- *Corresponding Author:
- Shuying Ma
Department of Otorhinolaryngology-Head and Neck Surgery, The Second Hospital of Tianjin Medical University, Hexi, Tianjin, 300211, China
E-mail: zhangyuantj@163.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “141-149” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To speculate an autophagy gene, secretion associated Ras related guanosine triphosphatase 1B related signaling pathway for nasopharyngeal carcinoma based on both in vitro and in vivo experiments. 120 nasopharyngeal carcinoma biopsies (pathologically confirmed) were analyzed and the differentially expressed genes were explored. The internal molecular mechanism was further investigated using the human nasopharyngeal carcinoma cell lines, CNE1, HONE1 and C666-1. The cell proliferation capacity examination and the metabolic assays were performed in CNE1 cell line. The subcutaneous xenograft tumor mice model was also established. Secretion associated Ras related guanosine triphosphatase 1B demonstrated a remarkable decreased activity in nasopharyngeal carcinoma tissues compared with sibling paracancerous tissues. The key components in mammalian target of rapamycin complex 1 but not mammalian target of rapamycin complex 2 were greatly enhanced in nasopharyngeal carcinoma tissues. Moreover, the secretion associated Ras related guanosine triphosphatase 1B displayed a significant decreasing expression pattern and the mammalian target of rapamycin complex 1 kept an upward trend as the tumor, node and metastases stage progressed. The clinical significances for nasopharyngeal carcinoma tumor progression were calculated based on statistical analysis. The cell proliferation assay suggested that secretion associated Ras related guanosine triphosphatase 1B manipulated nasopharyngeal carcinoma cell proliferation via mammalian target of rapamycin complex 1/p70 ribosomal protein kinase 1 dependent signaling pathway. At the same time, transfection of secretion associated Ras related guanosine triphosphatase 1B small interfering ribonucleic acid could significantly enhanced the glycolytic capacity and glycolytic reserve of nasopharyngeal carcinoma cells compared with negative control. Silencing of secretion associated Ras related guanosine triphosphatase 1B promoted xenograft tumour growth, which could be greatly suppressed by rapamycin treatment in a dosage-dependent manner. The study shed a variety of insights for nasopharyngeal carcinoma from an innovative direction.
Keywords
Nasopharyngeal carcinoma, silencing of secretion associated Ras related guanosine triphosphatase 1B, mammalian target of rapamycin complex 1, human nasopharyngeal carcinoma cell lines
Nasopharyngeal Carcinoma (NPC) represents a squamous cell carcinoma arising from the epithelial lining of the nasopharynx. Based on the report of the International Agency for Research on Cancer, 129 079 new cases and 2987 associated deaths of NPC were discovered in 2018[1]. NPC is heterogeneous considering the geographical distribution, which is particularly prevalent in East and Southeast Asia comprising more than 70 % of new cases worldwide[2]. Accumulating evidences have suggested multiple risk factors (also the regional disparities) for NPC, including exposures to harmful environmental factors, lifestyle change, different dietary habits, ethnicity, as well as internal genetic diversity especially the ones associated with Epstein Barr Virus (EBV) which is suggested as the primary cause for the disorder[3].
Currently, the clinical strategies for NPC focus on radiotherapy, chemotherapy, combination of both, immunotherapy with a Programmed Cell Death Protein 1 (PD-1) inhibitor, liquid biopsy with plasma EBV and minimally invasive surgery[4]. The traditional surgical excision is normally applicable and considered as the last option for advanced NPC with metastatic status, given the close proximity of nasopharynx to brain stem cell area, nerves and major blood vessels. For radiotherapy, the Overall Survival (OS) rate of patients with NPC was improved dramatically. However, the long-term survivors of patients often suffer severe adverse reactions with this treatment. Within it, the most common acute adverse events in NPC patients during radiotherapy include dermatitis and oral mucositis[5]. Compared with radiotherapy alone, concurrent chemoradiotherapy could dramatically improve the 5 y OS and Progression-Free Survival (PFS) of stage II NPC patients[6]. Moreover, compared with radiotherapy alone, the concurrent chemoradiotherapy was associated with reduced distant metastasis rate but did not significantly improve the local control rate. While, it is worth noting that the primary disadvantage of adjuvant chemotherapy is poor tolerability.
