- *Corresponding Author:
- X. D. She
- Department of Obstetrics and Gynecology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences
and Medicine, University of Science and Technology of China, Hefei, Anhui 230001, China
E-mail: shexiangdong119@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “19-24” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focused on analyzing the differences in efficacy of different doses of mifepristone in the treatment of uterine fibroids. 117 uterine fibroid patients admitted between September 2018 and September 2022 were selected and divided into two groups including 55 patients (control group) receiving 25.0 mg mifepristone oral therapy and 62 patients (research group) receiving 12.5 mg mifepristone oral therapy, both once daily for three menstrual cycles. Clinical evaluation included efficacy, occurrence of adverse drug reactions (dizziness, nausea, vomiting, and elevated alanine aminotransferase level), uterine volume, fibroid volume, and the levels of estradiol, luteinizing hormone, follicle-stimulating hormone, insulin-like growth factor-1, and transforming growth factor-beta 1. The data showed a similar total effective rate in the two groups. However, a markedly lower incidence of adverse drug reactions was determined in the research group vs. the control group. Besides, after treatment the research group had evident reductions in uterine volume, fibroid volume, as well as estradiol, luteinizing hormone, follicle-stimulating hormone, insulin-like growth factor-1, and transforming growth factor-beta 1 levels, which were statistically decreased compared with the control group. Therefore, low-dose mifepristone is equivalent to the conventional dose in treating uterine fibroids, with obvious advantages in medication safety, which will not only significantly reduce the volume of uterus and fibroids, but also inhibit the levels of serum sex hormones and disease progression, thus effectively inhibiting disease deterioration.
Keywords
Uterine fibroids, mifepristone, oral therapy, adverse drug reactions
Uterine Fibroids (UFs) are benign tumors of the reproductive organs that negatively affect a woman's fertility and quality of life, and may also contribute to adverse pregnancy outcomes in women[1,2]. The pathological mechanism of UFs is complex, which may be associated with multiple small and medium-sized leiomyomas caused by Mediator of Ribonucleic Acid (RNA) polymerase II transcription subunit 12 (MED12) gene mutations, or epigenetic disorders attributed to High Mobility Group protein 2 (HMGA2) overexpression-induced chromosomal instability or aberration[3]. Age, diet, obesity, caffeine, hypertension, family history and premenopausal status are shown to increase the risk of UFs[4,5]. In addition, most patients with the disease have atypical symptoms, while 30 % present with experience anemia, pain, frequent urination, menorrhagia, pelvic discomfort, constipation or infertility, abnormal uterine bleeding, and other typical clinical symptoms[6,7]. The major treatment strategies for UF patients are surgery and pharmacotherapy. The former may be associated with a higher reoperation rate and potential long-term sequelae; while the latter, though it may lead to some side effects, it is more acceptable and therapeutic to women as a non-surgical long-term treatment regimen[8-10]. This study aims to provide better treatment options for UF patients from a drug treatment perspective.
Mifepristone (Mif), a derivative of 19-nortestosterone, has anti-progesterone and anti-glucocorticoid activities and can be used for miscarriage management, emergency contraception, as well as for reducing fibroid volume and relieving symptoms[11,12]. A rat experiment showed that the role of Mif in drug abortion may be related to increase in expression levels of Malondialdehyde (MDA), activated caspase 3, caspase 9 and recombinant B-cell lymphoma 2 (Bcl-2)-associated X protein (BAX), as well as the down-regulation of Superoxide Dismutase (SOD) and Bcl-2[13]. Besides, compared with RFA intervention alone, Mif combined with ultrasound- guided Radiofrequency Ablation (RFA) for large UFs has been indicated to not only significantly reduce the mean ablation time and mean number of punctures, but also improve the average resolution rate of UFs, the quality of life and symptom scores without causing major complications, suggesting that Mif also has certain therapeutic potential in large UFs[14]. The dosage of Mif in the treatment of UFs is still controversial. This study attempts to analyze the difference in efficacy of different doses of Mif in UF treatment, so as to provide a reliable basis for clinical administration of Mif in UFs.
