- Corresponding Author:
- P. Mishra
Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India.
E-mail: pmishra51@rediffmail.com
| Date of Submission | 16 December 2004 |
| Date of Revision | 06 July 2005 |
| Date of Acceptance | 29 May 2006 |
| Indian J Pharm Sci, 2006, 68 (3): 365-368 |
Abstract
Two simple spectrophotometric methods for the determination of aspirin and clopidogrel in pharmaceutical formulations have been developed. First method is based on the additivity of absorbances. Second method is based on the determination of graphical absorbance ratio at two selected wavelengths, one being the isoabsorptive point for the two drugs (225 nm) and the other being the absorption maximum of hydrolysed aspirin (235.7 nm). Beer Lambert's law is obeyed for both the drugs in the concentration range 4-18 mg/ml. Both the methods were found to be simple, rapid, accurate and can be adopted in routine analysis of drugs in formulations. The accuracy and reproducibility of the proposed method was statistically validated by recovery studies.
Acetylsalicylic acid (Aspirin) and clopidogrel hydrogen sulphate [S-(α)( 2- chlorophenyl)-6,7-dihydrothieno (3,2-C) pyridine-5 (4H) acetic acid methyl ester sulphate] are antiplatelet agents approved by the Food and Drug Administration, USA, for use in secondary prevention of heart attacks and stroke. Several spectrophotometric methods [1] and several HPLC methods [2-9] have been reported for estimation of aspirin, whereas only HPLC methods are reported for estimation of clopidogrel in pharmaceutical dosage forms [10] and for its metabolite in plasma and serum [11]. Since clopidogrel and aspirin are marketed in combination and as no simultaneous methods are reported for the estimation of these drugs in combined dosage forms, we present two methods for their simultaneous estimation.
A GBC Cintra 10 UV/Vis spectrophotometer with 10 mm matched quartz cells was used for experiments. The chemicals used were of analytical grade. Sulphuric acid (Qualigens) (1 N) was prepared using distilled water. The commercially available tablets of clopidogrel and aspirin combination Deplatt A (Torrent, Ahmedabad) and Clopivas AP (Osaka, Satara, MH) and capsules Combiplet (Sun Pharma, Vadodara) were procured from local market. Clopidogrel (Dr. Reddy’s Laboratories, Hyderabad) and Aspirin (Sischochem, Mumbai) were used as standard samples without further purification.
Stock solutions of clopidogrel and aspirin were prepared by dissolving 100 mg (accurately weighed) each of standard clopidogrel and standard aspirin separately in 100 ml of methanol. Working standard solutions (A) and (B) were further prepared by heating 1 ml of stock solutions of clopidogrel and aspirin, respectively with 1 ml H2SO4 for 30 min on water bath.
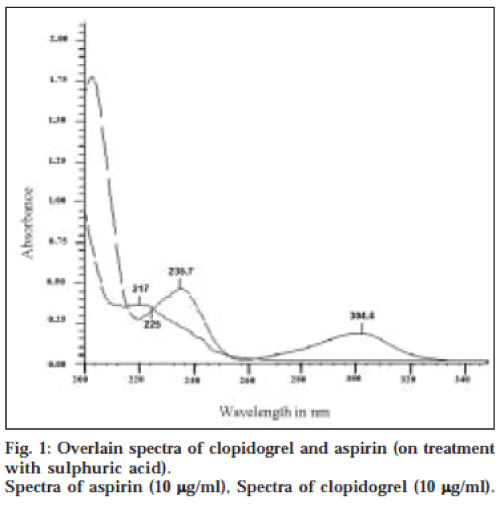
Method I is based on simultaneous equations method of Vierodt [12]. Aspirin shows two absorption maxima at 235.7 nm and 304.4 nm after acidic hydrolysis. Clopidogrel also absorbs at 235.7 nm but shows no absorption at 304.4 nm in the same condition. Calibration curve for clopidogrel and aspirin was prepared in the concentration range 4-18 μg/ml (range for which Beer Lambert’s law followed) at 235.7 nm and for aspirin at 304.4 nm. The absorptivity coefficients were determined in this range and their average value taken. The overlain spectra of clopidogrel and aspirin are represented in fig. 1. A set of two simultaneous equations was developed using these absorptivity coefficients. These are: A1=0.0170 Cy…(1); and A2=0.0196 Cx+0.0418 Cy…(2), where A1 and A2 are absorbances at 304.4 nm and 235.7 nm, respectively, and Cx and Cy are concentrations of clopidogrel and aspirin, respectively.
