- *Corresponding Author:
- S. K. Liu
Department of Endocrinology, Tianjin Second People's Hospital, Tianjin 300000, China
E-mail: yuopenzhang7510@163.com
| This article was originally published in a special issue,“Recent Developments in Biomedical Research and PharmaceuticalSciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “171-175” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The research was to investigate the effect of semaglutide on glycolipid metabolism in patients with both type 2 diabetes mellitus and non-alcoholic fatty liver disease. A total of 150 patients with both type 2 diabetes mellitus and non-alcoholic fatty liver disease admitted to our hospital from January 2020 to June 2020 were selected. They were divided into 2 groups using random number table, with 75 cases in each. The control group was treated with metformin hydrochloride tablets twice/d and 0.5 g/time, and the observation group was treated with semaglutide by hypodermic injection in the morning for a course of 3 mo. After 3 mo of treatment, both groups showed lower levels of 2 h postprandial blood glucose, fasting blood glucose, glycated hemoglobin A1c and homeostatic model assessment of insulin resistance, than before (all p<0.05), with distinctly lower levels in the observation group than in the control group. Fasting insulin levels in the observation group were significantly increased compared to that in the control group, with significant differences (p<0.05). Both groups showed decreased levels of triglyceride, total cholesterol and low-density lipoprotein cholesterol after treatment than before; with distinctly lower levels in the observation group than in the control group. After treatment, the level of high-density lipoprotein cholesterol in the observation group was higher than that in the control group. Both groups showed decreased levels of aspartate aminotransferase, alanine aminotransferase and gamma-glutamyl transpeptidase than before (p<0.05), with distinctly lower level in the observation group than in the control group. The observation group showed more significantly increased patients with mild result of color ultrasound and decreased patients with moderate to severe results than the control group. The treatment of semaglutide for patients with both type 2 diabetes mellitus and non-alcoholic fatty liver disease significantly alleviated the glycolipid metabolism and can significantly improve the fatty liver classification and the liver serum level of alanine aminotransferase, aspartate aminotransferase and gamma-glutamyl transpeptidase, which has considerable clinical application value.
Keywords
Type 2 diabetes mellitus, non-alcoholic fatty liver disease, semaglutide, glycolipid metabolism
In recent years, with the change of people’s diet and lifestyle, the incidence of Non-Alcoholic Fatty Liver Disease (NAFLD) in China has been increasing by year. This disease is a benign lesion of the liver, which can be reversed through effective intervention and treatment. NAFLD is one of the common complications of Type 2 Diabetes Mellitus (T2DM) and these two diseases are causal to each other, making the condition difficult to control and affecting the quality of prognosis. Studies have reported that the Insulin Resistance (IR) of patients with both T2DM and NAFLD is more significant, increasing the difficulty of controlling glycolipid metabolism and the risk of diabetes-related complications and cardiovascular and cerebrovascular diseases[1]. At present, controlling IR and glycolipid metabolism disorders is of great value in ameliorating diabetes, reversing NAFLD and blocking the progression of chronic liver disease[2]. Semaglutide is a commonly used hypoglycemic drug in clinic, with exact efficacy on T2DM confirmed by many studies. However there are few reports mentioning the regulatory effect of this drug on glycolipid metabolism in patients with T2DM combined with NAFLD[3,4]. This study investigated the effect of semaglutide on glycolipid metabolism in patients with both T2DM and NAFLD.
Materials and Methods
General materials:
A total of 150 patients with both T2DM and NAFLD admitted to our hospital from January 2020 to June 2022 were selected. They were divided into 2 groups according to the random number table method, with 75 cases in each.
Control group: 34 female and 41 male; age (56.85±7.34) y, ranging 41 y-72 y; course of disease (6.39±1.44) y, ranging from (2-12) y; Fasting Plasma Glucose (FPG) (8.65±2.88) mmol/l; 2 h Postprandial Blood Glucose (2hPBG) (13.68±1.76) mmol/l and Triglyceride (TG) (2.63±0.86) mmol/l.
Observation group: 31 female patients and 44 male; age (56.91±7.37), ranging 42 y-72 y; duration (6.43±1.39) y, ranging 3 y-12 y; FPG (8.54±3.28) mmol/l; 2hPBG (13.42±1.55) mmol/l and TG (2.45±0.76) mmol/l. There was no significant difference in the general data between the 2 groups (p<0.05). This research conformed to the requirements of medical ethics principles.