Materials and Methods
Patients and clinical data:
117 UF patients treated at The First Affiliated Hospital of USTC between September 2018 and September 2022 were selected, of which 55 patients in the control group received 25.0 mg of Mif and 62 patients in the research group received 12.5 mg of Mif. In the control group, the age, UF diameter, and gravidity were (37.42±6.84) y, (3.61±1.06) cm and 2.53±0.84, respectively. Patients in the research were (36.53±5.98) y old, with an UF diameter of (3.69±1.08) cm and a gravidity of 2.35±1.01. The age, UF diameter, gravidity, and other information of the two groups were clinically comparable (p>0.05). This study was approved by the Ethics Committee of The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China.
Inclusion criteria:
Women diagnosed as UF[15]; non-menopausal women with symptoms such as menstrual disorders, dysmenorrhea, abdominal tenderness and mass; women with no contraindications to Mif; presence of surgical contraindications; patients with normal mental and cognitive abilities; non-lactating or pregnant patients and patients who provided written informed consent are included in the study.
Exclusion criteria:
Patients with cardio-cerebrovascular diseases or hepatic and renal insufficiency; presence of endometrial lesions, other tumors, or lesions of the cervix, uterus or adnexa uteri and patients with defective medical records are excluded from the study.
Research methods:
The control group received 25.0 mg of Mif (Shanghai New Hualian Pharmaceutical Co., Ltd., Batch no.: H10950202), which was orally administered once daily starting on the 1st d of the menstrual cycle. The research group used Mif in a similar way as the control group, except that the dosage was 12.5 mg. Both groups were treated continuously for 3 menstrual cycles.
Outcome measures:
Data on efficacy, occurrence of Adverse Drug Reactions (ADRs), uterine volume, fibroid volume, Estradiol (E2), Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), Insulin- like Growth Factor-1 (IGF-1) and Transforming Growth Factor-Beta 1 (TGF-β1) were collected for comparative analyses. Among them, the efficacy was assessed as significantly effective indicates reduction in fibroid volume >50 % compared with the baseline (before treatment), the basically recovered menstrual flow and menstrual cycle; effectiveness indicates a 20 %-50 % decrease in fibroid volume compared to the baseline, as well as improved but not recovered menstrual flow and menstrual cycle; ineffectiveness indicates a <20 % reduction in fibroid volume with no improvement in menstrual flow or menstrual cycle. ADRs mainly included dizziness, nausea and vomiting and elevated Alanine Aminotransferase (ALT) level, and the incidence rate was calculated. Changes in uterine volume and fibroid volume before and after treatment were detected by color Doppler ultrasound. E2, LH, FSH, all sex hormone indices, were also measured. Before detection, fasting cubital venous blood was collected from each patient and centrifuged to obtain serum samples for the measurement of the above sex hormone indices using radioimmunoassay. The quantification of IGF-1 and TGF-β1 was employed by Enzyme-Linked Immunosorbent Assay (ELISA) and microplate reader.
Statistical analyses:
Measurement data were statistically described by mean±Standard Error of the Mean (SEM), and the comparison between groups was performed by the independent sample t test. Count data were represented by frequency (%), and the comparison between groups was made by the Chi-square (χ2) test. Data were analyzed using GraphPad Prism 7.0 and p<0.05 is considered as statistically significant.
Results and Discussion
The general data of two groups of UF patients was compared as shown in Table 1. No notable differences were identified between the research and control groups in terms of age, UF diameter, gravidity, course of disease, and myoma type (p>0.05).
| Characteristics | Control group (n=55) | Research group (n=62) | χ2/t value | p value |
|---|---|---|---|---|
| Age (years) | 37.42±6.84 | 36.53±5.98 | 0.751 | 0.454 |
| UF diameter (cm) | 3.61±1.06 | 3.69±1.08 | 0.403 | 0.687 |
| Gravidity (times) | 2.53±0.84 | 2.35±1.01 | 1.04 | 0.3 |
| Course of disease (years) | 2.95±1.01 | 2.61±1.05 | 1.78 | 0.078 |
| Myoma type | ||||
| Mixed myoma | 5 (9.09) | 7 (11.29) | 0.931 | 0.818 |
| Submucous myoma | 6 (10.91) | 10 (16.13) | ||
| Subserous myoma | 5 (9.09) | 5 (8.06) | ||
| Intramural myoma | 39 (70.91) | 40 (64.52) |
Table 1: Comparative Analysis of General Data of Two Groups of UF Patients
After treatment with Mif at different doses, the efficacy analysis of two groups of UF patients was compared as shown in Table 2. The two groups showed no statistical significance in the total effective rate (p>0.05).
