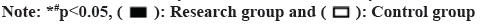- *Corresponding Author:
- Chujuan Li
Department of Gynecologic Oncology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province 430079, China
E-mail: li359928810@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “243-249” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To examine empagliflozin potential for treating acute decompensated heart failure. 108 acute decompensated heart failure patients who were treated in our hospital from January 2022 to January 2023 were randomly split into two groups, each with 54 patients as research group and the control group. The research group supplemented the control group with empagliflozin, while the control group continued to receive traditional anti-acute decompensated heart failure therapy. Comparisons were made between the two groups clinical effectiveness, cardiac and renal function markers, health status, and follow-up outcomes. The research group’s total treatment effectiveness was better than the control group’s (p<0.05). Following treatment, the research group had lower left ventricular end-systolic volume, left ventricular end-diastolic volume, serum creatinine, and blood urea nitrogen than the control group (p<0.05); the research group also had lower left ventricular ejection fraction, glomerular filtration rate, and the Kansas city cardiomyopathy questionnaire. Between the two groups, there was no discernible difference in the rate of decline (p>0.05). The research group outperformed the control group in terms of mortality and the risk of re-hospitalization within 60 d (p<0.05). Engramine is a highly valuable therapy option for acute decompensated heart failure since it is efficient, can enhance cardiac function, reduce renal impairment, and improve patient health.
Keywords
Acute decompensated heart failure, empagliflozin, cardiac function, renal function
A clinical illness known as Acute Heart Failure (AHF) occurs when the heart’s anatomical or functional defects induce a sudden decline in cardiac output, leading to hypoperfusion and stasis in tissues and organs[1,2]. Acute Decompensated Heart Failure (ADHF) is a common type of AHF characterized by volume retention and congestion, often accompanied by impaired renal function and diuretic resistance[3]. Relief of congestion is the main goal of treatment for patients with ADHF and failure to effectively relieve congestion can result in a poor prognosis and increased readmission rates. Diuretics are commonly used to relieve congestion in ADHF. However, clinical studies have revealed that their use is linked to worsening renal function[4], increased sympathetic activity[5], and elevated blood uric acid levels, all of which can have a negative impact on ADHF patients' prognoses. Therefore, finding new therapeutic strategies to effectively treat ADHF is a major need in the current clinical management of ADHF.Empagliflozin, a Sodium-Glucose Cotransport Protein 2 Inhibitor (SGLT2), is an oral hypoglycemic agent with cardiovascular benefits. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG)[6], which was completed in 2015, showed that heart failure hospitalization was significantly reduced by empagliflozin by 35 %, and the risk of the primary composite outcome, which comprised cardiovascular death, non-fatal Myocardial Infarction (MI), and non-fatal stroke, was significantly decreased by 14 %. Engramlizine is beneficial in treating heart failure of any ejection fraction type, according to a number of clinical trials[7-10]. We hypothesize that the use of empagliflozin in the treatment of ADHF would also be effective, but there is a paucity of studies on the benefit of empagliflozin in the treatment of ADHF to confirm that empagliflozin is an effective treatment option for ADHF. Based on this, we prospectively evaluated the benefit of empagliflozin in ADHF with the aim of providing a reliable and feasible reference for the clinical management of ADHF.
Materials and Methods
Experimental preparation:
Selecting 108 patients with AHF treated at our hospital between January 2022 and January 2023, a control group (n=54, receiving conventional anti-ADHF treatment) and a research group (n=54, adding engramine to conventional anti-ADHF treatment). The trial was approved by our medical ethics committee. A written informed consent form is signed by each patient included in the trial.
Inclusion and exclusion criteria:
Inclusion criteria met the diagnostic criteria for AHF and between 18 y-85 y of age.
Patients with chronic renal disease and a glomerular filtration rate of <30 ml/min/1.73 m2; patients requiring hemodialysis due to end-stage renal failure or acute kidney damage; patients treated with SGLT2 inhibitors within 3 mo prior to study entry and patients with allergic reactions to engramine and patients with AHF without signs of congestion were excluded.
Methods:
The control group's patients were given traditional anti-ADHF therapy, including cardioplegia, diuretics, nitrates, valsartan and beta (β) blockers in the conventional group.
The research group was supplemented with empagliflozin on top of the control group. Empagliflozin (Manufacturer: BoehringerIngelheimPharmaGmbH&Co.KG, Approval No: State Drug Administration J20171073, specification: 10 mg×10 s) was administered orally, 10 mg/dose, 1 time/d. Patients in both groups were treated continuously for 30 d. Death, serious drug-related adverse reactions were included as termination events for the experiment.
