- *Corresponding Author:
- M. Guo
Department of Rehabilitation, Binzhou Medical University Hospital, Binzhou, Shandong 256603, China
E-mail: guomengkf@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “31-37” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the analgesic effect of electro-acupuncture on neuropathic pain model rats and to clarify the mechanism of electro-acupuncture analgesia from the perspective of vesicular glutamate transporter 2/toll like receptor 4 signal pathway. A total of 30 specific pathogen free grade adult male Sprague-Dawley rats were selected. The rats were randomly divided into model group (chronic constriction injury), sham operation group, electro-acupuncture group, non-electro-acupuncture group (sham electro-acupuncture), Chicago sky blue 6B group and lipopolysaccharide group, with 5 rats in each group. The neuropathic pain model of chronic constriction injury of sciatic nerve was established. The “Huantiao” and “Yanglingquan” points were selected for electroacupuncture stimulation for 14 d. The mechanical foot contraction threshold, thermal pain, vesicular glutamate transporter 2/toll like receptor 4 signal pathway protein levels and inflammatory factor level of rats in each group were compared and analyzed. Compared with the sham operation group, the mechanical pain and thermal pain thresholds of the model group decreased significantly (p<0.01), the unilateral levels of vesicular glutamate transporter 2 and toll like receptor 4 and the release of interalukin-6 in the spinal cord increased significantly (p<0.01), while interalukin-10 decreased significantly (p<0.01); compared with the model group, electroacupuncture and intrathecal injection of Chicago sky blue 6B could significantly increase the pain threshold of neuropathic pain rats (p<0.01), reduce the release of interalukin-6 and the level of vesicular glutamate transporter 2 protein in the spinal cord and increase the level of interlukin-10; intrathecal injection of lipopolysaccharide decreased the pain threshold in rats (p<0.01). Electro-acupuncture can alleviate the pain sensitivity of neuropathic pain rats and its occurrence and development is closely related to the activation of vesicular glutamate transporter 2/toll like receptor 4 signal pathway in the spinal dorsal horn.
Keywords
Neuropathic pain, electroacupuncture, type 2 vesicular glutamate transporter, toll like receptor 4
Neuropathic pain, as a chronic disease that puzzles patients all over the world, is mainly caused by peripheral and central nervous system diseases. Numerous studies have shown that patients with neuropathic pain are accompanied by a variety of complications, the most common of which are anxiety, depression and sleep disorders. These diseases have a complex two-way relationship, which has a significant impact on patient’s daily activities and health status[1,2]. It is reported that the quality of life of patients with neuropathic pain is significantly lower than that of the general population and those without neuropathic pain[3], which increases the burden and health care costs[4] and causes social problems[5,6]. At present, ther is no effective method to treat neuropathic pain in clinic. Electro-Acupuncture (EA) in the treatment of neuropathic pain has the characteristics of less adverse reactions and significant efficacy, but its mechanism needs to be further clarified. Glutamate is one of the most important neurotransmitters that mediate pain transmission and pain information regulation. Vesicular Glutamate Transporter 2 (VGLUT2) is located in spinal cord neurons and plays an important role in mediating the release of glutamate[7]. Toll Like Receptor 4 (TLR4) is a key receptor in inflammatory and immune responses and is specifically expressed in microglia, indicating that it may be involved in the occurrence and maintenance of neuropathic pain[8]. Therefore, the purpose of this study is to explore the analgesic effect of EA on neuropathic pain model rats and to clarify the mechanism of EA analgesia from the perspective of VGLUT2/TLR4 signal pathway, so as to provide some basic research evidence for clinical EA treatment of neuropathic pain.
Materials and Methods
Experimental animal:
In this study, 30 adult male Sprague-Dawley (SD) rats of Specific Pathogen Free (SPF) grade, weighing about 180~220 g, were provided by Shanghai Zhanhui Biotechnology Co., Ltd. after 1 w of adaptive feeding in separate cages in the experimental animal center of our hospital, the rats were weighed and numbered. All experiments were conducted in accordance with the provisions of the international guidelines for animal protection and use.