ADRs of two groups of UF patients under the influence of different doses of Mif were compared as shown in Table 3. The ADRs such as dizziness, nausea and vomiting, and elevated ALT level were observed and counted, and an obviously lower overall incidence was determined in the research group compared to the control group (0.00 % vs. 9.09 %, p<0.05).
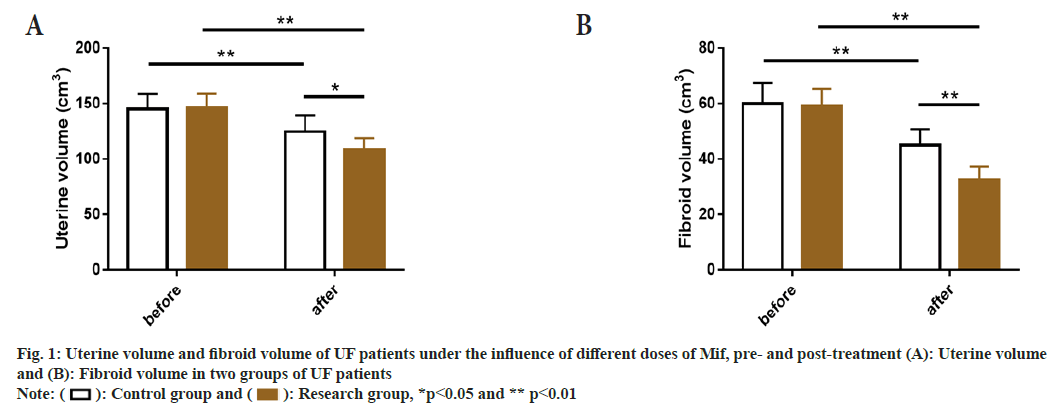
The uterine volume and fibroid volume were similar between the two groups at baseline (before treatment) (p>0.05), but they decreased significantly in both groups after treatment (p<0.05), with even more significant decrease in the research group (p<0.05) compared to the control group (fig. 1).
| Group | n | Marked effectiveness | Effectiveness | Ineffectiveness | Total effective rate |
|---|---|---|---|---|---|
| Control | 55 | 27 (49.09) | 25 (45.45) | 3 (5.45) | 52 (94.55) |
| Research | 62 | 27 (43.55) | 31 (50.00) | 4 (6.45) | 58 (93.55) |
| χ2 | 0.052 | ||||
| p | 0.821 |
Table 2: Efficacy Analysis of MIF at Different Doses on UF Patients after Treatment
| Group | n | Dizziness | Nausea and vomiting | Elevated ALT level | Total |
|---|---|---|---|---|---|
| Control | 55 | 2 (3.64) | 2 (3.64) | 1 (1.82) | 5 (9.09) |
| Research | 62 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| χ2 | 5.888 | ||||
| p | 0.015 |
Table 3: ADR’S of UF Patients under the Influence of Different Doses of MIF
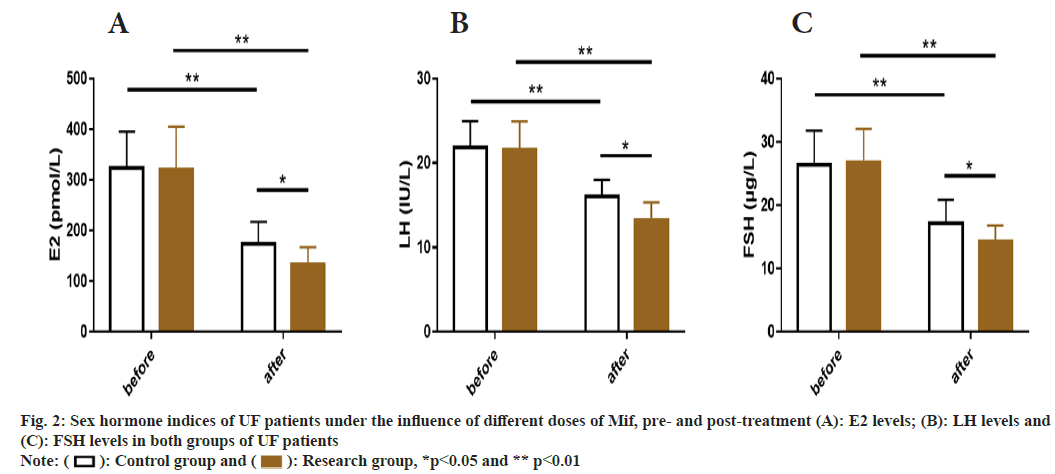
Before treatment, the E2, LH, and FSH, all sex hormone indicators showed no significant inter- group differences (p>0.05), but the aforementioned indicators in both groups were significantly reduced after treatment (p<0.05), with even lower E2, LH, and FSH levels in the research group as compared to the control group (p<0.05) (fig. 2).
