- *Corresponding Author:
- S. Xiong
Key Laboratory of Drug Metabolism and Pharmacokinetics, Beijing Institute of Pharmacology and Toxicology, Beijing-100850
E-mail: shanxiong83@sohu.com
| Date of Submission | 16 October 2015 |
| Date of Revision | 09 November 2016 |
| Date of Acceptance | 25 March 2017 |
| Indian J Pharm Sci 2017;79(2):303-306 |
Abstract
Morroniside is a well-known iridoid plant glycoside. Attempts were made to study the effects of gender and multiple oral dosing on the pharmacokinetics and bioavailability of morroniside in beagle dogs. The concentration of morroniside in plasma was determined using a LC-MS/MS method. Main pharmacokinetic parameters were estimated by DAS 2.0 pharmacokinetic software. Statistical analysis was performed using student t-test with P-values less than 0.05 as the level of significance. No significant gender difference was observed in the pharmacokinetic behaviour of morroniside and no differences were observed in the pharmacokinetic parameters following single and multiple administrations of morroniside. The absolute bioavailability after the oral administration of morroniside in beagle dogs was found to be 7.47±0.65%.
Keywords
Morroniside, LC-MS/MS, pharmacokinetics, bioavailability, beagle dogs
Morroniside, an iridoid glycoside (Figure 1), is the main active ingredient from Cornus officinalis Sieb. et Zucc. and Sambucus williamsii Hance, both of which are rich sources of iridoid glycosides and have been used as traditional Chinese medicinal herbs for centuries. Various pharmacologic studies have indicated that morroniside has therapeutic effects on diabetic angiopathies, renal damage, lipid metabolism and inflammation and bone resorption [1-4]. In recent studies, morroniside showed the protective actions against the cytotoxicity produced by exposure to H2O2 in human SH-SY5Y cells [5,6]. In addition, morroniside could protect ischemia/reperfusion-induced brain injury by decreased the caspase-3 activity, reduced the infarction volume and minimized oxidative stress [7].
There has been active interest in recent years in developing and optimizing analytical methods for determination of morroniside in rat plasma samples using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection [8]. However, only few reports have addressed the pharmacokinetics of morroniside in beagle dogs [9]. In our previous work, the analysis of morroniside in beagle dog plasma using liquid chromatography tandem-mass spectrometry (LC-MS/MS) has been systematically validated [9].
However, up to now, there were no reports on the pharmacokinetic properties including gender-related pharmacokinetic differences, pharmacokinetic behaviour after multiple dosing, and oral bioavailability of morroniside in beagle dogs. Therefore, it was necessary for an intensive investigation on pharmacokinetics of morroniside. In the present study, the pharmacokinetics of morroniside after oral administration were systematically investigated using a well-validated sensitive LC-MS/MS method. The key pharmacokinetic issues mentioned above would be well addressed in support of the development of morroniside as a candidate drug.
Morroniside was extracted and purified from sarcocarp of C. officinalis, in the Department of Pharmacology, Xuanwu Hospital of Capital Medical University and its purity was over 98.5% by HPLC analysis. Paeoniflorin, used as the internal standard (IS), was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). HPLC-grade methanol and acetonitrile were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Sodium formate was obtained from Beijing Chemical Reagent Company. All other chemicals were of analytic grade or better.
All analysis was performed with an API 5000 triplequadrupole mass spectrometer (Applied Biosystems/ MDS SCIEX, Foster City, CA, USA) coupled with electrospray ionization (ESI) source, which was operated in the positive ion mode. The liquid chromatography system consisted of a Shimadzu DGU-20A5 degasser, two Shimadzu LC-20AD pumps, and a Shimadzu SIL-20AHT auto sampler (Shimadzu Scientific Instruments, Columbia, MD, USA).