Thanks to decades of efforts, the population screening and diagnosis methods have been greatly improved. At the same time, the underlined molecular mechanisms of pathogenesis have been better understood. Moreover, the widespread utilization of intensity-modulated radiotherapy and optimization of chemotherapy strategies (including induction, concurrent as well as adjuvant) have contributed to significantly enhanced survival time following reduced toxicities[7]. Even with these breakthroughs, the mortality of NPC still displays an upward trend annually. The poor prognosis of NPC is primarily due to the lacking of awareness of the salient symptoms of the disorder. The early symptoms of NPC are minor, such as headaches and nose hemorrhage with limited therapeutic options. To this end, surgical excision is recommended to be the only choice for NPC patients with advanced Tumor, Nodes and Metastases (TNM) stage. It is beneficial to explore underlined molecular mechanism for NPC pathogenesis, for the sake of identifying early diagnosis biomarker and novel effective therapeutic therapies.
Silencing of Secretion Associated Ras related Guanosine Triphosphatase 1B (SAR1B) is characterized as a Coat Protein Complex II (COPII) component and plays a pivotal role in the process of COPII-dependent vesicular transportation of proteins from the Endoplasmic Reticulum (ER) to the Golgi apparatus[8]. SAR1B was first identified as a key gene for the rare recessive disorder Chylomicron Retention Disease (CMRD), a developmental disorder found in infancy[9]. Yet, the connections between SAR1B and cancer are still poorly understood. Previously, it was demonstrated that SAR1B was overexpressed in Colorectal Cancer (CRC) specimens and this was associated with shorter OS time in patients with CRC[10]. Except for CRC, the knowledge of SAR1B for NPC is restricted. The mammalian Target of Rapamycin (mTOR) is a central signaling cassette, which integrates environmental cues to modulate cell proliferation and lipid metabolism. Dysfunctions of mTOR signaling have been shown to be involved in a variety of human cancers[11]. The mTOR kinase node is functional through two interacted signaling axis, mTOR Complex 1 (mTORC1) and 2 (mTORC2).
In this study, based on a large sample of NPC patient’s tissues, we in-depth analyzed the differentially expressed gene profile for the disease. The SAR1B was approved to be a potential prognostic biomarker and therapeutic target for NPC progression, which regulated cell proliferation via mTORC1 dependent and mTORC2 independent signaling casual. The internal molecular mechanism was systematically explored using NPC cell line and mice xenograft tumor model. All the outcomes here provided beneficial references for future NPC study.
Materials and Methods
Subjects:
The Gene Expression Omnibus (GEO) chips numbering GSE62328 and GSE132112 were used for this study. Exclude 5 individuals, 120 NPC patients with complete clinical information were recruited in the following analysis. The limma package in R language (version: 3.5.2) was developed to analyze the differentially expressed genes.
Cell lines:
The human NPC cell lines CNE1, HONE1 and C666-1 were all purchased from American Type Culture Collection (ATCC). The Roswell Park Memorial Institute (RPMI)-1640 (Hyclone, United States of America (USA)) supplemented with 10 % Fetal Bovine Serum (FBS, Gibco, USA) plus 1 % penicillin/streptomycin (Solarbio) was used for the cell culturing respectively. For small interfering RNA (siRNA) transfection, a specific SAR1B siRNA (50 nmol/l) purchased from OriGene, as well as corresponding Negative Control (NC) were utilized. The LipofectamineTM 3000 was used for plasmid transfection. 10-3 mM of rapamycin (MedChemExpress (MCE), China, AY-22989) was used for mTORC1 inhibition in cells. 20 nM of PF- 4708671 (Biolab, China, M00742) was used for p70 Ribosomal Protein Kinase 1 (S6K1) inhibition in cells.
Cell proliferation assay:
For the cell proliferation capacity examination, the CNE1 cells were cultured with F-12K medium with 100 ng/ml siRNA to reach a final concentration of 2×103 cells/well and grown for another 24 h. The Cell Count Kit-8 (CCK-8) solution (10 μl, Beyotime, Shanghai, China) was included in the medium for 2 h incubation. The final results were calculated using a spectrophotometer with an absorbance at 450 nm.
Metabolic assays:
The metabolic assays were performed as previously described[12]. The Extracellular Acidification Rate (ECAR) in the CNE1 cells was examined using the XF24 extracellular flux analyzer (Seahorse Bioscience). The ECAR values were normalized to cell number. At the same time, the glycolytic capacity was defined as the difference between ECAR following the injection of 1 μM oligomycin (Seahorse Bioscience) and the basal ECAR reading. Meanwhile, the glycolytic reserve was defined as the difference in ECAR between the glucose and oligomycin injections.