Method II is a graphical absorbance ratio method. This method is based on the method used by Ghanem et al. [13], which takes advantage of iso-absorptive point14 of the two drugs, i.e., the wavelength of equal absorptivity of the two components of the mixture. The isoabsorptive point was found to be 225 nm in this case (λ1) (fig. 1). The other wavelength selected is the absorption maximum of one of the components. In this case, it was 235.7 nm, the absorption maximum of salicylic acid (λ2). The concentrations of the two components are related to the ratio of the absorbances at these two wavelengths. The absorbance of the mixture was noted at 235.7 nm and 225 nm. Calibration curves of aspirin and clopidogrel were plotted in the concentration range 4-18 μg/ml (range for which Beer Lambert’s law was obeyed). The absorptivity coefficients were determined for both the drugs and the average value was taken. These values and the absorbance ratio were used to develop a set of two equations [14]. A1=0.0280 (Cx+Cy) (3); A1=0.0280 Cx [-0.79/ (QM-1.49)] (4), where QM=A2/A1, A1 is absorbance at 225 nm and A2 is absorbance at 235.7 nm. Cx and Cy are concentrations of clopidogrel and aspirin, respectively.
Twenty tablets of Clopivas AP were weighed and average weight was determined (before and after removing the coating). The tablets were finely powdered and powder equivalent to 100 mg of clopidogrel and 100 mg of aspirin was taken. In case of Deplatt A, powder equivalent to 100 mg of clopidogrel and 200 mg of aspirin was taken after determination of the average weight of tablet.
Average weight of twenty Combiplet capsules was determined and the capsule contents were emptied. Average weight of empty shells was again taken to determine the weight of the powder in each capsule. Powder obtained was mixed thoroughly; powder equivalent to 100 mg of clopidogrel and 100 mg of aspirin was taken.
In all the above three cases, the equivalent amount of powder taken was extracted with 4×20 ml of methanol and volume was made up to 100 ml to give the stock solutions. Working standard solutions were made in all the three cases by heating suitable dilutions of the stock with 1 ml H2SO4 (1N). These were further suitably diluted and the absorbances were taken at different wavelengths as stated above .Using the equations 1, 2, 3, and 4, the concentrations were determined.
Both the methods were found to be accurate, simple, and rapid for routine simultaneous analysis of the formulations. In the first method, the content of the aspirin was directly found from the first equation at 304.4 nm, and substitution of this in second equation gives the concentration of clopidogrel. In the second method, the absorbance ratio and the absorptivity coefficients were determined, and the values were substituted in the equation given above to give the results. The reproducibility, repeatability, and accuracy of these methods were found to be good, which is evidenced by low values of standard deviation, percent relative standard deviation, and standard error (Table 1). The percent range of error (within 95% confidence limits) shows precision of the methods. To test the accuracy and reproducibility of the proposed methods, recovery experiments were performed by adding known amount of the drugs to the pre-analyzed formulations and reanalyzing the mixture by proposed methods [15] Thus it can be concluded that the methods developed in the present investigation are simple, sensitive, accurate, and precise. Hence, these can be successfully applied for simultaneous estimation of clopidogrel and aspirin in pharmaceutical formulations.