Inclusion criteria: Meeting the criteria for T2DM in basic Guidelines for diagnosis and treatment of type 2 diabetes[5]; meeting the guidelines for NAFLD in Guidelines for prevention of nonalcoholic fatty liver disease[6]; first diagnosed patient; tolerance to research drugs and being informed with signed approval.
Exclusion criteria: Diagnosed with other major diseases such as hepatitis; treated with weight loss drugs in the short term; diagnosed with malignancy or moderate or severe liver damage and poor compliance.
Methods:
Before treatment, the patient’s blood sugar, blood lipid and other indicators were measured. Patients were educated with health and disease knowledge, and told to reasonably eat and take healthy exercise. The control group orally took metformin hydrochloride tablets twice/d and 0.5 g/time with a course of 3 mo. In the observation group, semaglutide injection was injected in the morning. The initial dose was 0.6 mg/d, which was increased to 1.2 mg/d according to the patient’s specific situation. For those who still need further reduction in blood glucose, the dose could be increased to 1.8 mg/d after 1 w, with the maximum daily dose of 1.8 mg for a course of 3 mo. The patient’s condition was observed and the dose could be adjusted to 0.6 mg/d if not tolerated, and increased when the symptoms are reduced. After discharge, the patient was instructed to take medication according to the doctor’s advice, continuously monitor blood sugar and take regular follow-up with records. The adverse reactions were predicted, with timely treatment.
Outcome measures:
Before and 3 mo after treatment, blood was collected in the morning 8 h after fasting and immediately sent for testing to determine FPG, 2hPBG, Glycated Hemoglobin A1c (HbA1c), Fasting Insulin (FINS), Homeostatic Model Assessment of IR (HOMA-IR), Total Cholesterol (TC), TG and Low-Density Lipoprotein Cholesterol (LDL-C), High-Density Lipoprotein Cholesterol (HDL-C), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and Gamma-Glutamyl Transpeptidase (GGT). The degree of liver lesions was determined by fasting liver ultrasound (performed by the same sonologist before and after treatment) and the occurrence of adverse reactions during treatment was recorded in detail. FPG and 2hPBG were determined by hexokinase method, HbA1c by high performance liquid chromatography, FINS by chemiluminescence, TC by cholesterol oxidase, TG by Glycerine Phosphate Oxidase Peroxidase (GPO-PAP), and LDL-C and HDL-C by direct method.
Mild NAFLD: Detected with regular morphology, fine and uniform parenchymal light point, enhanced echo, no obvious far-field echo attenuation, increased parenchymal liver and kidney echo contrast, clear intrahepatic tube system and no abnormity in hepatic vein Color Doppler Flow Imaging (CDFI).
Moderate NAFLD: Detected with full shape, increased volume, fine and uniform parenchymal light point, enhanced echo, far-field echo attenuation in 1/3 of the whole liver, increased parenchymal liver and kidney echo contrast, not so clear intrahepatic tube system and thinner blood flow in hepatic vein CDFI.
Severe NAFLD: Detected with full shape, increased volume, fine and uniform parenchymal light point, enhanced echo, far-field echo attenuation in 1/2 of the whole liver, significantly increased parenchymal liver and kidney echo contrast, not so clear intrahepatic tube system and thinner or less filling blood flow in hepatic vein CDFI.
HOMA-IR=FPG (mmol/l)×FINS (mIU/l)/22.5
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 statistical software was used for data analysis, with variables data expressed as mean±standard deviation and tested by t-test, and attributes data expressed as N (%) and tested by Chi-square (χ2) test. p<0.05 was considered as statistical significance.
Results and Discussion
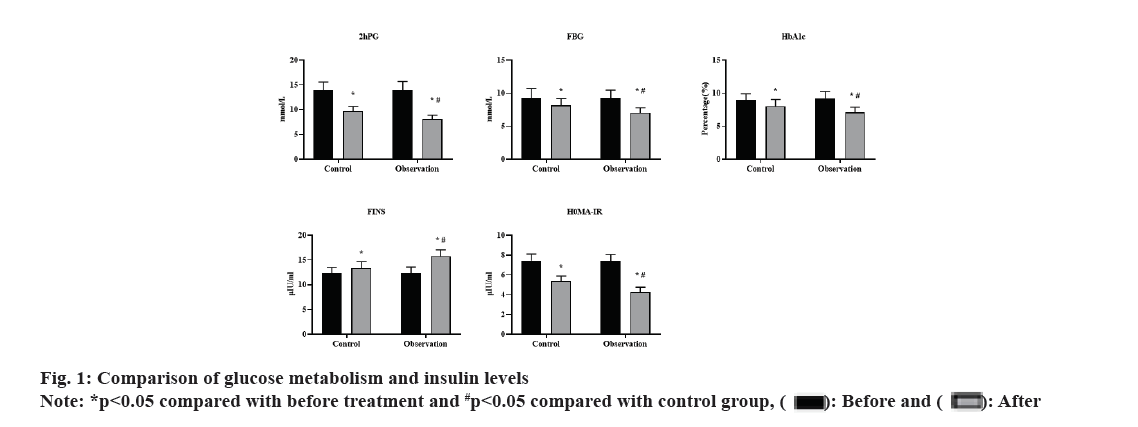
Before treatment, there was no significant difference in 2hPG, Fasting Blood Glucose (FBG), HbA1c, FINS and HOMA-IR levels between the 2 groups (p>0.05). After 3 mo of treatment, both groups showed decreased levels of 2hPG, FBG, HbA1c and HOMA-IR than before, with significantly lower levels in the observation group than in the control group (all p<0.05). FINS level was significantly higher in the observation group than in the control group, with significant differences (p<0.05). These results were shown in fig. 1.
