- *Corresponding Author:
- A. A. Shirwaikar
Departments of Pharmaceutical Quality Assurance, Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal–576 104, India
E-mail: arunshirwaikar@yahoo.co.in
| Date of Submission | 13 December 2006 |
| Date of Revision | 28 September 2007 |
| Date of Acceptance | 13 April 2008 |
| Indian J. Pharm. Sci., 2008, 70 (2): 236-238 |
Abstract
A novel, simple, sensitive and rapid spectrophotometric method has been developed for simultaneous estimation of ambroxol hydrochloride and levocetirizine dihydrochloride. The method involved solving simultaneous equations based on measurement of absorbance at two wavelengths 242 nm and 231 nm, the g max of ambroxol hydrochloride and levocetirizine dihydrochloride, respectively. Beer's law was obeyed in the concentration range 10-50 μg/ml and 8-24 μg/ml for ambroxol hydrochloride and levocetirizine dihydrochloride respectively. Results of the method were validated statistically and by recovery studies.
Keywords
Ambroxol hydrochloride, levocetirizine dihydrochloride, λ max, spectrophotometric method
Ambroxol hydrochloride (AMB) is chemically, trans- 4-((2-amino-3,5-dibromobenzyl) amino) cyclohexanol hydrochloride. Levocetirizine dihydrochloride (LEVC) is chemically, (RS)-2-{4-[(R)-p-chloro-α-phenylbenzyl]- 1-piperazinyl} ethoxyacetic acid dihydrochloride [1]. AMB reduces bronchial hyper-reactivity and acts as a mucolytic and cough suppressant [1]. LEVC is usually used in allergic conditions including rhinitis1. Combination of AMB and LEVC is used for the treatment of bronchitis. These two drugs are not official in any pharmacopoeia; hence no official method is available for the simultaneous estimation of AMB and LEVC in formulations. Capillary electrophoresis [2-4], spectrometry [5], gas chromatography [6,7], LC with potentiometric detection [8], MS detection [9] and UV detection [10-13] methods have been reported for the estimation of AMB. However, no references have been found for simultaneous determination of AMB and LEVC in pharmaceutical formulations. A successful attempt has been made to estimate these two drugs simultaneously by spectrophotometric analysis.
A Shimadzu UV/Vis spectrophotometer, model-1601 (Japan) was employed with spectral bandwidth of 0.1 nm and a wavelength accuracy of ±0.5 nm with automatic wavelength correction with a pair of 3 mm quartz cells. AMB and LEVC (Aristo Pharma Ltd.), methanol (Merck India Ltd., Mumbai) and distilled water were used in the present study.
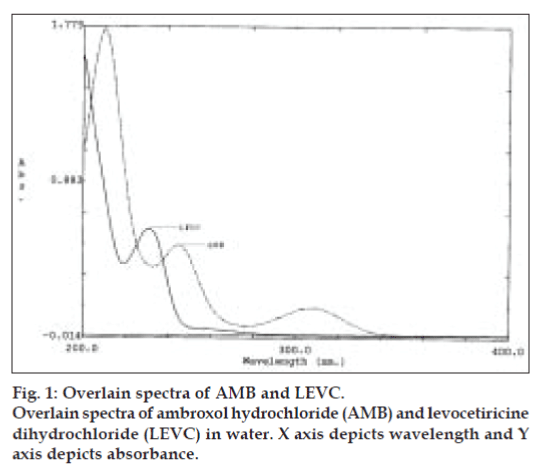
Stock solutions (500 μg/ml) of AMB and LEVC were prepared by dissolving separately in 20 ml of water in a 100 ml clean volumetric flask, and the volume was made up to 100 ml with distilled water. The maximum absorbance of AMB and LEVC was obtained at 244 nm (λ2) and 231 nm (λ1), respectively. AMB and LEVC showed linearity with absorbance in the range of 10–50 μg/ml and 8–24 μg/ml at their respective maxima, which were validated by least square method. Coefficients of correlation were found to be 0.9992 for AMB and 0.9993 for LEVC. For simultaneous estimation of AMB and LEVC, a series of standard solutions in concentration range of 2 to 24 μg/ml, were prepared by diluting appropriate volumes of the standard stock solutions. The scanning of solutions of AMB and LEVC were carried out in the range of 200 to 400 nm against water as blank for obtaining the overlain spectra that are used in the analysis (fig. 1). Absorbance and absorptivities of series of standard solutions were recorded at selected wavelengths λ1 and λ2.
