- *Corresponding Author:
- E. M. Jessy
Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, Manipal, Karnataka - 576 104, India
E-mail: jessyjamesmathew@yahoo.co.in
| Date of Submission | 17 January 2006 |
| Date of Revision | 6 February 2007 |
| Date of Acceptance | 22 June 2007 |
| Indian J Pharm Sci 2007, 69 (3): 476-478 |
Abstract
In view of the potent antimicrobial and antiinflammatory activities exhibited by quinazolin-4-(3H)-ones, a series of novel 2, 3-disubstituted-3,1-quinazolin-4-(3H) ones have been synthesized. The synthesized compounds were screened for antibacterial and antiinflammatory activities. Compounds 3a and 3l showed significant antiinflammatory activity comparable to ibuprofen, while the compound 3J showed antibacterial activity comparable to ciprofloxacin.
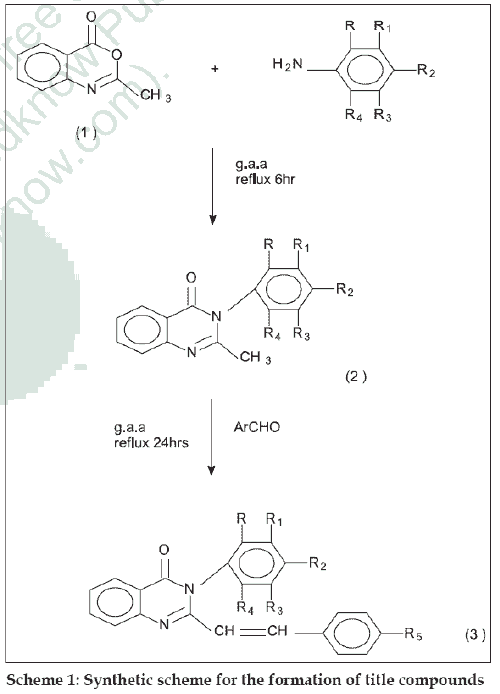
Substituted quinazolin-4-(3H)-ones exhibit a wide range of activities such as antibacterial [1], antiviral [2], analgesic, antiinflammatory [3], antihistamine [4], antihypertensive [5], antiparkinsonian [6], antitubercular [7] and anticancer8. In quinazolin-4-(3H)-one moiety, the presence of an aryl group at position three enhances antibacterial and tuberculostatic activities. It has also been observed that the incorporation of styryl moiety at position two in quinazolone imparts prominent chemotherapeutic activities. In view of these observations it was thought of interest to synthesize a new series of 2,3-disubstituted quinazolin- 4-(3H)-ones and to evaluate its antibacterial and antiinflammatory activities. 3-substituted quinazolin- 4-(3H)-ones were prepared by the condensation of 2-methyl benzoxazin-4-one with various aryl amines. These compounds were treated with various aromatic aldehydes in the presence of glacial acetic acid to get different 2,3-disubstituted quinazolin-4-(3H)-ones. The synthetic scheme leading to the formation of targeted compounds is depicted in scheme 1.
The melting points of the compounds were determined on a Toshniwal electric melting point apparatus and the values were uncorrected. IR spectra of the compounds were recorded on Shimadzu-FTIR 8300 using KBr disc method. 1H NMR spectra were recorded on Joel-GSX 400, IIT Chennai using DMSO as solvent. Mass spectra were recorded on Shimadzu- GCMS 50508.All the chemicals used were of LR and AR grade and were procured from S. D. Fine Chem. Ltd., Mumbai and E. Merck, Delhi.
The starting material 2-methyl benzoxazin-4-one was synthesized from anthranilic acid [9]. For the synthesis of 2-methyl-3-aryl quinazolin-4-(3H)-ones, an equimolar (0.01 mol) mixture of 2-methyl-1,3-benzoxazin-4-one and aryl amine was refluxed for 6 h with glacial acetic acid. The mixture was cooled to room temperature and poured into crushed ice; the solid thus obtained was recrystallized from ethanol. IR (KBr) cm-1: 1660 (C=O str), 1469 (C-N str), 788(C-Cl str).
