- *Corresponding Author:
- M. H. Boskabady
Applied Physiology Research Centre and Department of Physiology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
E-mail: mhboskabady@hotmail.com
| Date of Submission | 28 October 2012 |
| Date of Decision | 28 March 13 |
| Date of Acceptance | 18 April 13 |
| Indian J Pharm Sci 2013;75(4):400-405 |
Abstract
Different pharmacological effects of Achillea millefolium including its relaxant effect on smooth muscle have been shown previously. In the present study the stimulatory effect of the plant extract on β-adrenoceptor of tracheal muscle was examined in order to investigate one possible mechanism for its observed relaxant effect. Effect of three concentrations of hydroethanol extract, 10 nM propranolol, and saline on β-adrenoceptor was tested in two experimental groups including; nonincubated tracheal smooth muscles (group 1) and incubated tracheal smooth muscle with chlorpheniramine (group 2). Concentration response curves to isoprenaline were performed in precontracted tracheal smooth muscle in the presence of the extract, propranolol and saline. Values of EC 50 and CR-1 were measured. Leftward shifts in isoprenaline curves were observed in the presence of medium and high concentrations of the extract compared with saline in both groups. The values of EC 50 obtained in the presence of medium and high concentrations of the extract only in group 1 were nonsignificantly lower than that of saline. The values of CR-1 obtained in the presence of all concentrations of the extract in both groups were negative and significantly different with that of propranolol. The results indicated a small stimulatory effect of the extract on ί2-adrenoceptors.
Keywords
Achillea millefolium, hydroethanol extract, β‑adrenoceptor, guinea pig, trachea, EC50
The genus Achillea belong to Asteraceae family which is found in different areas including Iran [1,2]. Due to potential medicinal uses of A. millefolium (also named yarrow) [3,4], it is included in the national pharmacopoeias of countries such as Germany, France, and Switzerland and also included in the list of medicinal plants of Iranian traditional medicine (Ministry of Health and Medical Education (Iran)) [5]. The main components of the plant are: Flavonoids, phenolic acids, alkaloids, terpens (cineol, borneol, pinens, camphor, azulen), tannins, cis‑carveol, achillin, and leucosis [6,7]. The observed pharmacological effects for A. millefolium are antioxidant and antimicrobial activity [8], antiinflammatory [9], antihypertensive and bronchodilatory [10], gastrointestinal, antispasmodic [11‑13], diuretic, urinary antiseptic [12], astringent, and antihemorrhagic effects [14]. The effect of A. millefolium extract on reduction of the tonicity of isolated guinea pig trachea, ileum and pulmonary artery for its antitussive effect [7,15] and the relaxant effect on tracheal smooth muscle [16] were also shown.
To examine one possible mechanism for observed relaxant effect of the plant on smooth muscle [10‑14,16], in the present study, the effect of hydroethanol extract from A. millefolium on β‑adrenoceptor of guinea pig trachea was examined. According to similar previous studies [17,18], the effect of the extract on β‑adrenoceptor was evaluated by the effect of the extract on concentration response curve to isoprenalin (a β‑adrenoceptor agonist drug) compared to propranolol (a β‑adrenoceptor antagonist drug) and saline (as negative control).
Materials and Methods
A. millefolium was collected from Nishabour town (Khorasan province, Iran) and identified at the herbarium of Ferdowsi University (voucher No. 1357‑2216‑6), and dried with in the absence of daylight. Fifty grams of powdered plant was added to 700 ml of 50% ethanol (350 ml distilled water and 350 ml ethanol) and Soxhlet apparatus was used to prepare hydroethanol extract [19,20]. Under reduced pressure, the solvent was filtered. By adding distilled water to the dried extract, the plant ingredient concentration in the final extract was adjusted to 0.1 g/ml. The extract was prepared each week and stored in refrigerator.
Isoprenaline sulfate, methacholine hydrochloride, propranolol, and chlorpheniramine maleate were purchased from Sigma Chemical Ltd UK. Isoprenaline is a nonselective β1, β2‑adrenoceptor agonist which causes bronchodilatation. Methacholine is a long‑acting muscarinic cholinergic agonist which when inhaled causes bronchoconstriction. Propranolol is a competitive β‑adrenoceptor antagonist which in asthmatic subjects causes bronchoconstriction. Chlorpheniramine is a competitive antagonist at the histamine (H1) receptor in airway smooth muscle. In the present study, methacholine was used to induce contraction of tracheal smooth muscle but isoprenaline was used to stimulate β2 adrenoceptor and cause relaxation of smooth muscle. Therefore, isoprenaline is a functional antagonist for methacholine induced muscle contraction in the present study.