An Inertsil ODS-SP column (2.1×50 mm, 5 μm) was adopted, with a mobile phase consisted of 1 mM sodium formate (solvent A) and acetonitrile (solvent B) at a flow rate of 0.4 ml/min and the total run time was 6 min at room temperature. The gradient elution program was as follows: 5% B going to 20% B from 0-0.5 min, 20% B going to 70% B from 0.5-1.0 min, 70% B going to 95% B from 1.0-3.0 min, 95% B going to 5% B from 3.0-3.2 min and finally 5% B from 3.2- 6.0 min.
The mass spectrometer was operated in the multiple reactions monitoring (MRM) scan type. The transitions from molecular ion to dominant product ion m/z 429→267 [M+Na]+ and m/z 503→341 [M+Na]+ were monitored for morroniside and IS, respectively. The ESI source was operated with ion spray voltage at 5000 V and heater temperature at 550°. The optimized working parameters for mass detection were as follows: CUR 25 l/h, CAD 12 l/h, Gas1 45 l/h, Gas2 45 l/h, DP 180 V, EP 10 V, CE 35 V and CXP 15 V. Analyst 1.5.2 software (AB SCIEX, Foster City, CA, USA) was used for system control and data processing [9].
Twelve beagle dogs (nine males and three females), weighing 10.0 ± 2.0 kg were purchased from Beijing Marshall Biological Technology Co., Ltd. (Beijing, China, Certificate number. SCXK, Beijing, 2011-0003). Room environment was maintained continuously at a temperature of 20-25°, relative humidity of 40-70%, 10-15 air changes per hour and a 12 h fluorescent light/12 h dark cycle. All dogs were fed with standard dog diet and had free access to water. The animal experiments were conducted at the Beijing Center for Drug Safety Evaluation followed the protocol of the Institutional Animal Care and Use Committee of the Centre, which was in compliance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International.
Dogs were fasted for at least 12 h with free access to water prior to the pharmacokinetic studies [10-13]. Three male and three female dogs were used to determine gender-related difference of morroniside. The dogs were administered with morroniside at a dose of 15 mg/kg intragastrically (i.g.). Blood samples, about 1 ml each collected through hind leg vein, were placed in heparinized tubes pre-dose and at 5, 15, 30, 45, 60, 120, 240, 360, 480, and 720 min post-dose.
The dogs in multiple (7 times, q8h) administrations group (n=3) were also administered a dose of 15 mg/ kg. Blood samples of multiple doses were immediately collected after the first and seventh oral administration at the same time point as single dose study.
In the single intravenous administration group, morroniside was administered intravenously (i.v.) through the fore leg vein at a dose of 5 mg/kg. Blood samples were collected from the hind leg vein into heparinized tubes at 0, 2, 5, 15, 30, 45, 60, 120, 240, 360, 480, and 720 min after administration.
All plasma samples were harvested by centrifugation at 3000 rpm/min for 15 min at 4° and stored below –20° until LC-MS/MS analysis. Samples were prepared by using the standard protein precipitation method consisted of adding 50 μl of plasma to a centrifuge vial, followed by 50 μl of purified water and 350 μl of methanol containing 100 ng/ml paeoniflorin. The tubes were vortex-mixed for 1 min and centrifuged at 14 000 rpm/min for 10 min and 10 μl of the supernatant was analysed [9].
Absolute bioavailability (F%) of morroniside was calculated using the following Eqn., F% = (AUC(0-∞, i.g.)×Di.v./AUC(0-∞, i.v.)×Di.g.)×100, (1), where AUC(0–∞, i.g.)and AUC(0–∞, i.v.) represent the area under the concentration–time curve from zero to infinity after morroniside oral and i.v. administration, respectively, whereas Di.g. and Di.v. represent the dose of morroniside oral and i.v. administration, respectively.
Data were given as mean ± standard deviation (SD). Statistical analyses were performed using the SPSS 18.0 software package (SPSS Inc., Chicago, IL, USA) and pharmacokinetic parameters for morroniside were calculated by non-compartmental analysis using the Drug and Statistic (DAS) 2.0 pharmacokinetic software (Mathematical Pharmacology Professional Committee of China, Shanghai, China). Statistical analysis of the data was performed using student t-test. A different of P<0.05 was considered as statistically significant.