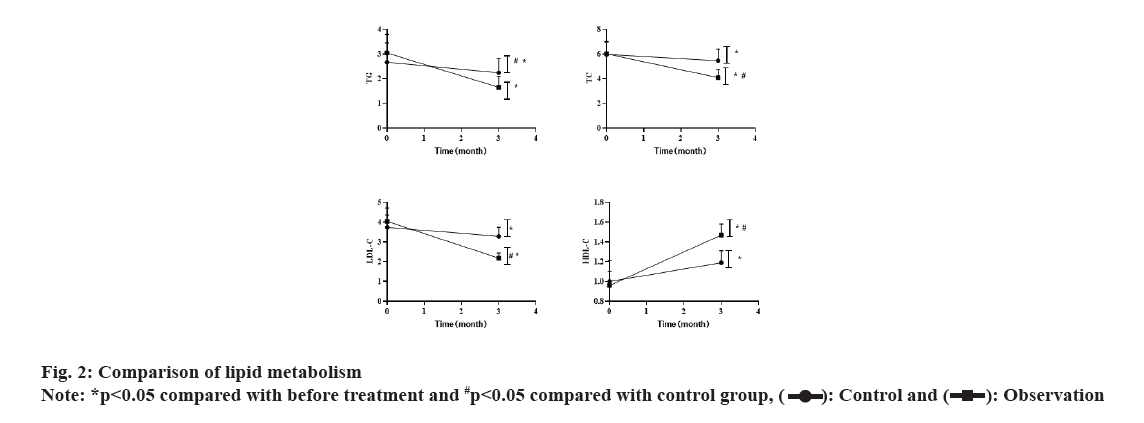
Before the treatment, there was no significant difference in the levels of TG, TC, LDL-C and HDL-C between the 2 groups (p>0.05). After 3 mo of treatment, both groups showed decreased levels of TG, TC and LDL-C (both p<0.05), with significant lower levels in the observation group than in the control group (p<0.05). HDL-C level was significantly increased in the observation group than before. These results were shown in fig. 2.
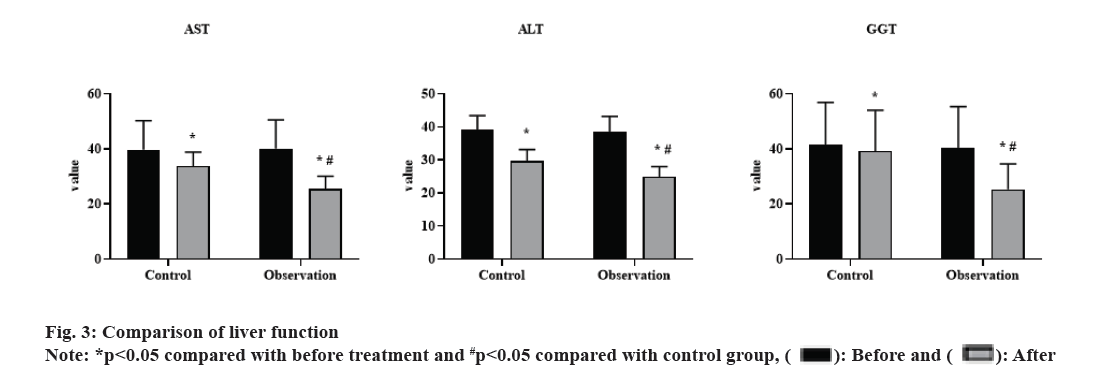
Before treatment, there was no significant difference in the levels of AST, ALT and GGT between the 2 groups (p>0.05). After 3 mo of treatment, both groups showed decreased levels of AST, ALT and GGT (p<0.05), with significantly lower levels in the observation group than in the control group (p<0.05). These results were shown in fig. 3.