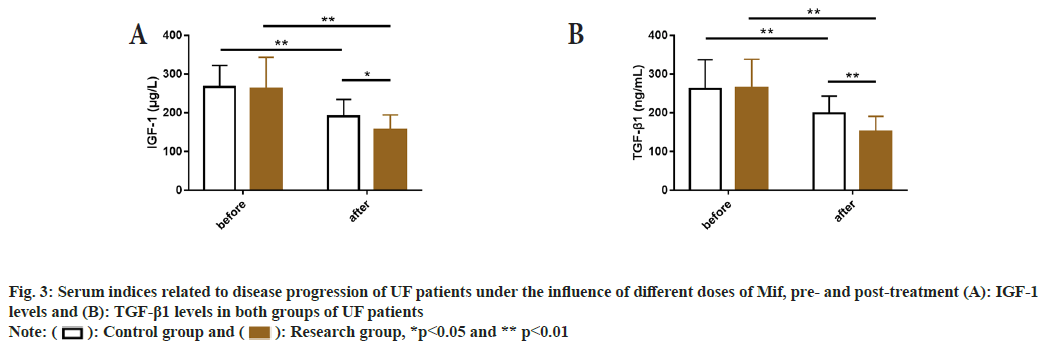
Before treatment, serum indicators related to disease progression, such as IGF-1 and TGF-β1, were detected and no significant differences were found between two groups (p>0.05); but these indices reduced significantly after treatment (p<0.05), with even lower levels in the research group (p<0.05) compared to the control group (fig. 3).
UFs are benign lesions of endometrial glands and stroma located in the myometrium that may involve inflammation, fibrosis, neuroangiogenesis and abnormal sex steroid hormones[16]. Moreover, the lifetime prevalence of UFs in women can be as high as 80 %, and the disease appears in patients during perimenopause or menopause, it usually presents only as abnormal uterine bleeding[17]. Therefore, it is necessary to increase research investment from the treatment of UF to alleviate the clinical symptoms of UF patients and reduce the risk of UFs.
Many previous studies have conducted comparative analysis of the therapeutic effect of 5.0 mg and 2.5 mg Mif in UF, confirming that 2.5 mg Mif can achieve considerable effects on improving patients’ quality of life despite a small reduction in the volume of fibroids[18]. Another study pointed out that 5.0 mg and 2.5 mg Mif have similar effects in reducing the fibroid volume, and the improvement in symptoms was sustained for 1 y[19]. A total of 117 UF patients were enrolled in this study, with patients receiving 25.0 mg Mif and those receiving 12.5 mg Mif being assigned to the control group and the research group respectively, to comparatively analyze the effects of different doses of Mif on the clinical efficacy of UFs. Our results showed that the total effective rate of the research group was equivalent to that of the control group (93.55 % vs. 94.55 %), suggesting that Low-Dose (LD) Mif (12.5 mg) is as effective as conventional dose Mif (25.0 mg) in UF patients. The therapeutic mechanism of Mif in UF has been reported to be strongly related to its inhibition of the formation of Extracellular Matrix (ECM) in UFs, which is manifested by its down-regulation of ECM components such as Collagen Alpha 1(I) gene (COL1A1), fibronectin, versican, and dermatopontin in human leiomyoma cells[20]. It has also been shown that the anti-UF therapeutic effect of Mif may be regulated by syndecan-1, and the inhibitory effect on UF cell proliferation and ECM can be enhanced when syndecan-1 is knocked down[21]. Subsequently, the incidence of ADRs such as dizziness, nausea and vomiting, and elevated ALT levels was found to be markedly lower in the research group vs. the control group (0.00 % vs. 9.09 %), indicating that LD-Mif has a better clinical safety profile than the conventional dose.