Main reagents and instruments:
Reagent: Chloral hydrate, paraformaldehyde (National Pharmaceutical reagent); penicillin (Hangzhou Jinuo); VGLUT2 antibody, TLR4 antibody, VGLUT inhibitor Chicago Sky Blue 6B (CSB6B) and TLR4 activator Lipopolysaccharide (LPS) (Abcam); TRIzol™ reagent (Invitrogen); Polymerase Chain Reaction (PCR) reverse transcription kit, fluorescent quantitative PCR kit (Takara).
Instrument: Fluorescence quantitative PCR instrument 96 lightcycler (Roche); ultra-low temperature refrigerator (Sanyo); frozen microtome, laser confocal microscope (Leica); high speed cryogenic centrifuge (Eppendorf). Use 4-0 chrome catgut (Shanghai PudongJinhuan medical supplies Co., Ltd.). Huatuo brand disposable acupuncture needle (0.28 mm×15 mm), EA therapeutic apparatus (Suzhou Medical Supplies Factory Co., Ltd.).
Preparation and grouping of rat model of neuropathic pain:
The rats were randomly divided into model group (Chronic Restriction Injury (CCI)), sham operation group, EA, non-EA group (sham EA), CSB6B group and LPS group, with 5 rats in each group. In the model group, the neuropathic pain model of CCI of sciatic nerve was established; in sham operation group, only skin was cut without ligation of sciatic nerve; in the EA group, after 7 d of successful modeling (P7), EA was performed at Huantiao and Yanglingquan points, once a day, and continued to intervene until P14; the rats in the non EA group were only needled at the above acupoints without switching on the EA therapeutic apparatus; in CSB6B group, CSB6B (dissolved in 8 % Dimethyl Sulfoxide (DMSO) and injected at 2 mg/ kg) was injected into the spinal cord sheath of P7, P10 and P14 respectively; in LPS group, LPS (dissolved in 8 % DMSO, 0.1 mg/kg) was injected into the spinal cord sheath of P7, P10 and P14 once respectively. The preparation process of CCI neuropathic pain model of sciatic nerve is as follows, after intraperitoneal injection of 10 % chloral hydrate anesthesia (4 ml/kg), the skin of the right hind limb was cut, the muscle was passively separated and the sciatic nerve was exposed. The middle segment of the sciatic nerve was loosened and bound with 4-0 chromium catgut for four times, resulting in a chronic CCI neuropathic pain rat model, after operation, a proper amount of penicillin powder was sprinkled on the wound to prevent infection. After the model was established, the behavioral changes of mechanical pain and thermal pain were observed on the 7th (P7) and 14th (P14) d after operation.
Western blot analysis:
After 21 d, the left spinal cord (L4~L6) of rats was taken and frozen at -80°. The spinal cord was lysed with Radio Immunoprecipitation Assay (RIPA) lysate. The protein concentration was determined by Bicinchoninic Acid (BCA) assay method. Each sample was quantified as 30 μg. Then add Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) protein loading buffer, mix well, boil the protein for 5 min and denature the protein. The denatured protein samples were separated by 10 % SDS-PAGE gel electrophoresis under the conditions of 40 v~100 v and the constant flow membrane was carried out on the Polyvinylidene Difluoride (PVDF) membrane. After the membrane transfer, 5 % skimmed milk was sealed at room temperature for 1 h and the first antibody was incubated overnight at 4°. On the 2 d, Tris Buffered Saline with Tween (TBST) was washed for 3 times, each time for 10 min. The second antibody was incubated for 2 h at room temperature. TBST was washed for 3 times, each time for 5 min. Enhanced Chemiluminescence (ECL) chromogenic solution was configured at 1:50, exposed, observed the results and statistically analyzed the gray value.
Observation indicators:
Mechanical foot retraction behavior test put the rats into the von Frey filament pain meter, adapt to the environment for 15 min and start the measurement after the rat’s behavior state is completely stable. Use fibers with different bending forces to stimulate the skin in the middle of the rat’s paw and gradually exert force until the rats have reflexive foot contraction, shaking feet, jumping and other positive behaviors and then stop stimulating. The time interval between two consecutive stimuli shall not be <1 min and each stimulus shall last for about 5 s, instantly record the data displayed on the computer. Measure 3 times continuously and take the average value.
