- *Corresponding Author:
- D. Selvakumar
Department of pharmaceutical chemistry, SB college of pharmacy, Anaikuttam, Sivakasi - 626 130, India
E-mail: deselva123@rediffmail.com
| Date of Submission | 10 April 2007 |
| Date of Revision | 8 March 2007 |
| Date of Acceptance | 10 August 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 586-589 |
Abstract
4,6-Diaryl substituted-4,5-dihydro-2-amino pyrimidines have been synthesized by the reaction of chalcones with guanidine nitrate. Their chemical structures have been confirmed by means of IR and NMR data and by elemental analysis. The compounds were screened for antibacterial activity against 16 gram-positive bacterial strains in minimum inhibitory concentration. The minimum inhibitory concentration of the compound II has found to be better than the reference standards taken for the antibacterial screening. Sulphamethoxazole and trimethoprim were taken as reference standards.
Keywords
Dihydropyrimidines, Antimicrobial, Chalcones, Minimum inhibitory concentration
Emergence of resistant bacterial strains towards the existing antibacterial agents is one of the major reasons for the search and development of newer molecules to defend them. Not many drugs with pyrimidine nucleus are available in current antibacterial chemotherapy. In accordance with the availability of the earlier drugs having pyrimidine nucleus like trimethoprim and pyrimethamine for the chemotherapy of bacterial diseases, we aimed to synthesize the molecules having pyrimidine nucleus with more potency.
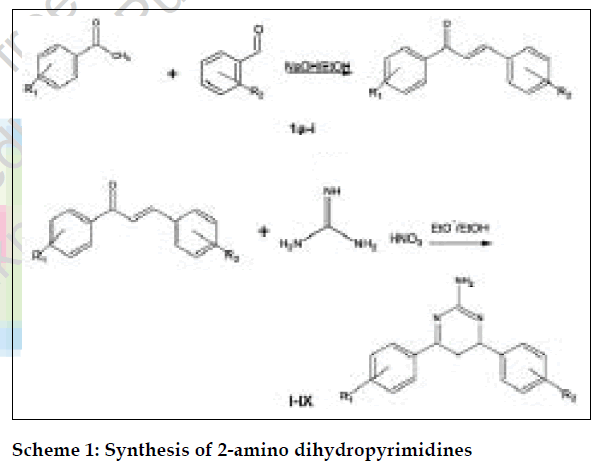
In our continuous search for newer antibacterial agents, we have synthesized a series of compounds with dihydropyrimidine nucleus and evaluated for antibacterial activity against gram-positive strains. The chalcones [1-6] were prepared by Claisen-Schmidt condensation and then treated with guanidine [7,8] to get 2-aminodihydropyrimidines (Scheme 1). The amino group was then converted in to respective ureas by treating the 2-aminopyrimidines with sodium cyanate [9,10].
The preparation of 4,6-diaryl substituted 4,5-dihydro-2- aminopyrimidines was initiated by treating the chalcones and substituted chalcones, which have been prepared earlier by treating the corresponding aromatic ketones with corresponding aromatic aldehydes in the presence of sodium hydroxide, with guanidine [10]. The synthesized 4,6-diarylsubstituted-4,5-dihydro-2-aminopyrimidines exhibited characteristic primary amino band at 3400- 3300 cm-1 .The stretching peak of C=N appeared at 1600-1510 cm-1. The absorption band at 855-800 cm-1 was characteristic of aromatic C-H bending vibrations. The 1H-NMR spectrum also revealed that the amine proton attached to the phenyl ring at δ 8.30, singlet (2H) disappeared in D2O exchange and the aromatic protons appeared between δ 6.90-7.90 as a multiplet in all synthesized compounds.
Melting points are uncorrected. All the compounds were subjected to elemental analysis (C, H, and N) and the measured values agreed within ±0.4% with the calculated ones. The purity of the compounds was confirmed by thin layer chromatography (TLC, Silica gel; solvent system; benzene:chloroform, 9:1, 7:3). The spots were developed in TLC chamber and visualized with an ultraviolet lamp.1H-NMR spectra were recorded on a Jeol FX 90Q Fourier transform spectrometer. The IR spectra were recorded on a Jasco infrared spectrophotometer.
To a 20 ml solution of 20% sodium hydroxide in distilled water, acetophenone (0.01mol) was added and dissolved. To this add benzaldehyde (0.01 mol) in small amounts with continuous stirring with the help of a mechanical stirrer for about 2 h. The mixture was left in the refrigerator for 24 h. Then the yellowish product thus formed is filtered, dried and recrystallized from ethanol. (mp-54º, yield-65%).
To the freshly prepared solution of sodium ethoxide (0.02 mol sodium in 50 ml ethanol), chalcones (0.01 mol) and guanidine nitrate (0.01 mol) were added. The mixture was refluxed for 3-6 h. The resulting mixture was then concentrated to half of its volume and poured over crushed ice. The solid thus separated was collected, washed with cold ethanol and dried.