Tissue preparation
Guinea pigs (400‑700 g, both sexes) were killed by sharp head blowing and their tracheal chains were prepared and suspended in a 10 ml organ bath (organ bath 61300, Bio Science Palmer‑Washington, Sheerness, Kent, U.K.) containing Krebs‑Henseliet solution according previous study [20]. The tissue was allowed to equilibrate for at least 1 h while it was washed with Krebs solution every 15 min.
Concentration-response curves
A detailed protocol for concentration response curves is given in fig. 1. The stimulatory effect of A. millefolium on ß2‑adrenoceptors was examined by producing the cumulative log concentration‑response curve of isoprenaline‑induced relaxation of precontracted tracheal chains by 10 μM methacholine 7 min after the exposure of tissue to one of the test solutions including; 10 nM propranolol, three concentrations of hydroethanol extract from A. millefolium (0.2, 0.4, and 0.8 mg/ml) and 0.2 ml saline. The doses of the extract [16,21] and time of exposure of tissue to test solutions [17,18] were chosen according our previous studies in the same extract. The consecutive concentrations of isoprenaline were added every 2 min (5 nM-1000 μM); and the percentage of relaxation due to each concentration in proportion to the maximum relaxation obtained in the presence of saline was plotted against log concentration of isoprenaline. The effective concentration of isoprenaline causing 50% of maximum response (EC50) was measured using the log concentration‑response curve of the corresponding experiment [18].
Fig. 1: Schematic representation of treatment schedule. OB: Organ bath, CRC: Concentration response curve, Isopre: Isoprenaline. Test solutions were, saline, propranolol and three concentrations of the extract. In group 2 experiments, tissues were incubated with chlorpheniramine during two last tissue washout (last 30 min of tissue resting) and during performing CRC to Isopre.
The shift of cumulative log concentration‑response curves in the presence of different concentrations of the extract and propranolol (comparing the EC50 obtained in the presence of each solution with that of saline), isoprenaline maximum responses, and the index of the competitive antagonism [concentration ratio minus one (CR‑1)=(EC50 obtained in the presence of effective solutions/EC50 obtained in the presence of saline)‑1], were measured according to previous study [18].
The study was performed in two different experimental conditions of nonincubated tracheal chains (group 1, n=5) and tracheal chains incubated with 1 μM chlorpheniramine, 30 min prior to the beginning and while obtaining the isoprenaline curve, (group 2, n=4). Experiments were performed randomly, giving the tracheal chain 1 h resting period between two experiments while washing it every 15 min with Krebs‑Henseliet solution. In all experiments contractions were measured using an isotonic transducer (MLT0202, AD Instruments, Australia) which was connected to a power lab system (PowerLab 8/30, ML870, AD Instruments, Australia).
Statistical analysis
All data were expressed as mean±SEM. Data obtained in the presence of the extract and propranolol were compared with those of saline and the (CR‑1) obtained in the presence of extract with those of propranolol using paired t test. Comparison of data between two groups was made using unpaired Student “t” test. Significance was accepted at P<0.05.