Subcutaneous xenograft tumor growth:
The subcutaneous xenograft tumor mice model was established as previously reported[13]. CNE1 cells with either NC or SAR1B siRNA analogue transfection were subcutaneously implanted into the right back flank of female Bagg and Albino (BALB)/c nude mice to develop tumors. The rapamycin at 1.5 mg/kg mouse weight (low dose) or 4.5 mg/kg mouse weight (high dose) was applied via intraperitoneal injection. Mice were randomly allocated into groups and n=10 for each group. The experiments period was 20 d.
Statistical analysis:
The Statistical Analysis Software (SAS) 11.0 software was established for data collection and analysis in this study. The student t-test was used to compare the differences between NPC cancerous and paracancerous tissues. The chi-squared test assisted in analyzing the relationship between particular regulatory factors and the clinical characteristics of NPC patients. The p-value less than 0.05 were considered statistically significant. The data presented in the figures were mean±Standard Deviation (SD), *p<0.05; **p<0.01; ***p<0.001; NS-Not Statistically Significant.
Results and Discussion
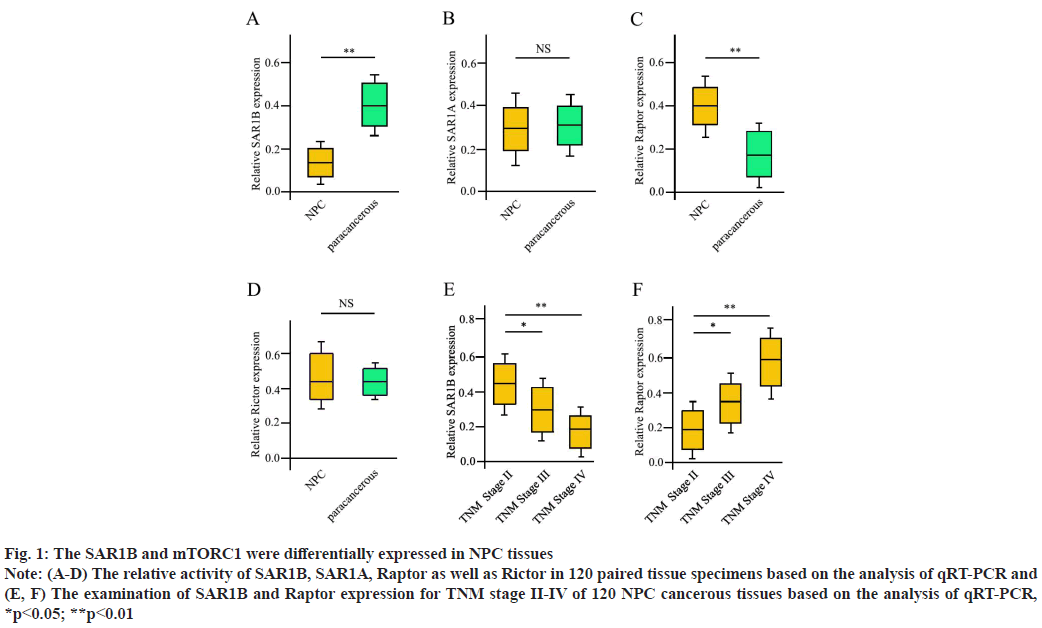
SAR1B and mTORC1 demonstrated differential expression pattern for NPC progression. First, we compared each single gene expression between NPC tissues and sibling paired paracancerous tissues. The gene SAR1B demonstrated a remarkable decrease in activity in NPC tissues compared with sibling paracancerous tissues (fig. 1A). Interestingly, another isoform of SAR1 gene, SAR1A, did not show a significant difference (fig. 1B). The mTORC1 expression was attractive since several core components of it were up-regulated in NPC tissues (data not shown). The Regulatory associated protein of mTOR (Raptor), a scaffolding protein important for mTORC1 assembly and a specific component for mTORC1 but not mTORC2, was greatly enhanced in NPC tissues (fig. 1C), while, Rapamycin-insensitive companion of mTOR (Rictor), a specific component for mTORC2 did not demonstrate obvious difference between NPC tissues and paracancerous tissues (fig. 1D). Next, we further investigated the expression of SAR1B for different TNM stages of NPC patients. The SAR1B displayed a great decreasing pattern as the TNM stage advanced (fig. 1E). Inversely, the Raptor kept an upward trend as the TNM stage progressed (fig. 1F). Overall, these results indicated that SAR1B (not SAR1A) and mTORC1 (not mTORC2) were closely associated with NPC tumor progression.