| Method | Tablet brand | Tablet component | Label claim* mg/tab | Amount found* mg/tab | S.D.* | %R.S.D* | S.E.* |
|---|---|---|---|---|---|---|---|
| I | Combiplet | CLOP | 75 | 74.4±0.43 | 0.5411 | 0.7210 | 0.2209 |
| ASP | 75 | 76.4±0.44 | 0.5558 | 0.7410 | 0.2269 | ||
| Clopivas AP | CLOP | 75 | 74.5±0.39 | 0.4961 | 0.6661 | 0.2025 | |
| ASP | 75 | 76.32±0.37 | 0.4745 | 0.6217 | 0.1937 | ||
| Deplatt-A | CLOP | 75 | 74.0±0.15 | 0.1900 | 0.2567 | 0.0755 | |
| ASP | 150 | 151±0.31 | 0.3895 | 0.3895 | 0.1590 | ||
| I I | Combiplet | CLOP | 75 | 75.5±0.40 | 0.5055 | 0.6780 | 0.2064 |
| ASP | 75 | 75.7±0.47 | 0.5992 | 0.7917 | 0.2446 | ||
| Clopivas AP | CLOP | 75 | 75.6±0.45 | 0.5712 | 0.7560 | 0.2332 | |
| ASP | 75 | 75.8±0.37 | 0.4690 | 0.6191 | 0.1915 | ||
| Deplatt-A | CLOP | 75 | 75.6±0.29 | 0.2514 | 0.3327 | 0.1026 | |
| ASP | 150 | 150.0±0.49 | 0.3693 | 0.2464 | 0.1507 |
*Average of six determinations; SD=Standard deviation; % RSD=Relative standard deviation and SE=Standard error
Table 1: Compilation of Results of Statistical Analysis of Commercial Formulations
| Method | Tablet brand | Percent recovery ± S.D.* | |
|---|---|---|---|
| Clopidogrel | Aspirin | ||
| I | Combiplet | 97.1±0.90 | 99.6±0.93 |
| Clopivas | 99.3±0.92 | 101.8±0.95 | |
| Deplatt-A | 101.4±0.92 | 99.8±1.66 | |
| I I | Combiplet | 97.9±0.91 | 100.4±0.97 |
| Clopivas | 97.0±0.83 | 102.9±0.98 | |
| Deplatt-A | 98.7±0.94 | 100.6±1.86 | |
Table 2: Compilation of Results of Drug Recovery Study
Acknowledgements
The authors wish to thank Prof. N. K. Jain, Head of the Department, for providing necessary facilities; and Dr. Reddy’s Laboratories Ltd., Hyderabad, for supplying clopidogrel as a gift sample. The financial assistance received from UGC in the form of fellowship for postgraduate studies in Engineering and Technology is being gratefully acknowledged by one of the authors (AD).
References
- Sethi, P.D., Eds; In; Quantitative analysis of drugs in pharmaceutical formulations 3rd Edn, CBS publishers and distributors, New Delhi,1997,105.
- Terweij-groen, C.P., VahlkampT.,andKraak J.C., J. Chromatogr., 1978,145,115
- Cham, B.E., Ross-Lee, L., Bochner, F. and Imhoff, D.M., Clin.Chem., 1979, 25, 1420.
- Maulding, D.L. and Young, J.F., J. Pharm. Sci., 1980,69,1224.
- Berkersky, I., Boxenbaaum, H.G., Whitson, M.H., Puglisi, C.V., Pocelinko, R., and Kaplan, S.A., Anal. Lett., 1977,10,539.
- Harrison, L.I., Funk, M.L. and Ober, R.E., J. Pharm. Sci. , 1980,69,1268.
- Lo, L.Y. and Bye, A., J. Chromatogr. , 1980,181,473.
- Peng, G.W., Gadalla, M.A.F., Peng, A. and Chiou, W.L., J. Pharm.Sci .,1978, 67,710.
- Amick, E.N.andMason,W.D., Anal. Lett. , 1979,12,629.
- Mitakos, A. and Pander I. ,J. Pharm. Biomed. Anal., 2002, 431 438.
- Lagorce, P., Perez, Y., Ortiz, J., Necciari, J. and Bressolle, F., J.Chromatogr. B. Biomed. Sci. Appl., 1998, 720(1:2),107-117.
- Beckett, A.H. and Stenlake, J.B. In; Practical PharmaceuticalChemistry, 4th Edn. Vol. II, CBS Publishers and Distributors, New Delhi, 1997, 278.
- Ghanem, A., Meshali, M. and Foda, A., J. Pharm. Pharmacol., 1979, 31, 122.
- Pernarowski, M., Knevel, A.M. and Christian, J.E., J. Pharm. Sci., 1960, 50, 943.
- Reddy, N.R., Prabhavathi, K. and Chakravarthy, I.E., Indian J.Pharm. Sci., 2004, 66(2), 240-242.