The general procedure used for 2,3-disubstituted quinazolin-4-(3H)ones (3a-3l) is as follows. A mixture of appropriate quinazolone (0.1 mol) and various aromatic aldehydes (0.1 mol) in 50 ml glacial acetic acid was stirred and refluxed for 24 h. The reaction mixture was cooled and poured into ice cold water, filtered and dried to get the final product, which was recrystallized from ethanol. (Table 1) Compounds (3a-3l) IR (KBr) cm-1: 3678 (O-H str phenolic), 3075 (Olefinic CH=CH str), 2603 (O-H str in acid group), 1697 (C=O str), 1598-1571 (Several bands, Aromatic C=C str), 1352 (N-O str), 975 (Olefinic CH=CH str), 788 (C-Cl str). 1H NMR (DMSO) δ ppm: 8.7 to 7(11H, Ar-H), 6.51 (1H, Olefinic-H, Ph-CH=C-), 5.1 (1H, Olefinic-H, quinazolinnoyl-CH=C-), 11.4 (1H, Carboxylic acid group) 3.4 (3H, methyl group of –OCH3). Mass m/z value for compound 3b: M+ is 432, fragmented ions at 172, 156.5, 122; compound 3g: M+ is 420 fragmented ions at 386, 300.5, 266, 172.0.
| Compd code | R | R1 | R2 | R3 | R4 | R5 | Mol. formula | Mp (°C) | Rf* value | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | COOH | H | H | Cl | H | NO2 | C23H14N3O5Cl | 190 | 0.57 | 70 |
| 3b | COOH | H | H | Cl | H | OCH3 | C24H17N2O4Cl | 167 | 0.71 | 68 |
| 3c | COOH | H | H | Cl | H | F | C23H14N2O3ClF | 188 | 0.67 | 67 |
| 3d | COOH | H | H | Cl | H | OH | C23H15N2O4Cl | 213 | 0.61 | 65 |
| 3e | H | COOH | H | H | Cl | NO2 | C23H14N3O5Cl | 260 | 0.73 | 66 |
| 3f | H | COOH | H | H | Cl | OCH3 | C24H17N2O4Cl | 240 | 0.41 | 60 |
| 3g | H | COOH | H | H | Cl | F | C23H14N2O3ClF | 261 | 0.72 | 62 |
| 3h | H | COOH | H | H | Cl | OH | C23H15N2O4Cl | 246 | 0.57 | 64 |
| 3i | COOH | H | Br | H | H | NO2 | C23H14N3O5Br | 196 | 0.68 | 58 |
| 3j | COOH | H | Br | H | H | OCH3 | C24H17N2O4Br | 136 | 0.68 | 62 |
| 3k | COOH | H | Br | H | H | F | C23H14N2O3BrF | 147 | 0.74 | 63 |
| 3l | COOH | H | Br | H | H | OH | C24H15N2O4Br | 130 | 0.6 | 67 |
Table 1: Physical Data of 2,3-Disubstituted Quinazolin-4(3h)-Ones
Antiinflammatory activity was measured using the carrageenan-induced paw oedema test in rats [10]. Animals were divided into different group each consisting of six animals. Out of twelve synthesized compounds, six compounds (3a, 3c, 3f, 3g, 3i, 3l) were selected as test compounds and standard ibuprofen were administered orally at a dose of 200 mg/kg as an aqueous suspension in 1% CMC, while the control group was fed with the same volume of 1% CMC suspension. The paw volume were measured using Plethysmograph immediately before and 3 h after the carrageenan injection. The percent inhibition of paw volume was calculated by using the formula, Percent inhibition= (1-Vt/Vc)×100, where Vt is the mean volume of the test drug, Vc is the mean volume of the control (Table 2). The one-way ANOVA (Scheffe’s method) test was applied, and the test compounds were found to be significantly active compared to the control (P<0.05). The institutional animal ethics committee of kasthurba medical college has approved the experimental protocol (No. IACE/ KMC/023/2004-05).