Fig. 1: The SAR1B and mTORC1 were differentially expressed in NPC tissues
Note: (A-D) The relative activity of SAR1B, SAR1A, Raptor as well as Rictor in 120 paired tissue specimens based on the analysis of qRT-PCR and
(E, F) The examination of SAR1B and Raptor expression for TNM stage II-IV of 120 NPC cancerous tissues based on the analysis of qRT-PCR,
*p<0.05; **p<0.01
SAR1B and mTORC1 showed a clinical significance for NPC tumor progression. Since the low-activity of SAR1B and high-activity of mTORC1 were closely associated with NPC tumor progression, we further sub-grouped the 120 NPC patients into high SAR1B expression group (n=56) and low SAR1B expression group (n=64), according to the median of SAR1B expression. It could be indicated that there was a significant relationship between expression activity of SAR1B and lymph node metastasis (p=0.011), tumor size (p=0.010), and TNM stages (p=0.005), but not for age (p=0.136) and gender (p=0.179) (Table 1). In contrast to SAR1B, there was a remarkable correlation between high expression level of Raptor and occurrence of lymph node metastasis (p=0.001), increased tumor size (p=0.012), and advanced TNM stage (p=0.008) (Table 2), so that, these data supported the close connections between SAR1B and mTORC1 with NPC tumor progression clinically.
| Clinical features | N | High Raptor expression group (n=53) | Low Raptor expression group (n=67) | p |
|---|---|---|---|---|
| Age | 0.158 | |||
| >60 | 50 | 21 | 29 | |
| ≤60 | 70 | 32 | 38 | |
| Gender | 0.246 | |||
| Male | 89 | 41 | 48 | |
| Female | 31 | 12 | 19 | |
| Lymph node metastasis | ||||
| NO | 58 | 3 | 55 | 0.001 |
| YES | 62 | 50 | 12 | |
| Tumor size (cm) | ||||
| <5 | 40 | 7 | 33 | 0.012 |
| ≥5 | 80 | 46 | 34 | |
| TNM stage | ||||
| I | 68 | 40 | 28 | 0.008 |
| II | 34 | 11 | 23 | |
| III | 18 | 2 | 16 |
Table 2: The Correlation between Raptor Activity and Clinicopathological Characteristics of 120 NPC Patients
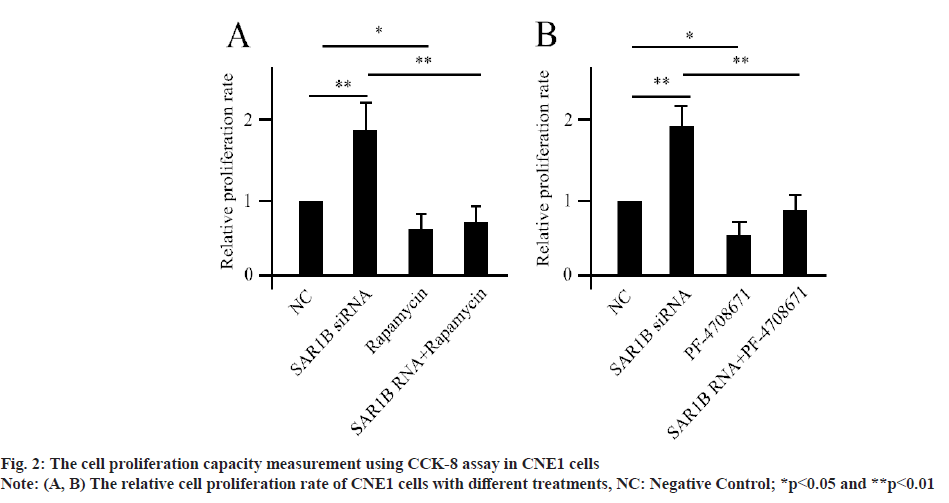
SAR1B modulated NPC cell proliferation through mTORC1 dependent signaling cascade. The human NPC cell lines-CNE1, HONE1 and C666-1 were established for in vitro analysis. Compared with control, silencing of SAR1B using siRNA transfection greatly enhanced the NPC cell proliferation (fig. 2A), since CNE1, HONE1 and C666-1 demonstrated similar results. We used CNE1 as a representative for the following analysis, while, co-treatment with rapamycin could significantly abolished the effects of SAR1B siRNA for NPC cell proliferation. Since mTORC1-mediated phosphorylation of S6K1 was the core for the cascade, we treated the NPC cells using S6K1 specific inhibitor (PF-4708671). The PF- 4708671 treated cells showed significantly decreased level of cell proliferation compared with control (fig. 2B). Moreover, the PF-4708671 treatment could attenuate the effects caused by SAR1B siRNA transfection. These data suggested that SAR1B manipulated NPC cell proliferation via mTORC1/ S6K1 dependent signaling pathway.