Thermal pain behavior test in a quiet environment, room temperature 20°±2°, put the rats into the glass lattice above the pain meter and move freely. After the rats were allowed to stand for 20 min, the Paw Withdraw Latency (PWL) of the rats was measured by irradiating the paw of the rats with the strong light of the 336 radiation calorimeter. The interval between each measurement shall not be <1 min. After the PWL of rats is basically stable, the average value of the last three measurement results shall be taken as the baseline. The heat source intensity was kept at a constant level during the measurement and the basic pain threshold was controlled at about 8~12 s to prevent thermal radiation scale in rats, the cut-off time of PWL was set as 20 s.
Detection of signal pathway related protein expression levels of VGLUT2 and TLR4 in each group were detected by Western blot.
Detection of inflammatory factor level, after the end of all experiments (P14), fresh spinal cord (L4-L5 segment) dorsal horn was quickly taken and placed in liquid nitrogen, and finally stored at -80° for testing. The expression of mature protein Interleukin (IL)-6 and IL-10 in spinal cord was detected by Enzyme-Linked Immunoassay (ELISA).
Statistical analysis:
Statistical Package for Social Sciences (SPSS) 22.0 was used for analysis and all measurement data were expressed in x±s. Independent sample t-test was used for intra group data. In inter group data comparison, single factor multiple analyses of variance was used for protein test results and two factors multiple analyses of variance was used for behavioral test results. p<0.05 was considered as significant difference.
Results and Discussion
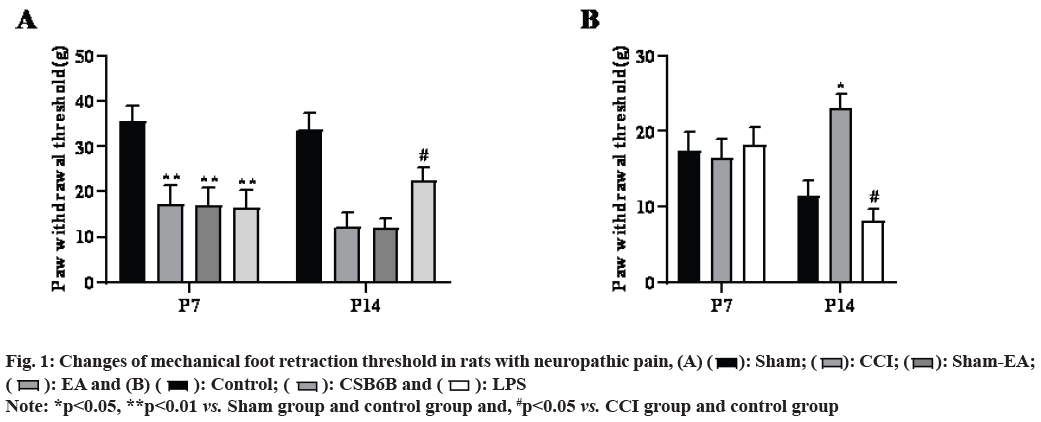
Compared with the sham operation group, the mechanical pain of model group (CCI) rats decreased significantly from the 7th and 14th d after modeling (p<0.01), compared with the model group, multiple EA can significantly improve the mechanical pain of CCI rats (p<0.05) and non EA does not change the pain behavior changes as shown in fig. 1A.
In the model group, multiple intrathecal injections of CSB6B significantly increased mechanical pain in rats (p<0.05), while multiple intrathecal injections of LPS further reduced mechanical pain in rats (p<0.05, fig. 1B).
Compared with the sham operation group, the thermal pain threshold of model group (CCI) rats decreased significantly from the 7th and 14th d after modeling (p<0.05), compared with the model group, multiple EA could significantly increase the thermal pain threshold of CCI rats (p<0.05) and non EA did not change the pain behavior changes as shown in fig. 2A.
Compared with the model group, multiple intrathecal injections of CSB6B significantly increased the thermal pain threshold of rats (p<0.05), while multiple intrathecal injections of LPS further reduced the thermal pain threshold of rats (p<0.05, fig. 2B).
On the 14th d after modeling, compared with the sham operation group, VGLUT2 and TLR4 proteins in the spinal dorsal horn of the model group increased significantly (p<0.05), compared with the model group, multiple EA could significantly reduce the VGLUT2 and TLR4 protein levels in the spinal dorsal horn (p<0.05) and intrathecal injection of CSB6B could significantly inhibit the VGLUT2 and TLR4 protein levels in the spinal dorsal horn (p<0.05). On the contrary, intrathecal injection of LPS further increased VGLUT2 and TLR4 protein levels in the spinal dorsal horn (p<0.05) as shown in fig. 3.