The product was recrystallized from hot ethanol. The melting points and percentage yields are presented in Table 1. The physical data and spectral properties were given in Table 2.
| Compound | Molecular formula | Elementalanalysis | Rf value | Solubility | |||||
|---|---|---|---|---|---|---|---|---|---|
| MW | %C | %H | %N | %CI | %O | ||||
| I | C16 H15 N3 | 249.317 | 77.08 | 6.06 | 16.85 | - | 0.67* | A1, C, D, E | |
| II | C16H14N3Cl | 283.762 | 67.72 | 4.97 | 14.81 | 12.49 | - | 0.59* | A1, C, D, E |
| III | C16H14N3Cl | 283.762 | 67.72 | 4.97 | 14.81 | 12.49 | - | 0.70* | A1, C, D, E |
| IV | C16 H15 N3O | 265.316 | 72.43 | 5.70 | 15.84 | 6.03 | 0.60** | A, B, C1, D, E | |
| V | C16H14N3OCl | 299.751 | 64.11 | 4.71 | 14.02 | 11.83 | 5.34 | 0.50** | A, B, C1, D, E |
| VI | C16H14N3OCl | 299.751 | 64.11 | 4.71 | 14.02 | 11.83 | 5.34 | 0.47** | A, B, C1, D, E |
| VII | C16H14N4O2 | 294.314 | 65.30 | 4.79 | 19.04 | - | 10.8 | 0.50* | C, D, E |
| VIII | C16H13N4O2Cl | 328.759 | 58.45 | 3.99 | 10.78 | 17.04 | 9.73 | 0.40* | C, D, E |
| IX | C16H13N4O2Cl | 328.759 | 58.45 | 3.99 | 10.78 | 17.04 | 9.73 | 0.40* | C, D, E |
The solvent systems for TLC are *benzene:chloroform (9:1), **benzene:chloroform (7:3). The solubilities of the synthesized compounds in different organic solvents A-ethanol; B-methanol; C-chloroform; D-DMSO; E-DMF; 1-slightly soluble
Table 1: Physical data of the synthesized compounds
| Comp | R | R1 | R2 | Yield (%) | M.P. (°C) | IR (cm-1) | 1H NMR (DMSO-d6) δ (ppm) |
|---|---|---|---|---|---|---|---|
| I | H | H | H | 44 | 63 | 3300(N-H), 1560(C=N) 830(aromatic C-H bending) | 7.0-7.3(m, 5H, Ar-H); 8.30(s, 2H, -NH2, D2O exchangeable), |
| II | H | H | 2-Cl | 49 | 67 | 3310(N-H), 1520(C=N) 820(aromatic C-H bending) | 7.4-7.7(m, 5H, Ar-H); 8.25(s, 2H, -NH2, D2O exchangeable), |
| III | H | H | 4-Cl | 51 | 75 | 3300(N-H), 1540(C=N) 830(aromatic C-H bending) | 6.9-7.2(m, 5H, Ar-H); 8.10(s, 2H, -NH2, D2O exchangeable), |
| IV | H | 4-OH | H | 48 | 158 | 3400(N-H), 1560(C=N) 830(aromatic C-H bending) | 7.3-7.5(m, 5H, Ar-H); 8.30(s, 2H, -NH2, D2O exchangeable), |
| V | H | 4-OH | 2-Cl | 52 | 164 | 3400(N-H), 1600(C=N) 825(aromatic C-H bending) | 7.0-7.2(m, 5H, Ar-H); 8.25(s, 2H, -NH2, D2O exchangeable), |
| VI | H | 4-OH | 4-Cl | 53 | 125 | 3360(N-H), 1560(C=N) 835(aromatic C-H bending) | 7.1-7.3(m, 5H, Ar-H); 8.8(s, 2H, -NH2, D2O exchangeable), |
| VII | H | 4-NO2 | H | 63 | 170 | 3380(N-H), 1540(C=N) 818(aromatic C-H bending) | 7.8-7.9(m, 5H, Ar-H); 8.6(s, 2H, -NH2, D2O exchangeable), |
| VIII | H | 4-NO2 | 2-Cl | 63 | 170 | 3320(N-H), 1580(C=N) 830(aromatic C-H bending) | 7.7-7.9(m, 5H, Ar-H); 8.20(s, 2H, -NH2, D2O exchangeable), |
| IX | H | 4-NO2 | 4-Cl | 67 | 159 | 3350(N-H), 1560(C=N) 850(aromatic C-H bending) | 7.0-7.2(m, 5H, Ar-H); 8.25(s, 2H, -NH2, D2O exchangeable), |
Table 2: Spectral properties of the synthesized compounds
All compounds were evaluated for antibacterial activity against 16 gram-positive bacterial strains. The bacterial strains were obtained from fresh cultures in the Department of microbiology, Institute of medical sciences, Banaras Hindu University. The agar dilution method (Barry, 1991) was performed using Mueller-Hinton agar medium. Suspensions of each microorganism were prepared to contain approximately 106 colony-forming units, cfu/ml. The test organisms were inoculated in plates with serially diluted compounds and incubated overnight in an incubator oven at 35º. The lowest concentration of a compound which inhibits the growth of the organism completely without visible growth was taken as minimum inhibitory concentration (MIC). The study was simultaneously performed for the reference standards trimethoprim and sulphamethoxazole.