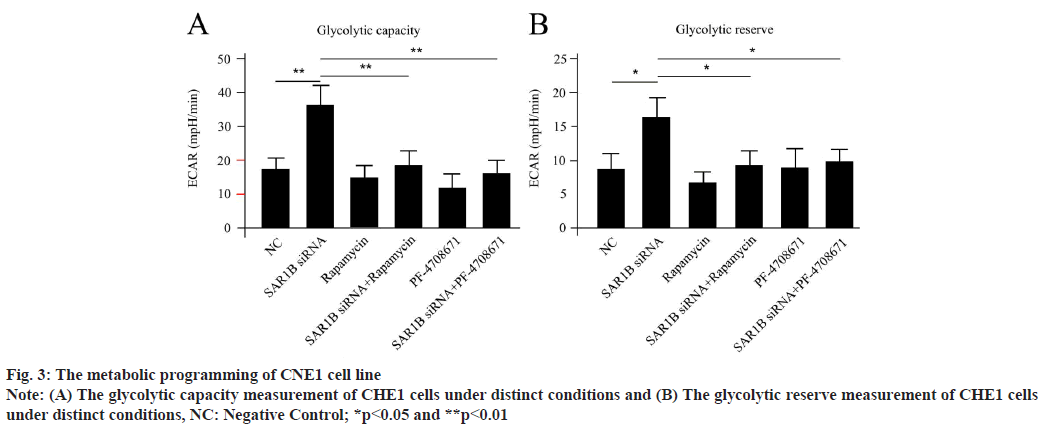
SAR1B manipulated metabolic programming of NPC cells via mTORC1 dependent signaling pathway. Since glucose metabolism was a wellknowntargeting process for mTORC1, we wonder if SAR1B could control the process in NPC cells. Driven by this question, we testified the metabolic phenotype of NPC cells. The CNE1 cells were differentiated in vitro. The oxygen consumption and lactate production were examined using an extracellular metabolic flux analyzer. Transfection of SAR1B siRNA could significantly enhance the glycolytic capacity and glycolytic reserve of NPC cells compared with negative control (fig. 3A and fig. 3B), which could be dramatically reversed by cotreatment of either rapamycin or PF-4708671. These outcomes suggested that silencing of SAR1B could obviously accelerate glucose metabolism progress in NPC cells, which was both mTORC1 and S6K1 dependent.
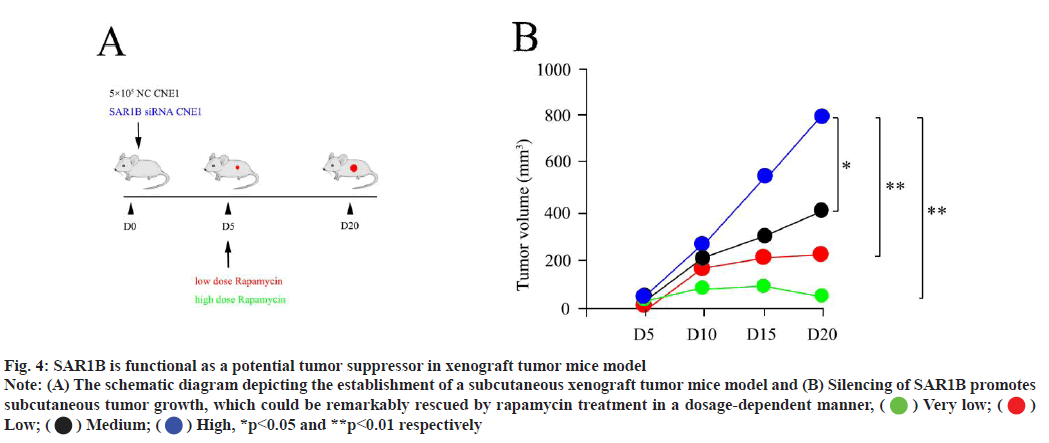
Blocking mTORC1 using rapamycin could inhibit subcutaneous tumor growth in vivo. In in vitro, our data proposed that SAR1B was functional as a negative regulator for NPC progression via mTORC1 signaling casual. Next, we sought to verify this outcome in a subcutaneous xenograft tumor mice model. We subcutaneously transplanted CNE1 control cells and CNE1 treatment cells (deficient in SAR1B) into nude mice to develop tumour growth (fig. 4A). Silencing of SAR1B promoted xenograft tumour growth, which could be remarkably suppressed by rapamycin treatment in a dosage-dependent manner (fig. 4B).
