can be seen from the guidelines that triple therapy is still the initial antithrombotic regimen for patients with CHD and AF after PCI and anticoagulation therapy runs through the entire antithrombotic regimen. At present, OACs mainly include warfarin and Novel Oral Anticoagulants (NOACs). As a traditional anticoagulant, warfarin has a significant anticoagulant effect, but it is required to monitor the INR regularly to adjust the dose during use and it is easily affected by food, drugs, etc., which leads to a higher incidence of clinical bleeding. As a NOAC and an inhibitor of coagulation factor Xa, rivaroxaban selectively blocks the active site of factor Xa and exerts its anticoagulant effect[12,13]. With a stable anticoagulation effect, it neither needs to monitor coagulation function nor will be affected by food or drugs. A clinical study compared the use of rivaroxaban and the dose-controlling use of warfarin in AF patients. The results showed that the two groups had similar incidences of ischaemic stroke, but the rivaroxaban group had a significantly lower incidence of bleeding events than the warfarin group[14]. This study compared, analysed and evaluated the use of rivaroxaban and warfarin combined with antiplatelets in the antithrombotic treatment of patients with CHD and AF after PCI. The results showed that the coagulation measures PT and aPTT at the 1st, 3rd and 12th mo after PCI were significantly higher than those before treatment in both groups, suggesting that both warfarin and rivaroxaban had significant anticoagulant effects. Further analysis showed that the PT and aPTT of the warfarin group were significantly higher than those of the experimental group after treatment, suggesting that warfarin had a greater effect on blood coagulation than rivaroxaban. The results of 12 mo follow-up showed that the incidence of bleeding events in the experimental group was significantly lower than that in the control group, while there was no significant difference in the incidence of thromboembolic events between the two groups. The research results of this paper are consistent with those of Gibson et al.[15]. In summary, compared with warfarin, rivaroxaban has comparable efficacy, but a lower bleeding incidence and a safer effect when combined with antiplatelets in the antithrombotic treatment of patients with CHD and AF after PCI and shows greater clinical benefits.
Author’s contributions:
Chenyang Fu and Huichao Pan contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374(17):1609-20.
[Crossref] [Google Scholar] [PubMed]
- Chinese Medical Association, Chinese Medical Journals Publishing House, Chinese Society of General Practice. Guideline for primary care of atrial fibrillation (2019). Chin J Gen Pract 2020;19(6):465-73.
- Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59(13):1200-54.
[Crossref] [Google Scholar] [PubMed]
- Li N, Yang XC. Advances in antithrombotic therapy of coronary heart disease with atrial fibrillation. Chin J Interv Cardiol 2017;25(7):410-2.
- Interventional cardiology group, Chinese society of Cardiology, Chinese journal of cardiology committee. Chinese guidelines for percutaneous coronary intervention (2016). Chin J Cardiovasc Dis 2016;44(4):271-7.
- Durko AP, Osnabrugge RL, van Mieghem NM, Milojevic M, Mylotte D, Nkomo VT, et al. Annual number of candidates for transcatheter aortic valve implantation per country: Current estimates and future projections. Eur Heart J 2018;39(28):2635-42.
[Crossref] [Google Scholar] [PubMed]
- Waksman R, Rogers T, Torguson R, Gordon P, Ehsan A, Wilson SR, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018;72(18):2095-105.
[Crossref] [Google Scholar] [PubMed]
- Lother A, Kaier K, Ahrens I, Bothe W, Wolf D, Zehender M, et al. Bleeding complications drive in-hospital mortality of patients with atrial fibrillation after transcatheter aortic valve replacement. Thromb Haemost 2020;120(11):1580-6.
[Crossref] [Google Scholar] [PubMed]
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J. Apixaban in patients with atrial fibrillation after transfemoral aortic valve replacement. JACC Cardiovasc Interv 2017;10(1):66-74.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Huang D. Atrial fibrillation: Current understanding and treatment recommendations: 2015. Chin J Card Pacing Electrophysiol 2015;29(5):377-415.
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol Pol 2016;74(12):1359-469.
[Crossref] [Google Scholar] [PubMed]
- Liu FY, Wang R, Chen Y, Liu SY, Cheng GH. Rivaroxaban versus dabigatran for anticoagulant therapy in patients with atrial fibrillation undergoing catheter ablation: A systematic review. Chin J Clin Pharmacol 2017;33(11):1032-5.
- Zhu Y, Xu C, Liu J. Randomized controlled trial of genotype?guided warfarin anticoagulation in Chinese elderly patients with nonvalvular atrial fibrillation. J Clin Pharm Ther 2020;45(6):1466-73.
[Crossref] [Google Scholar] [PubMed]
- Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2017;48(11):3040-8.
[Crossref] [Google Scholar] [PubMed]
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375(25):2423-34.
[Crossref] [Google Scholar] [PubMed]

 Control group
Control group 