Results and Discussion
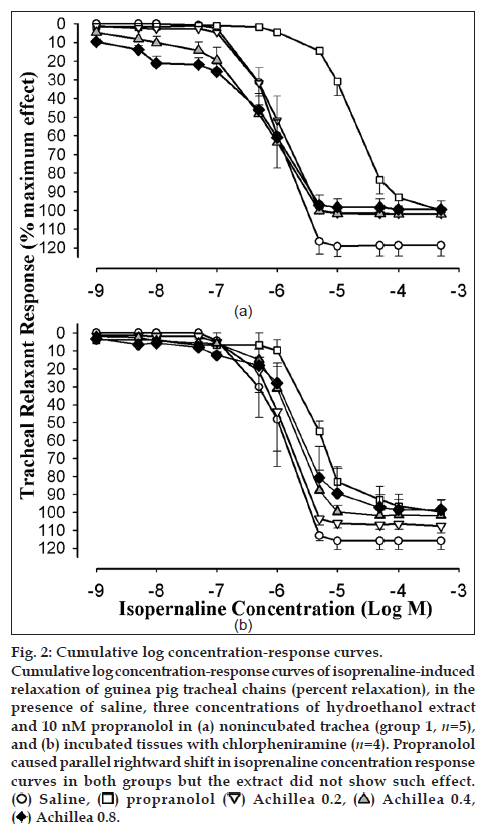
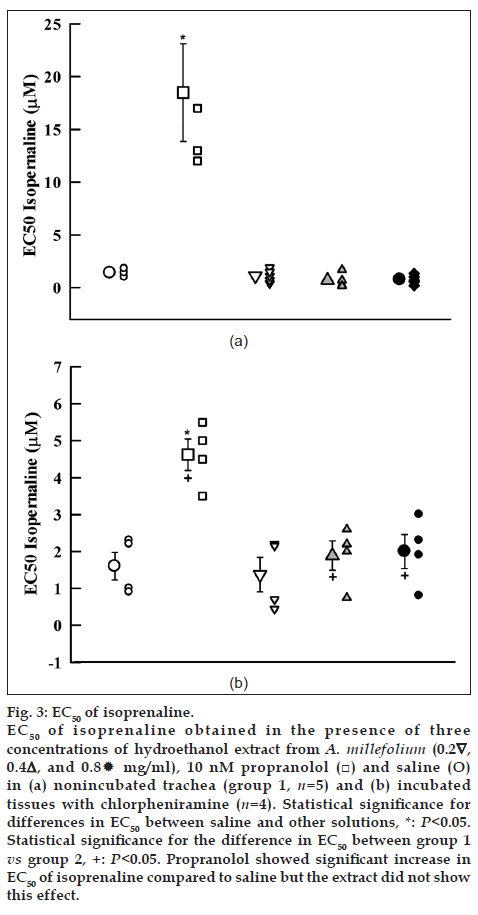
Cumulative log concentration‑response curves to isoprenaline obtained in the presence of medium and high concentrations of the extract showed leftward shift while the curve of propranolol showed clear rightward shift compared to isoprenaline curves produced in the presence of saline in both groups 1 and 2 (fig. 2a and b). EC50 obtained in the presence of propranolol was significantly higher than that of saline in both groups of experiments (P<0.05). However, the EC50 obtained in the presence of medium and high concentrations of the extract were nonsignificant and lower than those of saline in group 1 (Table 1, fig. 3a and b).
Fig. 2: Cumulative log concentration‑response curves. Cumulative log concentration‑response curves of isoprenaline‑induced relaxation of guinea pig tracheal chains (percent relaxation), in the presence of saline, three concentrations of hydroethanol extract and 10 nM propranolol in (a) nonincubated trachea (group 1, n=5), and (b) incubated tissues with chlorpheniramine (n=4). Propranolol caused parallel rightward shift in isoprenaline concentration response curves in both groups but the extract did not show such effect. (ο ) Saline, ( ❐) propranolol (▽ ) Achillea 0.2, (△ ) Achillea 0.4, ( ♦) Achillea 0.8.
Fig. 3: EC50 of isoprenaline. EC50 of isoprenaline obtained in the presence of three concentrations of hydroethanol extract from A. millefolium (0.2∇, 0.4△, and 0.8 mg/ml), 10 nM propranolol (□) and saline (O) in (a) nonincubated trachea (group 1, n=5) and (b) incubated tissues with chlorpheniramine (n=4). Statistical significance for differences in EC50 between saline and other solutions, *: P<0.05. Statistical significance for the difference in EC50 between group 1 vs group 2, +: P<0.05. Propranolol showed significant increase in EC50 of isoprenaline compared to saline but the extract did not show this effect.