Fig. 4: SAR1B is functional as a potential tumor suppressor in xenograft tumor mice model
Note: (A) The schematic diagram depicting the establishment of a subcutaneous xenograft tumor mice model and (B) Silencing of SAR1B promotes
subcutaneous tumor growth, which could be remarkably rescued by rapamycin treatment in a dosage-dependent manner,  Very low;
Very low;  Low;
Low;  Medium;
Medium;  High, *p<0.05 and **p<0.01 respectively
High, *p<0.05 and **p<0.01 respectively
NPC represents a distinct malignancy, compared with other types of epithelial head and neck cancers. Typically, NPC is characterized as an undifferentiated histologic subtype, closely associated with EBV status. Ecumenically, EBV-associated NPC patients demonstrate a specific geographical and demographic distribution, with a predisposition for Eastern and Southeastern Asia, as well as in Northern and Eastern Africa, and is more common in males than females[2]. Based on the large population of NPC patients in China, we deeply analyzed the differentially expressed genes for the tumor progression using a large patient sample. The first signaling cascade drived our attention was mTORC1. The mTOR, DEP Domaincontaining mTOR-Interacting Protein (DEPTOR), Raptor as well as Proline-Rich Akt Substrate of 40 kDa (PRAS40) all showed enhanced activities in NPC tissue specimen (fig. 1), while, Raptor is defined as a scaffolding protein for mTORC1 assembly, stability, as well as substrate specificity. Meanwhile, PRAS40, is characterized as an unique factor suppressing mTORC1 function[14]. Moreover, the mTORC2 specific regulators (such as Rictor and mammalian Stress-activated protein kinase-interacting protein 1 (mSin1)) did not display any significant differences (fig. 1D). All these allowed us to hypothesize that mTORC1 but not mTORC2 played a pivotal role in NPC progression. Accumulating evidences have supported the connections between mTORC1 and cancer. The direct evidence for the connection comes from tuberous sclerosis, a disorder caused by loss of Tuberous Sclerosis Complex (TSC) 1 or TSC2, consequently hyper-activating of mTORC1[15,16]. The mTORC1-mediated phosphorylation of S6K1 greatly enhances both purine and pyrimidine synthesis in cancer cells, which is required for cancer cells to rapidly duplicate[17,18]. Here, in this study, we proposed that mTORC1-S6K1 was also critical for NPC cell proliferation. Blocking either mTORC1 or S6K1 using chemical compounds could sufficiently attenuate cell proliferation in NPC cells (fig. 2).
Except for the functions in regulating cell growth and metabolism, mTORC1 also modulates autophagy in cancer cells. In fact, to some extent, autophagy is considered as a tumor suppressor by some researchers[19]. The autophagy is an orderly manipulated process for recycling of several cellular components. Indeed, the anterograde transport of vesicles from the ER to the golgi apparatus relies on COPII, which is composed of five cytosolic components, the coat-Guanosine Triphosphatases (GTPases) SAR1, Sec23, Sec24, Sec13 and Sec31[20,21]. So far, five SAR1 isoforms have been discovered[22]. Based on a dominant negative approach, it could be shown that distinct SAR1 isoforms exhibited cargo specificity even the high sequence of similarity between them[23-25]. For instance, SAR1A and SRA1B displayed obvious differences regarding the transport of Alpha (α)-amylase[23]; SAR1A and SAR1C differed in the transport of a membrane-bound transcription factor[24] and SAR1B and SAR1C differed in the transport of Protein S-acyltransferase 10 (PAT10) [25]. In this study, SAR1B but not SAR1A exhibited a close relationship with NPC development (fig. 1A and fig. 1B), supporting the SAR1B specificity for NPC. The SAR1B not only displayed decreased activity in NPC cancerous tissues compared with paracancerous tissues but also demonstrated reduced expression as TNM stage progressed (Table 1). This may be related with the distinct transportations of cargo components. Previously, Chen and his colleagues claimed that SAR1B was functional as a leucine sensor regulating mTORC1 signaling in response to intracellular levels of leucine[13]. Under conditions of leucine deficiency, SAR1B could suppress mTORC1 by physically targeting its activator GAP Activity Towards Rags 2 (GATOR2). While, in condition of leucine sufficiency, SAR1B binds to leucine, undergoes a conformational change and dissociates from GATOR2, causing the subsequently mTORC1 activation. Moreover, based on the bioinformatic analysis, they revealed that SAR1B deficiency correlated with the development of lung cancer. Using a similar animal model like us, they also found that silencing of SAR1B initiated mTORC1-dependent growth of lung tumor in mice and the tumor growth could be blocked with a dose dependent rapamycin (fig. 4B).