Efficacy assessment:
After treatment, It was deemed effective if clinical symptoms improved significantly, Left Ventricular Ejection Fraction (LVEF) improved by 20 % or more compared to that before treatment, and New York Heart Association (NYHA) classification improved by 2; if clinical symptoms improved, LVEF improved by 10 %-20 % compared to that before treatment, and NYHA classification improved by 1; and if the aforementioned criteria were not met after treatment, it was deemed ineffective.
Total treatment effectiveness=(Number of effective+number of ineffective)/total cases×100 %
Observed indicators:
Cardiac function indicators: Before and after treatment, Left Ventricular End-Systolic Volume (LVESV), Left Ventricular End-Diastolic Volume (LVEDV) and Left Ventricular Ejection Fraction (LVEF) were measured in both groups using a Sonos model color Doppler ultrasound machine manufactured by Philips.
Renal function indicators: 5 ml of venous blood from patients was taken before and after therapy, centrifuged, and the supernatant was then extracted. Serum creatinine (Scr) and Blood Urea Nitrogen (BUN) levels were measured using a fully automated immunoluminescence analyzer and Glomerular Filtration Rate (eGFR) was calculated.
The Kansas City Cardiomyopathy Questionnaire (KCCQ) was used to score the health status of both groups before and after treatment, using the KCCQ. The questionnaire was divided into five dimensions; physical limitations, symptoms, disease awareness, social dysfunction and quality of life, with each dimension scored on a scale of 0 to 100. Patients were followed up for 60 d and deterioration, death and re-hospitalization were recorded for both groups.
Statistical processing:
GraphPad Prism 9.0 was used for the graphing and Statistical Package for the Social Sciences (SPSS) 21.0 for the analysis. Count data were expressed as [n (%)] and compared using the Chi-square (χ2) test, whereas measurement data were expressed as (x±s) and compared using the t-test, Analysis of Variance (ANOVA), and Least Significant Difference (LSD) test. Differences were deemed statistically significant at p<0.05.
Results and Discussion
The basic information collected from the two groups for comparison showed no statistically significant difference (p>0.05, Table 1). During the study period, no patients experienced serious drug-related adverse reactions, no patients died and there were no trial discontinuations. Compared to the control group, the research group's total treatment effectiveness was higher (p<0.05, Table 2).
| Characteristics | Research group (n=54) | Control group (n=54) | χ2 or t | p |
|---|---|---|---|---|
| Gender, n (%) | 0.038 | 0.8456 | ||
| Male | 30 (55.56) | 31 (57.41) | ||
| Female | 24 (44.44) | 23 (42.59) | ||
| Age (year, x̄±s) | 67.42±1.81 | 67.48±1.85 | 0.170 | 0.865 |
| NYHA grading, n (%) | 0.041 | 0.839 | ||
| III | 36 (66.67) | 35 (64.81) | ||
| IV | 18 (33.33) | 17 (31.48) | ||
| LVEF (%, x̄±s) | 34.58±4.21 | 35.09±4.46 | 0.611 | 0.543 |
| BMI (kg/m2, x̄±s) | 23.53±2.14 | 24.03±2.08 | 1.231 | 0.221 |
| Heart rate (bmp) | 80±17 | 78±23 | 0.514 | 0.608 |