Furthermore, the post-treatment uterine volume and fibroid volume of the research group were significantly reduced than the baseline and the control group, indicating better effects of LD-Mif than the conventional Mif dose on reducing uterine volume and fibroid volume.
One study suggests that the reduction of fibroid volume by Mif may be associated with its competitive binding and antagonism to progesterone receptors[22]. In the study of Shen et al. Mif is beneficial to reduce uterine volume and fibroid volume in premenopausal UF patients while exerting a significant effect on relieving leiomyoma-related symptoms, consistent with our observations[23]. After testing the sex hormone indices such as E2, LH and FSH, it was found that their post-treatment levels in the research group were significantly reduced compared with the baseline and control group, suggesting that LD- Mif can significantly relieve the abnormalities of sex hormone indices in UF patients, similar to the research results of another study[24]. IGF-1, a hormone with similar structure to insulin, modulates cell growth, metabolism and programmed cell death, and is also involved in UF growth[25]. TGF-β1, one of the components of a polypeptide, is closely related to UF pathophysiology, and its overexpression will stimulate UF tumor growth, metastasis and metabolic enhancement to a certain extent[26] and both of them are closely related to UF progression. The measurement of serum indices related to disease progression (IGF-1 and TGF-β1) revealed their evidently reduced levels in both groups after treatment, with even lower levels in the research group, indicating that the LD-Mif treatment used in the research group can significantly inhibit the abnormal expression of IGF-1 and TGF-β1. According to Shen et al. the therapeutic effect of Mif in UF is related to its inhibition of IGF-1 signaling pathway, and overexpressing IGF-1 can significantly promote UF cell growth, suggesting that IGF-1 is a potential important target for Mif to play an anti-UF role[27].
In summary, LD-Mif has the same efficacy as the conventional Mif dose and a favorable clinical safety profile for the treatment of UFs, which can significantly reduce uterine volume and fibroid volume, and inhibit serum E2, LH and FSH, IGF-1, and TGF-β1 levels, which deserves clinical popularization. Our findings can provide new insights and choices for medication management in UF patients.
Conflict of interests:
The authors declared no conflict of interests.
References
- Tinelli A, Vinciguerra M, Malvasi A, Andji? M, Babovi? I, Spari? R. Uterine fibroids and diet. Int J Environ Res Public Health 2021;18(3):1-15.
[Crossref] [Google scholar] [PubMed]
- Metwally M, Raybould G, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev 2020;1(1):1-44.
[Crossref] [Google scholar] [PubMed]
- Baranov VS, Osinovskaya NS, Yarmolinskaya MI. Pathogenomics of uterine fibroids development. Int J Mol Sci 2019;20(24):1-12.
[Crossref] [Google scholar] [PubMed]
- Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2018;46:3-11.
[Crossref] [Google scholar] [PubMed]
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017;124(10):1501-12.
[Crossref] [Google scholar] [PubMed]
- Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet 2020;149(1):3-9.
[Crossref] [Google scholar] [PubMed]
- Al-Hendy A, Lukes AS, Poindexter III AN, Venturella R, Villarroel C, Critchley HO, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med 2021;384(7):630-42.
[Crossref] [Google scholar] [PubMed]
- Donnez J, Dolmans MM. Uterine fibroid management: From the present to the future. Hum Reprod Update 2016;22(6):665-86.
[Crossref] [Google scholar] [PubMed]
- Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy vs. ovarian conservation in the nurses’ health study. Obstet Gynecol 2013;121(4):709-16.
[Crossref] [Google scholar] [PubMed]
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: Burden and unmet medical need. Semin Reprod Med 2017;35(6):473-480.
[Crossref] [Google scholar] [PubMed]
- Karena ZV, Shah H, Vaghela H, Chauhan K, Desai PK, Chitalwala AR, et al. Clinical utility of mifepristone: Apprising the expanding horizons. Cureus 2022;14(8):1-12.