| Clinical features | N | High SAR1B expression group (n=56) | Low SAR1B expression group (n=64) | p |
|---|---|---|---|---|
| Age | 0.136 | |||
| >60 | 50 | 26 | 24 | |
| ≤60 | 70 | 30 | 40 | |
| Gender | 0.179 | |||
| Male | 89 | 42 | 47 | |
| Female | 31 | 14 | 17 | |
| Lymph node metastasis | 0.011 | |||
| No | 58 | 39 | 19 | |
| Yes | 62 | 17 | 45 | |
| Tumor size (cm) | 0.01 | |||
| <5 | 40 | 26 | 14 | |
| ≥5 | 80 | 30 | 50 | |
| TNM stage | 0.005 | |||
| I | 68 | 48 | 20 | |
| II | 34 | 6 | 28 | |
| III | 18 | 2 | 16 |
Table 1: The Correlation between SAR1B Activity and Clinicopathological Characteristics of 120 NPC Patients
Once activated, mTORC1 phosphorylates subsequent substrates, including S6K1. S6K1 is the key regulatory factor of both cap-dependent and cap-independent translation. mTORC1-dependent phosphorylation of S6K1 stimulates both purine and pyrimidine synthesis, which is necessary for cancer cells to rapidly duplicate their DNA. This could be the direct connection between mTORC1-S6K1 and cancer formation. The reprogramming of lipid and glucose metabolism has been identified as a newly recognized hallmark of malignancy. Enhanced lipid uptake, increased storage and accelerated usage of glucose, which occur in a variety of cancers and contribute to a rapid tumor growth[26,27]. As a core component for COPII, SAR1B is involved in autophagy. Here, we claimed that, in NPC cells, the glycolytic capacity and glycolytic reserve were both dramatically accelerated compared with control cells (fig. 2). At the same time, silencing of SAR1B hampered the increase of glycolytic capacity and glycolytic reserve. So that we could hypothesize that SAR1B-modulated autophagy process was closely associated with glycolytic capacity and glycolytic reserve, which then affected purine and pyrimidine synthesis and NPC tumor cell proliferation. These might explain the functions of SAR1B for NPC tumor progression and also the underlying connections between autophagy and tumorgenesis.
Collectively, in this study, we proposed SAR1B as a potential tumor depressor gene, which modulated glucose metabolism and cell proliferation through autophagy process targeting mTORC1, since the uncontrolled cell proliferation and acquisition of metabolic events were characterized by their hallmarks for cancer cells. All the work here suggested a promising target for treating NPC patients in the future and also shed numerous insights for future clinical study.
Author’s contributions:
Conception and design: Xuebing Liu and Shuying Ma; Administrative support: Lei Chen; Provision of study materials or patients: Lei Chen; Collection and assembly of data: Xuebing Liu and Lei Chen; Data analysis and interpretation: Shuying Ma and manuscript writing and final approval of manuscript: All authors.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
[Crossref] [Google scholar] [PubMed]
- Chen YP, Chan AT, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019;394:64-80.
[Crossref] [Google scholar] [PubMed]
- Lau L, Huang L, Fu E, Tan TC, Kong KO, Lim MY. Nasopharyngeal carcinoma in dermatomyositis. Clin Otolaryngol 2021;46(5):1082-8.
[Crossref] [Google scholar] [PubMed]
- Lam WJ, Chan JY. Recent advances in the management of nasopharyngeal carcinoma. F1000Res 2018;7:1829.