| Systolic blood pressure (mmHg) | 139.62±23.19 | 140.08±23.05 | 0.103 | 0.918 |
| Heart failure medication | ||||
| Renin-angiotensin inhibitor | 41 (75.93) | 40 (74.07) | 0.050 | 0.824 |
| Sacubitril/valsartan | 11 (20.37) | 13 (24.07) | 0.214 | 0.643 |
| Mineralocorticoid receptor antagonist | 16 (29.63) | 15 (27.78) | 0.045 | 0.832 |
| β-blocker | 38 (70.37) | 40 (74.07) | 0.185 | 0.668 |
| Previous treatment with loop diuretics | 29 (53.70) | 28 (51.85) | 0.037 | 0.847 |
| Pathogenesis of heart failure | 0.042 | 0.837 | ||
| Ischemic | 17 (31.48) | 18 (33.33) | ||
| Nonischemic | 37 (68.52) | 36 (66.67) | ||
Table 1: Basic Information of the two Groups of Patients
| Group | n | Markedly significant | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| Research | 54 | 28 (51.85) | 22 (40.74) | 4 (7.41) | 50 (92.59) |
| Control | 54 | 23 (42.59) | 18 (33.33) | 13 (24.07) | 41 (75.93) |
| χ 2 | - | - | - | - | 5.655 |
| p | - | - | - | - | 0.017 |
Table 2: Total Effective Rate of Treatment in both Groups [n (%)]
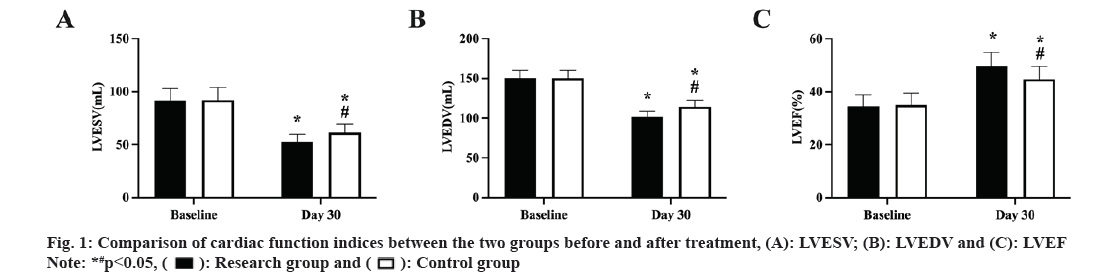
The cardiac function measures did not significantly differ between the two groups prior to therapy (p>0.05). After treatment, the study group's LVEF was larger than the control groups, and its LVESV and LVEDV were lower (p<0.05, fig. 1A-fig. 1C).
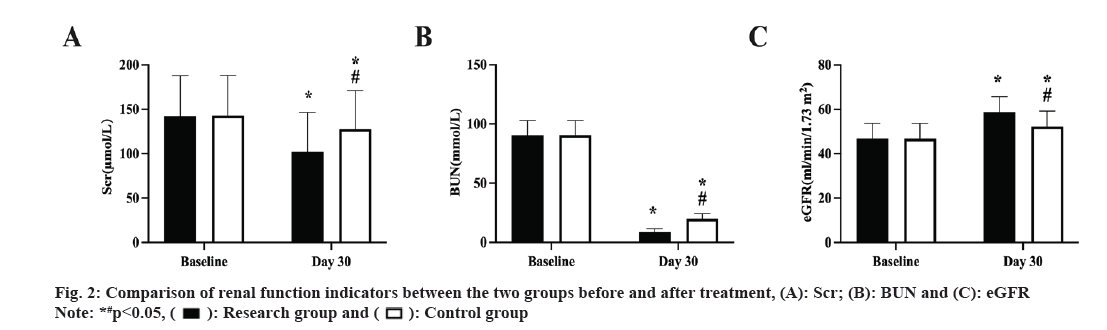
Prior to therapy, there was no discernible difference between the two groups renal function indicators (p>0.05), and both groups improved following treatment. The study group, however, had lower Scr and BUN, and higher eGFR than the control group (p<0.05, fig. 2A-fig. 2C).
The KCCQ scores of the two groups did not significantly differ before to therapy (p>0.05). All KCCQ scores in both groups were higher after treatment than they were before, with the research group scoring higher than the control group (p<0.05, fig. 3A-fig. 3E).
Two patients in the trial group and one in the control group were lost during the follow-up period. The rate of deterioration between the two groups did not differ significantly (p>0.05). The research group's death and readmission rates were lower than those of the control group. (p<0.05, Table 3).
| Group | n | Deterioration of disease | Re-hospitalization | Death |
|---|---|---|---|---|
| Research | 52 | 7 (12.96) | 3 (5.56) | 1 (1.85) |
| Control | 53 | 8 (14.81) | 10 (18.52) | 7 (12.96) |
| χ2 | - | 0.077 | 4.285 | 4.86 |
| P | - | 0.781 | 0.038 | 0.028 |
Table 3: Follow-Up Results of the two Groups [n (%)]
The prognosis of AHF has improved with the gradual improvement in diagnosis and treatment, but mortality remains high and is a key concern for clinicians. ADHF is an acute onset of symptoms and signs in addition to CHF and accounts for approximately 80 % of AHF[11]. In a prospective multicenter pilot research, Lassus et al.[12] reported a 1 y mortality rate of 21.7 % and a 5 y mortality rate of 44.4 % for patients with primary AHF. Patients with ADHF showed death rates of 33.3 % after 1 y and 75.6 % after 5 y. It is therefore important to investigate effective treatment strategies for ADHF.