| Solutions | Concentration | EC50 (µM) |
(CR‑1) | ||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | ||
| Saline | 1.21±0.29 | 1.35±0.52 | - | - | |
| Extract | 0.2 mg/ml | 1.10±0.27 | 1.37±0.47 | −0.26±0.11# | −0.21±0.13# |
| 0.4 mg/ml | 0.70±0.27 | 1.89±0.40 | −0.51±0.08# | 0.37±0.51# | |
| Propranolol | 0.8 mg/ml 10 nm |
0.76±0.19 18.5±4.63* |
2.00±0.46 4.62±0.43* |
−0.59±0.15# 8.06±0.73 |
0.45±0.63# 6.12±1.39 |
Values are presented as mean±SEM. Group 1: Experiments on nonincubated (n=5) and Group 2: Experiments on tracheal chains incubated with 1 μM chlorpheniramine (n=4). *P<0.05 is statistical difference between saline and other solutions. +P<0.05 is statistical difference between group 1 vs group 2. #P<0.05 is statistical difference in CR-1 between propranolol and the extract. The EC50 was not significantly different in three concentrations of the extract in both groups
Table 1: EC50 of Isoprenaline and CR-1 in the presence of Hydroethanol extract of A. millefolium, propranolol and saline in two groups
In both groups, there were no significant difference between maximum responses to isoprenaline in the presence of all concentrations of the extract and that of saline (Table 2). There was also no significant difference in maximum responses between two groups (Table 2). In the presence of all three concentrations of the extract, the slopes of isoprenaline‑response curves were not significantly different from those of saline in both groups 1 and 2 (Table 2). There was also no significant difference in the slopes obtained between two groups (Table 2).
| Solutions | Concentration | Maximum response | Slope | ||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | ||
| Saline | 0.51±0.10 | 0.36±0.05 | 0.90±0.06 | 0.87±0.07 | |
| Extract | 0.2mg/ml | 0.46±0.09 | 0.31±0.04 | 0.90±0.06 | 0.87±0.07 |
| 0.4mg/ml | 0.43±0.09 | 0.30±0.05 | 0.87±0.07 | 0.94±0.03 | |
| 0.8mg/ml | 0.43±0.10 | 0.32±0.07 | 0.90±0.06 | 0.92±0.04 | |
| Propranolol | 10nM | 0.37±0.10 | 0.34±0.11 | 0.98±0.02 | 0.99±0.00 |
Group 1: Experiments on nonincubated (n=5) and Group 2: Experiments on tracheal chains incubated with 1 μM chlorpheniramine (n=4). There was not any significant difference in maximum response and slope between saline and extract, two groups and three concentrations of the extract in both groups. For abbreviations see table 1.
Table 2: Maximum response to Isoprenaline and slope of Isoprenaline log concentration-response curves in presence of extract of A. millefolium, Propranolol and saline in the two groups
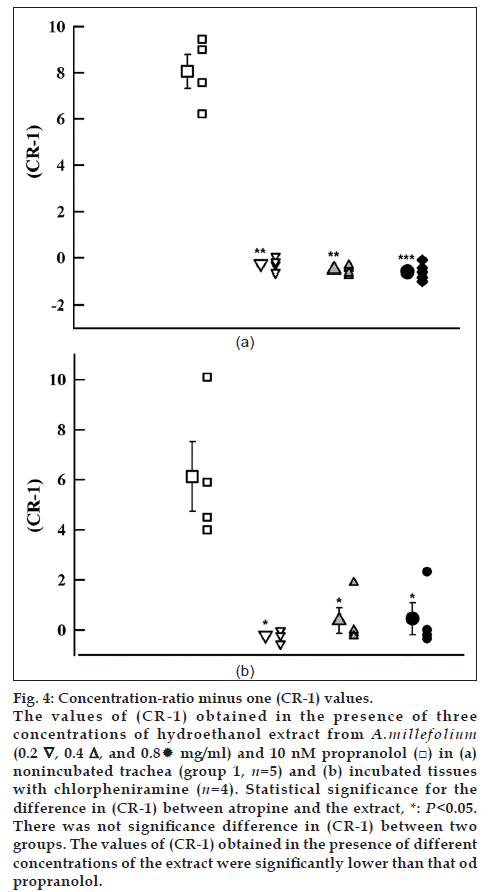
About shift in isoprenaline concentration‑response curves (CR‑1); the obtained values of (CR‑1) in the presence of all concentrations of the extract were negative in group 1 and their values were very small which were significantly different from that of propranolol in both groups (P<0.05) (fig. 4a and b, Table 1).