| Compound code | Dose mg/kg | Mean oedema volume (ml) | % Inhibition 3h |
|---|---|---|---|
| 3a | 200 | 0.165 ± 0.004a | 80.71 |
| 3c | 200 | 0.15 ± 0.006a | 66.6 |
| 3f | 200 | 0.156 ± 0.006a | 65.3 |
| 3g | 200 | 0.113 ± 0.003 | 74.76 |
| 3i | 200 | 0.168 ± 0.0033a | 62.62 |
| 3l | 200 | 0.103 ± 0.0004 | 77 |
| Control | - | 0.45 ± 0.181 | - |
Table 2: Antiinflammatory Activities of The Test Compounds
All the compounds synthesized (3a-3l) were screened for antibacterial activity using the cup-plate agar diffusion method [11] by measuring the zone of inhibition in mm. Ciprofloxacin (25 μg/ml) was used as standard for antibacterial activity. The compounds were screened for antibacterial activity against S. aureus, B. subtilis, P. aeruginosa, and E. coli in nutrient agar medium. This sterilized agar medium was poured in to Petri dishes and allowed to solidify. On the surface of the media microbial suspensions were spread with the help of sterilized triangular loop. A stainless steel cylinder of 8 mm diameter (pre-sterilized) was used to bore the cavities. All the synthesized compounds (25 μg/ml) were placed serially in the cavities with the help of micropipette and allowed to diffuse for 1 h. Dimethylformamide (DMF) was used as a solvent for all compounds and as control. The plates were incubated at 37o for 24 h. The zone of inhibition observed around the cups after incubation was measured. The results are presented in Table 3.
| Compound code | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E. coli | |
| 3a | - | 12 | 14 | - |
| 3b | - | - | - | - |
| 3c | - | - | 17 | - |
| 3d | - | - | 16 | - |
| 3e | - | 14 | 13 | - |
| 3f | - | - | - | 10 |
| 3g | - | 16 | - | - |
| 3h | - | - | - | - |
| 3i | - | 12 | - | - |
| 3j | 14 | 17 | - | 15 |
| 3k | - | - | - | - |
| 3l | - | - | - | - |
| Control | - | - | - | - |
| Standard | 23 | 22 | 20 | 23 |
Table 3: Antibacterial Activity of The Test Compounds
All the six compounds evaluated for antiinflammatory activity exhibited good activity ranging from 62.2 to 80.7% reduction in edema volume The compounds (3a-80.7%) and (3l-77%) showed significant antiinflammatory activity comparable to ibuprofen and the other compounds 3c, 3f, 3g, and 3i showed moderate activity. The test compounds were found to be significantly active when compared to control group.
The compound 3j showed good activity against S. aureus, B. subtilis and E. coli. Compounds 3a and 3e showed moderate activity against B. subtilis and P. aeruginosa and the compounds 3c and 3d have shown moderate activity against P. aeruginosa. Compounds 3b, 3h, 3k and 3l did not possess any encouraging activity.
Acknowledgements
The authors are thankful to Dr. Udupa, Principal, Manipal College of Pharmaceutical Sciences, Manipal, for providing necessary facilities to carry out the research. The authors are thankful to Dr. Vasanthkumar, H.O.D, Pharmacology, MAHE, Manipal and to Mr. J. Venkata Rao, for providing laboratory facilities to conduct Pharmacological and Microbiological activities. The authors are also thankful to IIT, Madras for providing NMR and mass spectral data.
References
- Srivastava, V.K., Singh, S., Gupti, A. and Shankar, K., Indian. J. Chem ., 1987, 26B, 652.
- Agnihotri, A.K. and Shukla, S.K., Arch. Pharm ., 1972, 315, 701.
- Alagarsamy, V., Pathak, U.S. and Goyal, R.K., Indian J. Pharm. Sci ., 2000, 62, 63.
- Lemura, R., Manabe, H. and Todayaki, S., Chem. Pharm. Bull ., 1989, 37, 2723.
- Vanhouttle, R.M., J. Cardiovasc. Pharmacol ., 1985, 7, 5105.
- Parmar, J.S. and Singh, S.P., J, Heterocyclic. Chem ., 1979, 16, 448.
- Joshi, V. and Chaudhary, R.P., Indian J. Chem., 1987, 26B, 602.
- Robba, M., Cagniant, S.M. and Leucomte, J.M., J. Heterocyclic. Chem ., 1975, 12, 525.
- Zentmyer, D.T. and Wagner, E.C., J. Org. Chem ., 1948, 14, 967.
- Winter, C.A., Risely, E.A. and Nu, G.N., Proc. Soc. Exp. Biol ., 1962, 111, 54719.
- Gillespie, S.H., Medical Microbiology-Illustrated. 1st edn., Butterworth Heinemann; 1994,1.