Cardiovascular disease is the primary killer of diabetics and a common complication of the condition, according to past study. Traditional glucose-lowering medications have minimal benefits on the heart and may even raise the chance of developing heart disease. With the prevalence of diabetes remaining high, it is critical to find drugs that combine both glucose-lowering and cardiovascular protection. As a result, the United States Food and Drug Administration (USFDA) ordered in 2008 that all new glucose-lowering medications must undergo cardiovascular safety testing[13]. As research progresses; a number of new glucose-lowering drugs, represented by SGLT2 inhibitors are gradually entering the clinic, playing different roles in lowering blood pressure and cardiovascular protection, while effectively lowering glucose. Among them, SGLT2 inhibitors have received widespread attention due to their excellent cardiovascular protective effects and are gradually becoming an optional treatment for cardiovascular diseases. One of the four SGLT2 inhibitors now in clinical usage in the US and approved by the USFDA is empagliflozin[14].
Our results show that the cardiac function indicators in the research group improved following treatment compared to those in the control group, demonstrating the considerable contribution of engramine to the enhancement of cardiac function in ADHF patients. After the onset of ADHF, the heart can experience dramatic changes in its energy metabolism. The heart becomes more dependent on glycolysis as an energy source as the disease worsens because mitochondrial oxidative metabolism keeps declining[15], while glucose oxidation in the mitochondria decreases in heart failure, leading to reduced energy production and inadequate blood supply to the heart[16,17]. Studies have shown that SGLT2 inhibitors, represented by empagliflozin, can reduce renal excretion of ketone bodies, elevate β-hydroxybutyric acid levels in the body, and provide the heart with energy-supplying substances, while ketone body oxidation not only improves cardiac oxidative stress, but also reduces mitochondrial oxidative stress, improves mitochondrial efficacy, stabilizes cell membrane potential, and improves the energy metabolism of the heart[18]. Meanwhile, empagliflozin has certain anti-cardiac fibrosis effects. Engramine can reduce myocardial fibrosis and provide cardio protective benefits by lowering the expression of transforming growth factor 1, type I collagen and type III collagen, according to animal studies[19]. In addition, empagliflozin also exerts anti-cardiac fibrosis effects by promoting the conversion of macrophages from M1 to M2, inhibiting Alpha (α)-smooth muscle actin, ligand tissue growth factor, matrix metalloproteinase 2 and human fibroblast activation[20,21].
Patients with ADHF are often treated with diuretics. It is undisputed that diuretics are effective in relieving the congestive symptoms of ADHF. However, most clinical studies have shown an association between diuretics and worsening renal function and even increased mortality[22,23]. Therefore, clinical use of diuretics for ADHF should be cautious. The findings of this study looked at how engramine affected renal function in ADHF patients. The findings demonstrated that all measures of renal function in the research group were superior to those in the control group, demonstrating that empagliflozin does not exacerbate or even worsen individuals with ADHF's compromised renal function. The reasons considered were; related studies suggest that empagliflozin may have a different role in regulating interstitial fluid (vs. intravascular volume), which may limit the reactive neurohumoral stimulation produced by the contraction of intravascular volume by conventional diuretics[24]. Meanwhile, empagliflozin reduces the affinity of renal proximal tubular SGLT2 for glucose, decreasing renal reabsorption of glucose and promoting urinary glucose excretion, as well as urinary sodium excretion[25]. Most patients with ADHF have renal impairment, and microRNA-21, which is expressed at high levels in the heart and kidney, is a major target for the treatment of renal impairment in ADHF. It has been reported in the literature that empagliflozin can inhibit microRNA-21 while acting as an effective diuretic, thereby inhibiting the process of renal fibrosis[26,27]. The effect of empagliflozin in improving cardiac function, which increases cardiac output and improves renal perfusion, together with its excellent hypoglycemic, anti-inflammatory and endothelial cell protective effects, may reduce renal impairment in ADHF patients.
KCCQ scores were used in this study to gauge the patients' health state. The results showed that after treatment, the research group's KCCQ ratings were considerably higher than those of the control group, showing that empagliflozin helped to enhance the health status of patients with ADHF and their quality of life. This was considered to be due to the improvement in physiological indicators such as cardiac function on the one hand, and the improvement in mind-set on the other.