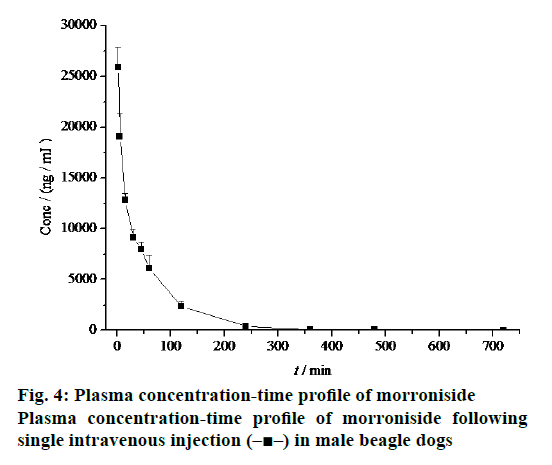
The mean plasma concentration-time profiles of morroniside in beagle dogs were shown in Figure 2. Morroniside was detected in beagle dog plasma 5 min after oral administration. There were no genderrelated differences in the pharmacokinetic profiles of morroniside. The pharmacokinetic parameters of morroniside were summarized in Table 1. No significant differences in Tmax (0.75 ± 0.25 h vs. 0.83 ± 0.14 h), Cmax (1886.67 ± 533.14 ng/ml vs. 1800.00 ± 391.28 ng/ml), AUC(0-∞) (4272.47 ± 838.34 ng·h/ml vs. 4146.29 ± 408.63 ng·h/ml), t1/2z (1.13 ± 0.14 h vs. 0.87 ± 0.04 h), CLz (3.60 ± 0.67 l/h/kg vs. 3.64 ± 0.34 l/h/kg) and MRT (1.93 ± 0.31 h vs. 2.04 ± 0.31 h) were observed between the male and female beagle dogs.
| Parameter/ data unit | Parameter value/mg·kg-1 | |||
|---|---|---|---|---|
| 15 (male, i.g., single) | 15 (female, i.g., single) | 15 (male, i.g., multiple) | 5 (female, i.v., single) | |
| Tmax/h | 0.75 ± 0.25 | 0.83 ± 0.14 | 0.57 ± 0.13 | 0.03 ± 0.00 |
| Cmax/ng·ml-1 | 1886.67 ± 533.14 | 1800.00 ± 391.28 | 1940.00 ± 289.31 | 25900.00 ± 1967.23 |
| AUC(0-t)/ ng·h·ml-1 | 4267.89 ± 834.60 | 4133.04 ± 406.93 | 4128.03 ± 1259.44 | 18933.22 ± 2021.30 |
| AUC(0-∞)/ ng·h·ml-1 | 4272.47 ± 838.34 | 4146.29 ± 408.63 | 4128.92 ± 1259.65 | 18945.09 ± 2007.92 |
| t1/2z/h | 1.13 ± 0.14 | 0.87 ± 0.04 | 0.97 ± 0.05 | 0.92 ± 0.06 |
| CLz/l·h-1·kg-1 | 3.60 ± 0.67 | 3.64 ± 0.34 | 3.91 ± 1.36 | 0.27 ± 0.03 |
| MRT/h | 1.93 ± 0.31 | 2.04 ± 0.31 | 1.60 ± 0.21 | 1.02 ± 0.05 |
| F/% | 7.47 ± 0.65 | |||
Table 1: Pharmacokinetic parameters of morroniside in beagle dogs
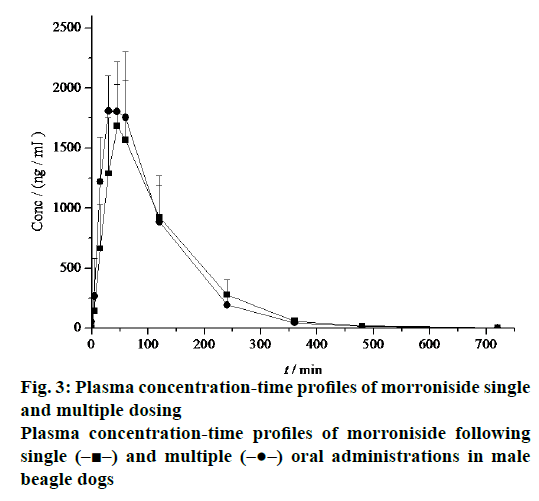
The mean plasma concentration-time profiles after single and multiple oral administrations of morroniside in beagle dogs were shown in Figure 3 and the derived pharmacokinetic parameters were presented in Table 1. Morroniside concentrations reached a maximum of 1940.00 ± 289.31 ng/ml, with the AUC(0-∞), t1/2z, CLz and MRT equal to 4128.92 ± 1259.65 ng·h/ml, 0.97 ± 0.05 h, 3.91 ± 1.36 l/h/kg and 1.60 ± 0.21 h following multiple oral administrations. The absorption of morroniside for multiple administrations was still very fast (Tmax, 0.57 ± 0.13 h). No significant deviation was observed in the difference of all the pharmacokinetic parameters following the single and multiple doses of morroniside in dogs.