Fig. 4: Concentration‑ratio minus one (CR‑1) values. The values of (CR‑1) obtained in the presence of three concentrations of hydroethanol extract from A.millefolium (0.2 ∇, 0.4△, and 0.8 mg/ml) and 10 nM propranolol (□) in (a) nonincubated trachea (group 1, n=5) and (b) incubated tissues with chlorpheniramine (n=4). Statistical significance for the difference in (CR‑1) between atropine and the extract, *: P<0.05. There was not significance difference in (CR‑1) between two groups. The values of (CR‑1) obtained in the presence of different concentrations of the extract were significantly lower than that od propranolol.
The observed relaxant effect for the extract of A. millefolium on smooth muscle [6,7,10,15] might be due to several different mechanisms. The most possible mechanisms responsible for the relaxant effect on tracheal smooth muscle are; muscarinic receptor inhibitory which was demonstrated in previous study [21] and the β‑adrenoceptor stimulatory effect which is shown by the results of the present study.
The parallel leftward shifts in isoprenaline log concentration‑response curves, obtained in the presence of the medium and high concentrations of hydroethanol extract compared to that of saline in group 1 indicated a possible stimulatory effect of the extract at β‑adrenoceptor of guinea pig trachea smooth muscle [22]. Maximum contraction effect to isoprenaline obtained in the presence of all concentration of the extract was not significantly different with that of saline. However, the EC50 obtained in the presence of the extract was not different with that of saline. These findings indicate a mild stimulatory effect of the extract on β‑adrenoceptor of tracheal smooth muscle [22].
To evaluate the contribution of histamine (H1) blocking effect on the effect seen for the extract in group 1, the effect of the plant on β-adrenoceptors was also examined on tracheal chains incubated with chlorpheniramine in group 2. The parallel leftward shift in isoprenaline-response curves in the presence of concentrations of the extract and nonsignificant difference in maximum responses to isoprenaline, compared to saline suggest a possible mild stimulatory effect of the extract on β-adrenoceptors. However, EC50 obtained in this group was increased compared to group 1. The data of group 2 may also indicate an inhibitory effect for the extract on histamine (H1) receptors.
The negative values of (CR‑1) of the extract in group 1 and their low in group 2 which were significantly lower than the values obtained in the presence of propranolol supported the stimulatory effect of the extract on β‑receptors. These results are supported by the reduction of the relaxant effect of the extract of this plant in tracheal chains incubated with propranolol and chlorpheniramine [16].
The other possible mechanism (s) responsible for the relaxant effect of A. millefolium should be examined in further studies. In addition, the extract should be standardized (i.e., active constituents, ash value, moisture contents should be determined) and the contribution of different constituents of the plant on its relaxant effect should be investigated. The extract of the plant contain several constituents which may have different pharmacological effects. Therefore, the effect of different constituents of the plant on β‑adrenoceptors should be evaluated in further studies. Although both isoprenaline and The other possible mechanism (s) responsible for the relaxant effect of A. millefolium should be examined in further studies. In addition, the extract should be standardized (i.e., active constituents, ash value, moisture contents should be determined) and the contribution of different constituents of the plant on its relaxant effect should be investigated. The extract of the plant contain several constituents which may have different pharmacological effects. Therefore, the effect of different constituents of the plant on β‑adrenoceptors should be evaluated in further studies. Although both isoprenaline and propranolol are nonselective β‑agonist and antagonist, the density of β2‑adrenoceptors in tracheal smooth muscle was much higher than β1 and α‑adrenoceptors. In addition the same agonist and antagonist were used in our similar studies with other plants [17,18]. However, it is recommended to examine this mechanism of action using selective agonist and antagonist drugs. Other limitation of the present study was its small sample size and some variation among data. However, significant differences between data were shown. With regard to relaxant effect of the plant on tracheal smooth muscles and its possible stimulatory effect on β‑adrenoceptors, the plant could be of therapeutic value in obstructive pulmonary diseases, like asthma, as a bronchdilatory drug. Therefore, the bronchdilatory effect of the plant should be examined in patients with obstructive pulmonary diseases in further studies. In conclusion, these results indicated a mild stimulatory effect of A. millefolium on β‑adrenoceptors of tracheal smooth muscle.
Acknowledgements
This study was financially supported by Mashhad University of Medical Sciences.
References
- Chevallier A. The Encyclopedia of Medicinal Plants: A Practical Reference Guide to over 550 Key Herbs and Their Medicinal Uses. London: Dorling Kindersley Publishing Inc; 1996.