Before treatment, there was no significant difference in NAFLD classification between 2 groups (p>0.05). After 3 mo of treatment, the number of mild patients significantly increased and those of moderate to severe patients significantly reduced in the observation group than in the control group, with significant differences (p<0.05). These results were shown in Table 1. During the treatment, there was no significant difference in the incidence of adverse reactions between two groups (χ2=0. 267, p=0. 301). These results were shown in Table 2.
| Group | n | Mild | Moderate | Severe | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| Control | 75 | 15 (20.00) | 28 (37.33) | 37 (49.33) | 35 (46.67) | 23 (30.67) | 12 (16.00) |
| Observation | 75 | 14 (18.67) | 47 (62.67)* | 40 (53.33) | 21 (28.00)* | 21 (28.00) | 7 (9.33)* |
Note: *p<0.05, compared to the control group
Table 1: Comparison Of NAFLD Classification by Ultrasound
| Group | Gastrointestinal reaction | Headache | Dizziness | Total incidence |
|---|---|---|---|---|
| Control | 1 (1.33) | 1 (1.33) | 2 (2.66) | 4 (5.32) |
| Observation | 1 (1.33) | 2 (2.66) | 2 (2.66) | 5 (6.65) |
Table 2: Incidence of Adverse Reactions
With the rapid development of China’s economic level and the consequential prosperous life, the diet structure and lifestyle have undergone great changes. The NAFLD and T2DM mainly caused by abnormal glycolipid metabolism will be a major obstacle threatening the health of Chinese people in the future. The liver is an important organ to regulate the balance of glycolipid metabolism and also the main target organ of insulin activity. The excessive deposition of TG in the liver is closely related to IR and glycolipid metabolism. The quantitative index to measure the severity of hepatic steatosis is liver enzyme, which is closely related to IR, FPG and blood lipid index. Especially, GGT is a predictor of diabetes and IR, second only to FBG[7]. The IR in the peripheral tissues, hyperinsulinemia and high blood sugar levels of T2DM patients weaken the inhibitory effect of insulin on fat and increase the de novo liver fat synthesis pathway, thus leading to increased levels of circulating Free Fatty Acid (FFA) and increased liver FFA source and finally the formation of NAFLD[8]. When hepatic steatosis occurred in NAFLD patients, the release of liver cytofactors and inflammatory signals affects the normal glucose metabolism process, further increasing IR in T2DM patients. Excess FFA levels in circulation can damage the normal utilization of peripheral glucose; promote liver glycogen hetereogenesis, aggravating IR of peripheral tissue, making it more difficult for T2DM patients to get smooth and effective control of blood sugar. Therefore, patients with both two diseases have more severe glycemic-lipid metabolism disorders and IR[9] than patients with only one. T2DM and NAFLD are closely related with IR as a bridge. They interact and promote the disorder of glycolipid metabolism, forming a negative cycle. The current intervention for patients with both T2DM and NAFLD is to treat these two diseases respectively, while many chemical drugs are unable to target the multiple causes of the disease and lack drug specificity with many defects[2,10]. Therefore, it is of great clinical significance to find new drugs that can control both blood glucose level and lipid regulation to improve the therapeutic effect of combined treatment for T2DM and NAFLD.