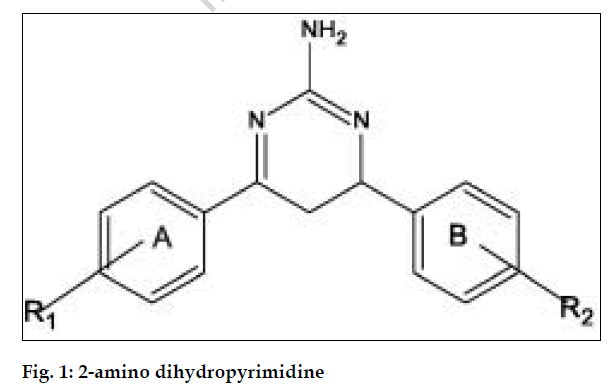
In the present study 4,6-diaryl substituted-4,5- dihydro-2-aminopyrimidines have been synthesized from chalcones condensed with guanidine nitrate. The chalcones were synthesized by the reaction of substituted acetophenones with substituted benzaldehydes in the presence of sodium hydroxide. All the compounds gave satisfactory elemental analysis (Table 2). The IR and 1H NMR spectra were consistent with the assigned structures. All the synthesized compounds were tested for in vitro antibacterial activity by agar dilution method. The MIC of the compounds against 16 gram-positive bacterial strains is presented in Table 3. Compounds I to IV showed very good activity against all bacterial strains than sulphamethoxazole and trimethoprim taken as reference standards. The compounds V to IX showed mild to moderate activity against all bacterial strains taken for the screening. When compared to the sulphamethoxazole and trimethoprim, the compound II exhibited very good activity against all tested bacterial strains. This compound in 5 μg/ml inhibits Salmonella paratyphi B, Vibrio cholerae 01 39, Vibrio cholerae 01, Bacillus subtilis, Shigella sonnei, Proteus rettgeri, Shigella boydii. The compounds with no ring A substitution exhibit better activity than those of substituted one. The compounds with no substitution in ring A, the antibacterial activity with respect to the substitution in ring B was in the order of 2-Cl>no substitution>4-Cl. The compound with nitro group substitution in para position exhibits reduced activity than their unsubstituted compounds (fig. 1).
The reference standards used were SM-sulphamethoxazole; TRIM-trimethoprim. (MIC’s in µg/ml)
Table 3: Antibacterial activity of the compounds
Acknowledgements
We express our sincere gratitude to the Department of Microbiology, Institute of Medical Sciences, BHU, for providing the microorganisms required for the investigation.
References
- Dimmock, J.R., Kandepu, N.M., Hetherington, M., Quail, J.W., Pugazhenthi, U., Sudom, A.M., Chamankhah, M., Rose, P., Pass, E., Allen, T.M., Halleran, S., Szydlowdki, J., Mutus, B., Tannous, M., Manavathu, E.K., Myers, T.G., De Clerq, E. and Balzarini, J., J. Med. Chem., 1998, 41, 1014.
- Li, R., Kenyon, G.L., Cohen, F.E, Chen, X, Gong, B., Dominguez J.N., Davidson, E., Kurzban, G., Miller R.E. and Nuzum E.O., J. Med. Chem., 1995, 38, 5031. Back to cited text no. 2
- Anto, R.J., Girija, K., Ramadasan, K., Satyanarayana, K. and Rao, M.N.A., J. Clin. Biochem. Nutr., 1994, 17, 73.
- De Vincenzo, R., Scambia, G., BenidettiPanici, P., Ranalletti, F.O., Bonanno, G., Ercoli, A., DelleMonache, F., Ferrari, F., Piantelli, M. and Mancuso, S., Anticancer drug Des., 1995, 10, 481.
- Anto, R.J., Girija, K., Rao, M.N.A., Subbaraju, V. and Ramadasan, K., Cancer Lett., 1995, 97, 33.
- Bullesteros J.F., Sanz M.J., Ubeda A., Miranda, M.A. and Alcaraz, M.A. J. Med. Chem., 1995, 38, 2794.
- Mishra, A. and Rajan, K.B. Indian J. Heterocyl. Chem., 1996, 6, 1.
- Jain, A.C. and Sinha, S.P., Indian J. Chem., Sec.B, 1994, 33,317.
- Jain, A.C., Singh, P.K. and Bhojak, N., Indian J. Chem., Sec.B, 1994, 33,372.
- Norman, R.O.C., The Principles of Organic Synthesis, II Edition, Chapman and Hill Pubs, 1978, 337.