In terms of overall benefit, our results show that the research group had a higher overall treatment efficiency than the control group, and lower readmission and mortality rates within 60 d of follow-up than the control group. Taken together, the above discussion confirms that patients with ADHF can benefit from treatment with empagliflozin.
Firstly, this study was limited by the number of patients and should be considered as a pilot study. Secondly, for various reasons, fewer cases were included in our study than were enrolled, so the applicability of the results of this study to ADHF in general may be questioned, but the inclusion and exclusion criteria of this study were strict but not harsh and to some extent representative of the characteristics of patients with ADHF currently receiving treatment. Finally, our study had few comparisons of biochemical parameters between the two groups of patients to confirm other mechanisms of benefit of empagliflozin in the treatment of ADHF.
Empagliflozin is effective in the treatment of ADHF, improving cardiac function, alleviating renal impairment and improving the health status of patients, and is of great value in clinical applications.
Author’s contributions:
Ya Liu and Jing Hu have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Mentz RJ, Oconnor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016;13(1):28-35.
[Crossref] [Google Scholar] [PubMed]
- Aguirre Tejedo A, Miro O. Precipitating factors in acute heart failure: A review. Emergencias 2017;29(3):185-193.
[Google Scholar] [PubMed]
- Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S et al . Recommendations on pre-hospital and early hospital management of acute heart failure: A consensus paper from the heart failure association of the European society of cardiology, the European society of emergency medicine and the society of academic emergency medicine–Short version. Eur Heart J 2015;36(30):1958-66.
[Crossref] [Google Scholar] [PubMed]
- Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B. EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur Heart J 2013;34(11):835-43.
- Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, et al. Haemoconcentration, renal function and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Eur J Heart Fail 2013;15(12):1401-11.
[Crossref] [Google Scholar] [PubMed]
- Zinman B, WaIIIler C, Lachin JM.Empaglinozin,cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med 2015;373(22):2117-28.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383(15):1413-24.
[Crossref] [Google Scholar] [PubMed]
- McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381(21):1995-2008.
[Crossref] [Google Scholar] [PubMed]
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385(16):1451-61.
[Crossref] [Google Scholar] [PubMed]
- Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation 2021;144(16):1284-94.
[Crossref] [Google Scholar] [PubMed]
- Njoroge JN, Teerlink JR. Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ Res 2021;128(10):1468-86.
[Crossref] [Google Scholar] [PubMed]
- Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola VP, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: The Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol 2013;168(3):2186-94.
[Crossref] [Google Scholar] [PubMed]
- Schnell O, Standl E, Catriinoju D. Report from the 1st cardiovascular outcome trial (CVOT) summit of the diabetes and cardiovascular disease EASD study group. Cardiovasc Diabetol 2016;15:33-8.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393(10166):31-9.
[Crossref] [Google Scholar] [PubMed]
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90(1):207-58.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, et al. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 2013;6(5):1039-48.
[Crossref] [Google Scholar] [PubMed]
- Neubauer S. The failing heart-An engine out of fuel. N Engl J Med 2007;356(11):1140-51.
[Crossref] [Google Scholar] [PubMed]
- Verma S, McMurray JJ, Cherney DZ. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: The search for the sweet spot in heart failure. JAMA Cardiol 2017;2(9):939-40.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhang J, Xue M, Li X, Han F, Liu X, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019;18:1-3.
[Crossref] [Google Scholar] [PubMed]
- Quagliariello V, de Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol 2021;20(1):150.
[Crossref] [Google Scholar] [PubMed]
- Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell 2022;13(5):336-59.
[Crossref] [Google Scholar] [PubMed]
- Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2020;75(10):1178-95.
[Crossref] [Google Scholar] [PubMed]
- Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol 2010;56(19):1527-34.
[Crossref] [Google Scholar] [PubMed]
- An D, Li SR, Luo F. Research progress on the application of sodium-glucose cotransport protein 2 inhibitors in heart failure patients with mildly reduced ejection fraction. Chin General Med 2022;5(21):2680-5.
- Liu DQ. Role of sodium-glucose co-transport protein 2 inhibitors in heart failure. Chin Cardiovasc J 2020;25(1):1-3.
- Huang CK, Bär C, Thum T. miR-21, mediator and potential therapeutic target in the cardiorenal syndrome. Front Pharmacol 2020;11:726.
[Crossref] [Google Scholar] [PubMed]
- Das NA, Carpenter AJ, Belenchia A, Aroor AR, Noda M, Siebenlist U, et al. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell Signal 2020;68:109506.
[Crossref] [Google Scholar] [PubMed]