On the 14th d after modeling, the results of spinal cord ELISA showed that compared with the sham operation group, the expression of mature protein IL-6 in the spinal dorsal horn of the model group was significantly increased and IL-10 was significantly decreased (p<0.01); compared with the model group, multiple EA could significantly reduce the expression of IL-6 and increase the expression of IL-10 in the spinal dorsal horn (p<0.01); intrathecal injection of CSB6B significantly inhibited the expression of spinal mature protein IL-6 and increased the expression of IL- 10 (p<0.01); in contrast, intrathecal injection of LPS activator (Muramyl Dipeptide (MDP)) further increased the expression of IL-16 and decreased the expression of IL-10 in the spinal cord (p<0.01) as shown in fig. 4.
Nociceptive stimulation of peripheral nerves is transmitted to the spinal cord and uploaded to the higher brain center after the integration of the spinal dorsal horn, resulting in the generation of pain behavior[9]. Pain research has always been a hot issue in the international academic community, in which the survival burden caused by chronic pain on patients has attracted extensive attention of researchers[10]. As one of the chronic pain types, neuropathic pain has been suffering from serious adverse reactions. Therefore, it is urgent to find more effective treatment. In China, EA is an organic combination of acupuncture points and modern science and technology. It is a traditional means to treat nervous system diseases. The previous work of our research group found that EA can alleviate the mechanical pain sensitivity of Spared Nerve Injury (SNI) rats by interfering with the activation of dorsal root ganglion and spinal cord microglia, blocking the release of cytokines and the expression of some related pain signal pathway molecules[11,12]. Based on the fact that VGLUT2/TLR4 signal pathway participates in the occurrence and development of neuropathic pain, it suggests that the mechanism of EA analgesia may play a role by interfering with VGLUT2/TLR4 signal pathway, but the specific mechanism of EA analgesia needs to be further explored.
VGLUTs are a family of glutamate transporters that control the release of glutamate from presynaptic neurons in the spinal dorsal horn. VGLUT1-3 is the most common marker of excitatory glutamatergic neurons, which is involved in the development of acute and chronic pain, while the absence of VGLUT1 has no effect on neuropathic pain[13]. The role of VGLUT3 in regulating neuropathic pain is still controversial[14]. Studies have shown that the pain threshold of VGLUT3 knockout mice is not significantly different from that of normal mice[15]. Previous studies have shown that VGLUT2 plays a key role in the transmission and expression of somatic pain information[16]. VGLUT2 is selectively expressed in glutamatergic neurons in the spinal cord. It is located at the axonal end and mediates the storage, release and reuptake of glutamate into the synaptic vesicles of pain neurons[17]. Therefore, VGLUT2 plays an ultimate role in maintaining extracellular glutamate concentration, glutamate receptor activation, neuronal plasticity and central sensitization. In the neuropathic pain induced by spinal nerve ligation, the expression level of VGLUT2 increased and the release of glutamate increased, leading to mechanical pain[18]. Meanwhile, VGLUT2 deficient mice show decreased mechanical pain after peripheral nerve injury or inflammation[19].
In this study, the L5 spinal nerve ligation method was used to prepare the rat model of neuropathic pain. The principle is that the nerve injury induced by spinal nerve ligation up regulates the expression level of VGLUT2, which increases the discharge activity of glutamatergic neurons and leads to mechanical pain. However, the effect on thermal pain perception in rats is relatively small, the possible reason is that the mechanical and thermal hypersensitivity induced by peripheral nerve injury mainly depends on different VGLUT2 primary afferent pathways[20]. Although more and more studies have proved the importance of glutamate transporter represented by VGLUT2 in the process of pain information transmission, whether the dysfunction of VGLUT2 related signal pathway is its mechanism in the occurrence and development of neuropathic pain remains to be further determined.
TLR4 is a member of the TLR family and is mainly expressed by microglia. It plays an important role in pathogen recognition and innate immune activation. It is a key regulator for activating the innate immune system.