The absorptivity values for AMB and LEVC are shown in Table 1. The optical characteristics and regression values for the calibration curve are presented in Table 2. The method employed simultaneous equations using Cramer’s rule and matrices (C1= λ2ε2×Aλ1-λ1ε2×Aλ2/λ1ε1×λ2ε2 −λ1ε2xλ2ε1and C2= λ1ε1×Aλ2−λ2ε1×Aλ1/λ1ε1×λ2ε2−λ1ε2×λ2ε1). A set of two simultaneous equations were framed using the mean of absorptivity values, given as Aλ1 = 211 C1+312 C2 and Aλ2 = 263 C1+71 C2, where, C1 and C2 are the concentrations of AMB and LEVC, respectively in simple solution (μg/ml). Aλ1 and Aλ2 are the absorbance of the sample solution measured at 231 and 244 nm, respectively.
| Concentration(µg/ml) | Absorptivity | ||||||
|---|---|---|---|---|---|---|---|
| AMB | LEVC | 231 nm | 244 nm | ||||
| AMB | LEVC | AMB | LEVC | ||||
| 2 | 2 | 210 | 310 | 261 | 70 | ||
| 4 | 4 | 212 | 311 | 261 | 73 | ||
| 6 | 6 | 212 | 314 | 264 | 72 | ||
| 8 | 8 | 209 | 312 | 262 | 71 | ||
| 10 | 10 | 208 | 312 | 263 | 71 | ||
| 12 | 12 | 211 | 313 | 263 | 70 | ||
| 14 | 14 | 212 | 310 | 261 | 72 | ||
| 16 | 16 | 212 | 312 | 262 | 70 | ||
| 20 | 20 | 212 | 311 | 265 | 71 | ||
| 24 | 24 | 211 | 310 | 262 | 71 | ||
| Mean | 211 | 312 | 263 | 71 | |||
| SD | 1.45 | 1.35 | 1.27 | 0.99 | |||
AMB and LEVC stands for ambroxol hydrochloride and levocetricine dihydrochloride, respectively
Table 1: Absorptivity values for ambroxol hydrochloride and levocetrizine dihydrochloride
| Parameters | AMB | LEVC |
| λmax | 244 nm | 231 nm |
| Beer’s Law range | 10-50 µg/ml | 8-24 µg/ml |
| Molar Absorptivity (0.001 | ||
| absorbance unit/mole. cm/dm3) | 9.944×103 | 1.4409×104 |
| Sandell’s sensitivity (µg/cm2/ | ||
| 0.001 absorbance unit) | 0.0379 | 0.0321 |
| Regression values: | ||
| Slope | 0.0262 | 0.0302 |
| Intercept | +0.0002 | +0.008 |
| Regression coefficient | 0.9992 | 0.9993 |
AMB and LEVC stands for ambroxol hydrochloride and levocetricine dihydrochloride, respectively
Table 2: Regression and optical characteristics of ambroxol hydrochloride and levocetirizine dihydrochloride
Twenty tablets were weighed accurately. The average weight was determined and then ground to a fine powder. A quantity equivalent to 75 mg of AMB and 5 mg of LEVC were transferred to a 100 ml volumetric ß ask. The contents were sonicated for 10 min with 50 ml of distilled water and the volume was made up with distilled water. The solution was then filtered through a Whatman filter paper No. 40. The solution was further diluted with distilled water, to give concentrations of 30 and 2 μg/ml of AMB and LEVC, respectively. The absorbance of the resulting solution was measured at 231 and 244 nm.