- Yassa N, Saeidnia S, Pirouzi R, Akbaripour M, Shafiee A. Three phenolic glycosides and immunological properties of Achilleamillefoliumfrom Iran, population of Golestan. Daru J Pharm Sci 2007;15:49‑52.
- Könemann B. The illustrated A‑Z of over 10,000 garden plants and how to cultivate them. Hong Kong: Gordon Cheers Publication; 1999. p. 51‑3.
- Chandler RF, Hooper SN, Harvey MJ. Ethnobotany and phytochemistry of yarrow, Achilleamillefolium, Compositae. Econ Bot 1982;36:203‑23.
- Shahbazi Y, Zadeh MS. Invitroassessment of antimicrobial eficacy of alcoholic extract of Achillea millefolium in comparison with penicillin derivatives. J Anim Vet Adv 2008;7:508‑11.
- Bimbirait K, Ragažinskien O, Maruška A, Kornyšova O. Comparison of the chemical composition of four yarrow (Achillea millefolium L.) morphotypes. Biologija 2008;54:208‑12.
- Raju D, Chitra V, Das K, Janiki P, Shankari M. Evaluation of anti‑asthmatic activity of aqueous extract of Achillea mellifolium Linn lowers. Arch Appl Sci Res 2009;1:287‑93.
- Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, etal. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan.(Asteraceae). J Ethnopharmacol 2003;87:215‑20.
- Goldberg AS, Evangelynne C, Eigen E. Isolation of the anti inflammatory principles from Achilleamillefolium(compositae). J Pharm Sci 2006;58:938‑41.
- Khan A, Gilani AH. Blood pressure lowering, cardiovascular inhibitory and bronchodilatory actions of Achilleamillefolium. Phytother Res 2011;25:577‑83.
- Bradley PR. British Herbal Compendium. British Herbal Medicine Association, Bournemouth, 1992. p. 190‑1.
- Bag A, Bhattacharyya S, Chattopadhyay R. Medicinal plants and urinary tract infections, an update. Pharmacogn Rev 2008;24:277‑84.
- Benedek B, Kopp B. AchilleamillefoliumL. s.l. revisited: Recent indings conirm the traditional use. Wien Med Wochenschr 2007;157:312‑4.
- Işcan G, Kirimer N, Kürkçüoglu M, Arabaci T, Küpeli E, Başer KH. Biological activity and composition of the essential oils of Achillea schischkinii Sosn. and Achillea aleppica DC. subsp. aleppica. J Agric Food Chem 2006;54:170‑3.
- Lemmens‑Gruber R, Marchart E, Rawnduzi P, Engel N, Benedek B, Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea‑pig ilea. Arzneimittelforschung 2006;56:582‑8.
- Boskabady MH, Eftekhar N, Kaveh M, Nemati A. Relaxant Effects of Achilleamillefoliumon Guinea‑pig Tracheal Chains. Pharmacologyonline 2009;3:893‑9.
- Boskabady MH, Kaveh M, Eftekhar N, Nemati A. Zataria multilora Boiss and Carvacrol Affect ß ‑adrenoceptors of Guinea Pig Trachea. Evid Based Complement Alternat Med 2011;2011:857124.
- Nemati H, Boskabady MH, Ahmadzadef Vostakolaei H. Stimulatory effect of Crocus sativus (saffron) on β2‑adrenoceptors of guinea pig tracheal chains. Phytomedicine 2008;15:1038‑45.
- Boskabady MH, Sheiravi N. Inhibitory effect of Nigella sativa on histamine (H1) receptors of isolated guinea pig tracheal chains. Pharm Biol 2002;40:596‑602.
- Boskabady MH, Aslani MR, Kiani S. Relaxant effects of Tymusvolgarion guinea pig tracheal chains and its possible mechanism (s). Phytother Res 2006;20:28‑33.
- Faizpour A, Boskabady MH, Bayrami G, Gholamnezhad Z, Shafei MN. The effect of hydro‑ethanol extract of Achilleamillefolliumon muscarinic receptors of guinea pig tracheal smooth muscle. Indian J Pharmacol 2013;4:13‑7.
- Linden A, Bergendal A, Ullman A, Skoogh BE, Lofdahl CG. Salmetrol, formetrol, and salbutamol in the isolated guinea‑pig trachea: Differences in maximum relaxant effect and potency but not in functional antagonism. Thorax 1993;48:547‑53.