The activation of TLR4 releases many neurotransmitters and regulators that can enhance neuronal excitability[21]. A large amount of evidence shows that TLR4 and its signal pathway play a key role in regulating inflammatory response and adaptive immune response, and are clearly involved in the aggravation and persistence of neuropathic pain[22]. Studies have shown that 39 TLR4 inhibitors can effectively treat neuropathic pain through molecular docking virtual screening[23]. Previous studies have confirmed that massage and acupuncture in traditional Chinese medicine can inhibit TLR4 pathway by activating the body’s immune function, resulting in the reduction of cytokine release, thus leading to the inhibition of hyperalgesia[24]. Therefore, in-depth study of the effect of EA on TLR4 expression may be of great significance to further clarify the activation of the immune system.
Studies have shown that in the occurrence and development of neuropathic pain, activation of spinal glial cells can release pro-inflammatory factors (IL-1 beta (β), IL-6, etc.) to maintain central sensitization and neuroinflammatory response in the spinal cord. In the spinal cord of neuropathic pain rats, activated VGLUT2 and TLR4 were mainly expressed in the superficial astrocytes of the spinal dorsal horn[25]. When stimulated by external stimuli, activated VGLUT2 can cause microglia activation, promote the expression of a variety of inflammatory factors and inhibit this pathway can effectively prevent glial cells from changing to M1 state[26]. In addition, TLR4 can also induce Janus Kinase 2 (JAK2)/Signal Transducer and Activator of Transcription 3 (STAT3) signal transduction in microglia during neuropathic pain[27]. Activated microglia can release inflammatory mediators such as Tumor Necrosis Factor alpha (TNF-α), IL-6 and IL-10, reduce cytokines such as IL-10 that protect neurons, maintain central sensitization and neuroinflammation, and show mechanical and thermal pain anaphylaxis. In this experiment, EA or VGLUT2 inhibitor can reduce the expression level of IL-6 and increase the expression level of IL-10 in spinal cord, and increase the pain threshold of neuropathic pain rats, so as to play an anti-inflammatory and analgesic role. However, intrathecal injection of classical TLR4 activator (LPS) can significantly activate VGLUT2/TLR4 signaling pathway, promote the release of spinal cord and IL-6, inhibit the expression of IL-10 and further induce pain sensitivity.
In conclusion, the occurrence and development of neuropathic pain are closely related to the activation of VGLUT2/TLR4 signal pathway in the spinal dorsal horn. The anti-inflammatory and analgesic mechanism of EA may be related to the inhibition of the activation of VGLUT2/TLR4 pathway in the spinal cord. In the future, VGLUT2, TLR4 and other gene knockout mice can be used to carry out in-depth experiments to further clarify the regulatory mechanism of EA antiinflammatory and analgesic.
Acknowledgement:
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2015PH004).
Conflict of interests:
The authors declared no conflict of interest.
References
- Nakagawa Y, Chiba K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Ther 2015;154:21-35.
- Guo JR, Wang H, Jin XJ, Jia DL, Zhou X, Tao Q. Effect and mechanism of inhibition of PI3K/Akt/mTOR signal pathway on chronic neuropathic pain and spinal microglia in a rat model of chronic constriction injury. Oncotarget 2017;8(32):52923-34.
[Crossref] [Google Scholar] [Pub Med]
- Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture–electroacupuncture on persistent pain. Anesthesiology 2014;120(2):482-503.
[Crossref] [Google Scholar] [Pub Med]
- Wu Q, Yue J, Lin L, Yu X, Zhou Y, Ying X, et al. Electroacupuncture may alleviate neuropathic pain via suppressing P2X7R expression. Mol Pain 2021;17:1744806921997654.
[Crossref] [Google Scholar] [Pub Med]
- Ju ZY, Wang K, Cui HS, Yao Y, Liu SM, Zhou J, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev 2017;12(12):CD012057.
[Crossref] [Google Scholar] [Pub Med]
- Zhang XH, Feng CC, Pei LJ, Zhang YN, Chen L, Wei XQ, et al. Electroacupuncture attenuates neuropathic pain and comorbid negative behavior: The involvement of the dopamine system in the amygdala. Front Neurosci 2021;15:657507.
[Crossref] [Google Scholar] [Pub Med]
- Cho YS, Ko HG, Han HM, Park SK, Moozhayil SJ, Choi SY, et al. Vesicular glutamate transporter-immunopositive axons that coexpress neuropeptides in the rat and human dental pulp.IntEndod J 2021;54(3):377-87.