As a new drug, semaglutide has significant hypoglycemic effect, extensive pharmacological effect and low incidence of adverse reactions[11]. Semaglutide is the product of gene recombination technology, which is released into the blood under the activity of sugar. Its mechanism of action is to stimulate basal endogenous insulin secretion and regulate glucose levels to achieve fair blood glucose control effect and promote cell proliferation and differentiation at the same time[12,13]. The results of this study showed that the glycolipid metabolism in the observation group were ameliorated more obviously than those in the control group, indicating that the combination therapy of metformin hydrochloride tablets and semaglutide for patients with both T2DM and NAFLD can significantly reduce the blood sugar and lipid levels and improve IR. In addition, the observation group showed a better amelioration in the NAFLD classification and levels of ALT, AST and GGT in liver serum, suggesting semaglutide has remarkable efficacy as combined treatment for T2DM and NAFLD. Previous[11] studies have pointed out that the application of semaglutide in patients with both T2DM and NAFLD plays an important role in regulating blood sugar, improving IR and ensuring drug safety, which is similar to the results of our research. Lipid metabolism disorders lead to liver fat environment imbalance, the excessive lipolysis of TG cause lipotoxicity, aggravating IR. After treatment, TG, TC, LDL-C was reduced, mainly because semaglutide can increase glucose dependent insulin secretion, effectively control glucagon, reduce appetite, inhibit gastric emptying, reduce weight, so as to ameliorate IR and control blood lipid levels. Visceral obesity, high liver hardness index, high fat controlled attenuation parameters are the important related factors of hepatic steatosis. After treatment, the liver hardness index and fat controlled attenuation parameters were reduced, because semaglutide can improve insulin sensitivity, effectively inhibits IR to prevent damage caused by hepatic steatosis to the body, reduce the production of liver glucose, thus improving liver hardness, controlling weight and effectively alleviating hepatic steatosis. In addition, while ameliorating IR, semaglutide can effectively inhibit the release of various inflammatory factors such as Interleukin 6 (IL-6) and Transforming Growth Factor (TGF), promote the recovery of fatty liver and then improve hepatic steatosis. By subcutaneous injection, the drug effect would last longer and the patients need only one daily subcutaneous injection to maintain fair hypoglycemic effect[14,15].
Conflict of interests:
The authors have declared no conflict of interests.
References
- Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat Rev Gastroenterol Hepatol 2021;18(9):599-612.
[Crossref] [Google Scholar] [PubMed]
- Dharmalingam M, Yamasandhi PG. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab 2018;22(3):421-8.
[Crossref] [Google Scholar] [PubMed]
- Viscardi AV, Reppert EJ, Kleinhenz MD, Wise P, Lin Z, Montgomery S, et al. Analgesic comparison of flunixin meglumine or meloxicam for soft-tissue surgery in sheep: A pilot study. Animals 2021;11(2):423.
[Crossref] [Google Scholar] [PubMed]
- Bussi S, Coppo A, Bonafè R, Rossi S, Colombo Serra S, Penard L, et al. Gadolinium clearance in the first 5 w after repeated intravenous administration of gadoteridol, gadoterate meglumine and gadobutrol to rats. J Magn Reson Imaging 2021;54(5):1636-44.
[Crossref] [Google Scholar] [PubMed]
- Javeed N, Matveyenko AV. Circadian etiology of type 2 diabetes mellitus. Physiology 2018;33(2):138-50.
[Crossref] [Google Scholar] [PubMed]
- Raza S, Rajak S, Upadhyay A, Tewari A, Sinha RA. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci 2021;26:206-37.
[Crossref] [Google Scholar] [PubMed]
- Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD and cardiovascular risk. Curr Diab Rep 2021;21(5):15.
[Crossref] [Google Scholar] [PubMed]
- Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Khamseh ME. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: A randomized, double-blind, placebo-controlled trial. Adv Ther 2020;37(11):4697-708.
[Crossref] [Google Scholar] [PubMed]
- Patoulias D. SGLT-2 inhibitors: Are they a promising treatment option in T2DM patients with NAFLD? Acta Med 2018;60(4):167-70.
- Zhou Q, Wang Y, Wang J, Liu Y, Qi D, Yao W, et al. Prevalence and risk factor analysis for the nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Medicine 2021;100(10):e24940.
[Crossref] [Google Scholar] [PubMed]
- Bravo-Nuevo A, Marcy A, Huang M, Kappler F, Mulgrew J, Laury-Kleintop L, et al. Meglumine exerts protective effects against features of metabolic syndrome and type II diabetes. PLoS One 2014;9(2):e90031.
[Crossref] [Google Scholar] [PubMed]
- Tsuchihara K, Ogura T, Fujioka R, Fujii S, Kuga W, Saito M, et al. Susceptibility of Snark-deficient mice to azoxymethane-induced colorectal tumorigenesis and the formation of aberrant crypt foci. Cancer Sci 2008;99(4):677-82.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Huang Y, Li K, Chen Y, Vanegas D, McLamore ES, et al. Leaf extract from Lithocarpus polystachyus Rehd. promote glycogen synthesis in T2DM mice. PLoS One 2016;11(11):e0166557.
[Crossref] [Google Scholar] [PubMed]
- Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci 2011;48(3):97-113.
[Crossref] [Google Scholar] [PubMed]
- Chen Z, Liu J, Zhou F, Li H, Zhang XJ, She ZG, et al. Nonalcoholic fatty liver disease: An emerging driver of cardiac arrhythmia. Circ Res 2021;128(11):1747-65.
[Crossref] [Google Scholar] [PubMed]