To study accuracy, reproducibility, and precision of the proposed methods, recovery studies were carried out at three different levels by addition of standard drug solution to preanalysed samples. Results of recovery studies were found to be satisfactory which are presented in Table 3.
| Drug in standard mixture solution (µg/ml) | % Recovery | Coefficient of variance (%) | |||
|---|---|---|---|---|---|
| AMB | LEVC | AMB | LEVC | AMB | LEVC |
| 2 | 2 | 99.28±0.341 | 98.88±0.555 | 0.311 | 0.491 |
| 4 | 4 | 99.52±0.254 | 99.42±0.308 | 0.209 | 0.256 |
| 8 | 6 | 99.13±0.205 | 99.03±0.404 | 0.322 | 0.460 |
AMB and LEVC stands for ambroxol hydrochloride and levocetricine dihydrochloride, respectively. The results are mean of three readings (n=3). % Recovery is expressed as mean ± standard deviation
Table 3: Recovery studies on ambroxol hydrochloride and levocetirizine dihydrochloride in syntheticmixture
The proposed method for simultaneous estimation of AMB and LEVC in combined sample solutions was found to be simple, accurate and reproducible. Beer’s law was obeyed in the concentration range of 10–50 μg/ml and 8-24 μg/ml for AMB and LEVC, respectively. Co-efficient of variation was found to be 0.9992 and 0.9993 for AMB and LEVC, respectively. The percentage recovery studies were found to be in the range of 99.13 to 99.52% and 98.88 to 99.42% for AMB and LEVC, respectively. Once the equations are determined, analysis requires only the measuring of the absorbance of the sample solution at two wavelengths selected, followed by a few simple calculations. It is a method that can be employed for routine analysis in quality control laboratories.
References
- Sweetman SC. Martindale, The Extra Pharmacopoeia, 34th ed. London: Pharmaceutical Press; 2004. p. 1114.
- Pospisilova M, Polasek M, Jokl V. Determination of ambroxol or bromhexine in pharmaceuticals by capillary isotachophoresis. J Pharm Biomed Anal 1997;24:421-8.
- Perez-Ruiz T, Martinez-Lozano C, Sanz A, Bravo E. Sensitive method for the determination of ambroxol in body fluids by capillary electrophoresis and fluorescence detection. J Chromatogr B 2000;742:205-10.
- Perez-Ruiz T, Martinez-Lozano C, Sanz A, Bravo E. Determination of bromhexine and ambroxol in pharmaceutical dosage forms, urine and blood serum. J Chromatogr B 1997;692:199-205.
- Dincer Z, Basan H, Goger NG. Quantitative determination of ambroxol in tablets by derivative UV spectrophotometric method and HPLC. J Pharm Biomed Anal 2003;31:867-72.
- Colombo L, Marcucci F, Marini GM, Poerfederici P, Mussini E. Determination of ambroxol in biological material by gas chromatography with electron-capture detection. J Chromatogr 1990;530:141-7.
- Schmid J. Assay of ambroxol in biological fluids by capillary gas-liquid chromatography. J Chromatogr 1987;414:65-75.
- Bazylak G, Nagels LJ. Simultaneous high-throughput determination of clenbuterol, ambroxol and bromhexine in pharmaceutical formulations by HPLC with potentiometric detection. J Pharm Biomed Anal 2003;32:887-903.
- Kim H, Yoo JY, Han SB, Lee HJ, Lee KR. Determination of ambroxol in human plasma using LC-MS/MS. J Pharm Biomed Anal 2003;32:209-16.
- Heinanen M, Barbas C. Validation of an HPLC method for the quantification of ambroxol hydrochloride and benzoic acid in a syrup as pharmaceutical form stress test for stability evaluation. J Pharm Biomed Anal 2001;24:1005-10.
- Koundorellis JE, Malliou ET, Broussali TA. High performance liquid chromatographic determination of ambroxol in the presence of different preservatives in pharmaceutical formulations. J Pharm Biomed Anal 2000;23:469-75.
- Nobilis M, Pastera J, Svoboda D, Kvetina J, Acek K. High-performance liquid chromatographic determination of ambroxol in human plasma. J Chromatogr 1992;581:251-5.
- Brizzi V, Pasetti U. High-performance liquid chromatographic determination of ambroxol in pharmaceuticals. J Pharm Biomed Anal 1990;8:107-9.