[Crossref] [Google scholar] [PubMed]
- Islam MS, Afrin S, Jones SI, Segars J. Selective progesterone receptor modulators-mechanisms and therapeutic utility. Endocr Rev 2020;41(5):1-52.
[Crossref] [Google scholar] [PubMed]
- Yu Q, Hu S, Hu S. Effect of mifepristone and lithospermum combination regimen on medical abortion in early pregnancy rats. J Obstet Gynaecol Res 2021;47(11):3789-96.
[Crossref] [Google scholar] [PubMed]
- Hai N, Hou Q, Dong X, Guo R. Comparison between radiofrequency ablation combined with mifepristone and radiofrequency ablation for large uterine fibroids. Int J Hyperthermia 2021;38(1):777-80.
[Crossref] [Google scholar] [PubMed]
- Lin Y, Wu RC, Huang YL, Chen K, Tseng SC, Wang CJ, et al. Uterine fibroid-like tumors: Spectrum of MR imaging findings and their differential diagnosis. Abdom Radiol 2022;47(6):2197-208.
[Crossref] [Google scholar] [PubMed]
- Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum Reprod Update 2020;26(3):392-411.
[Crossref] [Google scholar] [PubMed]
- Ulin M, Ali M, Chaudhry ZT, Al-Hendy A, Yang Q. Uterine fibroids in menopause and perimenopause. Menopause 2020;27(2):238-42.
[Crossref] [Google scholar] [PubMed]
- Carbonell JL, Acosta R, Pérez Y, Garcés R, Sánchez C, Tomasi G. Treatment of uterine myoma with 2.5 or 5 mg mifepristone daily during 3 months with 9 months posttreatment followup: Randomized clinical trial. ISRN Obstet Gynecol 2013;2013:1-9.
[Crossref] [Google scholar] [PubMed]
- Esteve JL, Acosta R, Pérez Y, Campos R, Hernández AV, Texidó CS. Treatment of uterine myoma with 5 or 10 mg mifepristone daily during 6 months, post-treatment evolution over 12 months: double-blind randomised clinical trial. Eur J Obstet Gynecol Reprod Biol 2012;161(2):202-8.
[Crossref] [Google scholar] [PubMed]
- Patel A, Malik M, Britten J, Cox J, Catherino WH. Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril 2016;105(4):1102-10.
[Crossref] [Google scholar] [PubMed]
- Shen X, Wang X. The function role and synergic effect of syndecan-1 for mifepristone in uterine leiomyoma. Cytotechnology 2021;73(2):179-87.
[Crossref] [Google scholar] [PubMed]
- Reis FM, Bloise E, Ortiga-Carvalho TM. Hormones and pathogenesis of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2016;34:13-24.
[Crossref] [Google scholar] [PubMed]
- Shen Q, Hua Y, Jiang W, Zhang W, Chen M, Zhu X. Effects of mifepristone on uterine leiomyoma in premenopausal women: A meta-analysis. Fertil Steril 2013;100(6):1722-6.
[Crossref] [Google scholar] [PubMed]
- Zhao X, Zhang C, Lou H, Wu C. Clinical efficacy and safety study of mifepristone with misoprostol treatment in patients with missed abortion. Evid Based Complement Alternat Med 2021;2021:1-7.
[Crossref] [Google scholar] [PubMed]
- Uimari O, Subramaniam KS, Vollenhoven B, Tapmeier TT. Uterine fibroids (leiomyomata) and heavy menstrual bleeding. Front Reprod Health 2022;4:1-9.
[Crossref] [Google scholar] [PubMed]
- Ciebiera M, W?odarczyk M, Wrzosek M, M?czekalski B, Nowicka G, ?ukaszuk K, et al. Role of transforming growth factor β in uterine fibroid biology. Int J Mol Sci 2017;18(11):1-16.
[Crossref] [Google scholar] [PubMed]
- Shen Q, Zou S, Sheng B, Zhao M, Sun LZ, Zhu X. Mifepristone inhibits IGF-1 signaling pathway in the treatment of uterine leiomyomas. Drug Des Devel Ther 2019;13:3161-70.
[Crossref] [Google scholar] [PubMed]
 Research group, *p<0.05 and ** p<0.01
Research group, *p<0.05 and ** p<0.01