[Crossref] [Google scholar] [PubMed]
- Chen YP, Lv JW, Mao YP, Li XM, Li JY, Wang YQ, et al. Unraveling tumour microenvironment heterogeneity in nasopharyngeal carcinoma identifies biologically distinct immune subtypes predicting prognosis and immunotherapy responses. Mol Cancer 2021;20(1):14.
[Crossref] [Google scholar] [PubMed]
- Albasri AM. Nasopharyngeal carcinoma metastasis to the breast. Saudi Med J 2020;41(10):1130-4.
[Crossref] [Google scholar] [PubMed]
- Lee HM, Okuda KS, González FE, Patel V. Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol 2019:11-34.
[Crossref] [Google scholar] [PubMed]
- Fryer LG, Jones B, Duncan EJ, Hutchison CE, Ozkan T, Williams PA, et al. The endoplasmic reticulum coat protein II transport machinery coordinates cellular lipid secretion and cholesterol biosynthesis. J Biol Chem 2014;289(7):4244-61.
[Crossref] [Google scholar] [PubMed]
- Charcosset M, Sassolas A, Peretti N, Roy CC, Deslandres C, Sinnett D, et al. Anderson or chylomicron retention disease: Molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol Genet Metab 2008;93(1):74-84.
[Crossref] [Google scholar] [PubMed]
- Lu Y, Zhou SK, Chen R, Jiang LX, Yang LL, Bi TN. Knockdown of SAR1B suppresses proliferation and induces apoptosis of RKO colorectal cancer cells. Oncol Lett 2020;20(5):186.
[Crossref] [Google scholar] [PubMed]
- Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol 2019;12(1):1-9.
[Crossref] [Google scholar] [PubMed]
- Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015;125(1):194-207.
[Crossref] [Google scholar] [PubMed]
- Chen J, Ou Y, Luo R, Wang J, Wang D, Guan J, et al. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature 2021;596(7871):281-4.
[Crossref] [Google scholar] [PubMed]
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017;36(16):2191-201.
[Crossref] [Google scholar] [PubMed]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 2004;166(2):213-23.
[Crossref] [Google scholar] [PubMed]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age-and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431(7005):200-5.
[Crossref] [Google scholar] [PubMed]
- Ben-Sahra I, Hoxhaj G, Ricoult SJ, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016;351(6274):728-33.
[Crossref] [Google scholar] [PubMed]
- Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013;339(6125):1320-3.
[Crossref] [Google scholar] [PubMed]
- White E. The role for autophagy in cancer. J Clin Invest 2015;125(1):42-6.
[Crossref] [Google scholar] [PubMed]
- Hawes C, Kiviniemi P, Kriechbaumer V. The endoplasmic reticulum: A dynamic and well‐connected organelle. J Integr Plant Biol 2015;57(1):50-62.
[Crossref] [Google scholar] [PubMed]
- Wadowska K, Bil-Lula I, Trembecki Ł, Śliwińska-Mossoń M. Genetic markers in lung cancer diagnosis: A review. Int J Mol Sci 2020;21(13):4569.
[Crossref] [Google scholar] [PubMed]
- Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol 2013;14(6):382-92.
[Crossref] [Google scholar] [PubMed]
- Zeng Y, Chung KP, Li B, Lai CM, Lam SK, Wang X, et al. Unique COPII component AtSar1a/AtSec23a pair is required for the distinct function of protein ER export in Arabidopsis thaliana. Proc Natl Acad Sci USA 2015;112(46):14360-5.
[Crossref] [Google scholar] [PubMed]
- Feng QN, Song SJ, Yu SX, Wang JG, Li S, Zhang Y. Adaptor protein-3-dependent vacuolar trafficking involves a subpopulation of COPII and HOPS tethering proteins. Plant Physiol 2017;174(3):1609-20.
[Crossref] [Google scholar] [PubMed]
- Hanton SL, Chatre L, Matheson LA, Rossi M, Held MA, Brandizzi F. Plant Sar1 isoforms with near-identical protein sequences exhibit different localisations and effects on secretion. Plant Mol Biol 2008;67:283-94.
[Crossref] [Google scholar] [PubMed]
- Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev Cell 2021;56(10):1363-93.
[Crossref] [Google scholar] [PubMed]
- Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun 2018;38(1):1-4.
[Crossref] [Google scholar] [PubMed]