[Crossref] [Google Scholar] [Pub Med]
- Gao YH, Wang JY, Han YJ, Liu JL. Spinal cord Toll like receptor 4 and its co-stimulatory molecule heat shock protein 90 mayparti-cipate in electroacupuncture analgesia in rats with chronic neuropathic pain. Zhen Ci Yan Jiu 2021;46(9):735-41.
[Crossref] [Google Scholar] [Pub Med]
- Zhang S, Tang H, Zhou J, Gu Y. Electroacupuncture attenuates neuropathic pain after brachial plexus injury. Neural Regen Res 2014;9(14):1365-70.
[Crossref] [Google Scholar] [Pub Med]
- Zhang H, Sun J, Xin X, Huo Z, Li D. Contralateral electroacupuncture relieves chronic neuropathic pain in rats with spared nerve injury. Medical Sci Monit 2018;24:2970-4.
[Crossref] [Google Scholar] [Pub Med]
- Kim W, Kim SK, Min BI. Mechanisms of electroacupuncture-induced analgesia on neuropathic pain in animal model. Evid Based Complement Alternat Med 2013;2013:436913.
[Crossref] [Google Scholar] [Pub Med]
- Chen SP, Kan Y, Zhang JL, Wang JY, Gao YH, Qiao LN, et al. Involvement of hippocampal acetylcholinergic receptors in electroacupuncture analgesia in neuropathic pain rats. Behav Brain Funct 2016;12(1):1-3.
[Crossref] [Google Scholar] [Pub Med]
- Leo S, Moechars D, Callaerts-Vegh Z, D’Hooge R, Meert T. Impairment of VGLUT2 but not VGLUT1 signaling reduces neuropathy-induced hypersensitivity. Eur J Pain 2009;13(10):1008-17.
[Crossref] [Google Scholar] [Pub Med]
- Draxler P, Honsek SD, Forsthuber L, Hadschieff V, Sandkühler J. VGluT3+ primary afferents play distinct roles in mechanical and cold hypersensitivity depending on pain etiology. J Neurosci 2014;34(36):12015-28.
[Crossref] [Google Scholar] [Pub Med]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 2009;462(7273):651-5.
[Crossref] [Google Scholar] [Pub Med]
- Liu Y, Samad OA, Zhang L, Duan B, Tong Q, Lopes C, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 2010;68(3):543-56.
[Crossref] [Google Scholar] [Pub Med]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci 2006;26(46):12055-66.
[Crossref] [Google Scholar] [Pub Med]
- Malet M, Brumovsky PR. VGLUTs and glutamate synthesis—focus on DRG neurons and pain. Biomolecules 2015;5(4):3416-37.
[Crossref] [Google Scholar] [Pub Med]
- Wang ZT, Yu G, Wang HS, Yi SP, Su RB, Gong ZH. Changes in VGLUT2 expression and function in pain-related supraspinal regions correlate with the pathogenesis of neuropathic pain in a mouse spared nerve injury model. Brain Res 2015;1624:515-24.
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, et al. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci USA 2010;107(51):22296-301.
[Crossref] [Google Scholar] [Pub Med]
- Li Q. Antagonists of toll like receptor 4 maybe a new strategy to counteract opioid-induced hyperalgesia and opioid tolerance. Med Hypotheses 2012;79(6):754-6.
[Crossref] [Google Scholar] [Pub Med]
- P?óciennikowska A, Hromada-Judycka A, Borz?cka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 2015;72(3):557-81.
[Crossref] [Google Scholar] [Pub Med]
- Zali H, Golchin A, Farahani M, Yazdani M, Ranjbar MM, Dabbagh A. FDA approved drugs repurposing of toll-like receptor 4 (TLR4) candidate for neuropathy. Iran J Pharm Res 2019;18(3):1639-47.
[Crossref] [Google Scholar] [Pub Med]
- Wang LT, Wang SJ, Hsu SH. Functional characterization of mammalian Wntless homolog in mammalian system. Kaohsiung J Med Sci 2012;28(7):355-61.
[Crossref] [Google Scholar] [Pub Med]
- Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, et al. Mu opioid receptor activation modulates toll like receptor 4 in murine macrophages. Brain Behav Immun 2012;26(3):480-8.
[Crossref] [Google Scholar] [Pub Med]
- Lee JW, Nam H, Kim LE, Jeon Y, Min H, Ha S, et al. TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia. Autophagy 2019;15(5):753-70.
[Crossref] [Google Scholar] [Pub Med]
- Vichaya EG, Ford BG, Quave CB, Rishi MR, Grossberg AJ, Dantzer R. Toll-like receptor 4 mediates the development of fatigue in the murine Lewis lung carcinoma model independently of activation of macrophages and microglia. Psychoneuroendocrinology 2020;122:104874.
[Crossref] [Google Scholar] [Pub Med]
 Sham-EA;
Sham-EA;  : EA and (B)
: EA and (B) 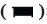 : Control;
: Control;  : CSB6B and
: CSB6B and  : LPS
: LPS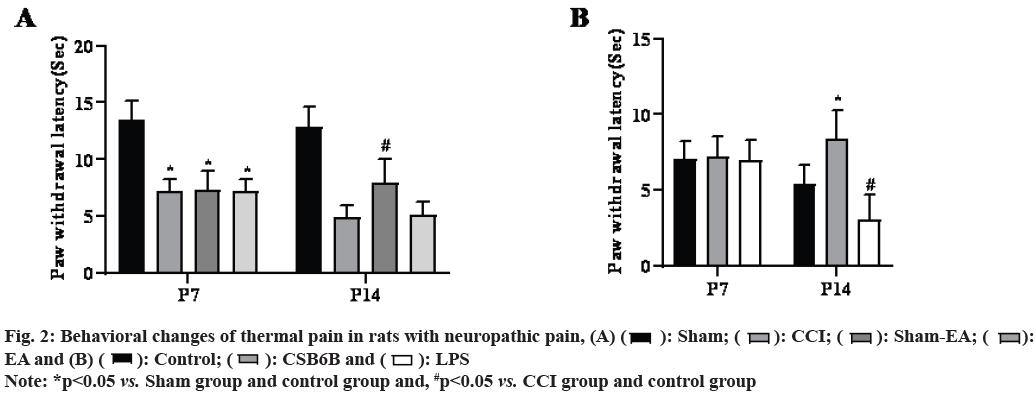
 : Sham;
: Sham;  : CCI;
: CCI;  : Sham-EA;
: Sham-EA;  : EA and (B)
: EA and (B)  : Control;
: Control;  : CSB6B and
: CSB6B and  : LPS
: LPS
 : Sham;
: Sham;  : CCI;
: CCI;  : EA;
: EA;  : CSB6B and
: CSB6B and  : LPS
: LPS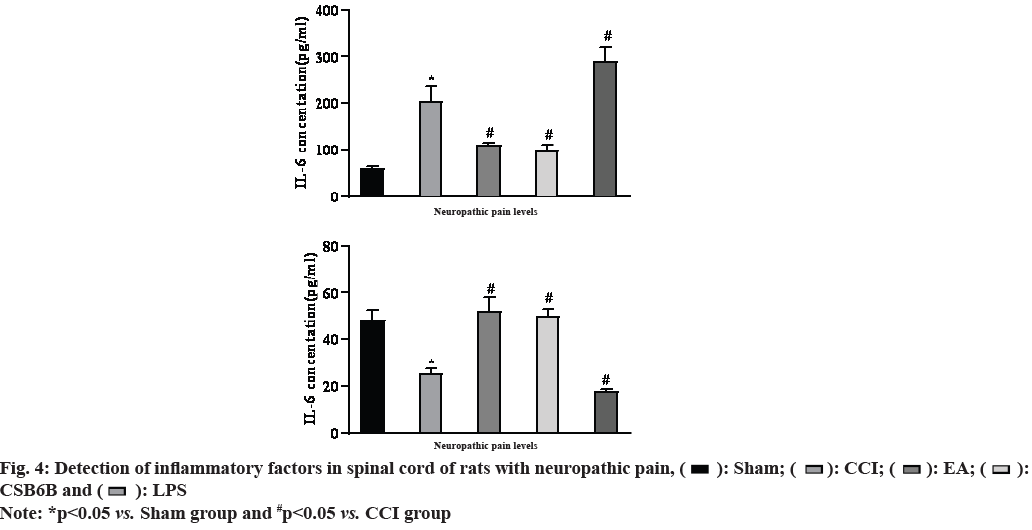
 : Sham;
: Sham;  : CCI;
: CCI;  : EA;
: EA;  : CSB6B and
: CSB6B and  : LPS
: LPS