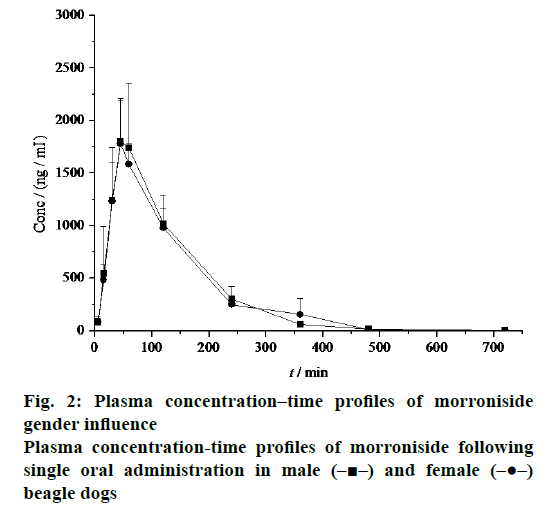
The mean plasma concentration-time profile after i.v. administration of morroniside to beagle dogs was shown in Figure 4 with the corresponding pharmacokinetic parameters given in Table 1. After dosage correction, the oral bioavailability of morroniside was calculated by comparing the corresponding values of AUC(0–∞). The
In summary, this was the first study to investigate the pharmacokinetic characteristics of morroniside including gender-dependent pharmacokinetic differences, results showed that oral administration of morroniside led only to an absolute bioavailability of (7.47 ± 0.65%).
pharmacokinetic behaviours after multiple oral administrations, and oral bioavailability in beagle dogs
Our results provided valuable pharmacokinetic information for rational use of morroniside. The pharmacokinetic parameters of morroniside were important to elucidate its pharmacological properties and further investigations were required to reveal the underlying mechanisms for absorption and metabolism of morroniside in vivo.
Conflict of interests
Authors report no conflict of interests.
Financial support and scholarship
This work has been financially supported by the Innovation Project of Shandong Academy of Medical Sciences and National Major Scientific and Technical Special Projects for Innovative Drug of China (No. 2012ZX09301003- 001-007).
References
- Xu HQ, Hao HP, Zhang X, Pan Y. Morroniside protects cultured human umbilical vein endothelial cells from damage by high ambient glucose. ActaPharmacol Sin 2004;25:412.
- Yokozawa T, Yamabe N, Kim HY, Kang KS, Hur JM, Park CH, et al. Protective effects of morroniside isolated from Cornifructusagainst renal damage in streptozotocin-induced diabetic rats. Biol Pharm Bull 2008;31:1422-8.
- Park CH, Yamabe N, Noh JS, Kang KS, Tanaka T, Yokozawa T. The beneficial effects of morroniside on the inflammatory response and lipid metabolism in the liver of db/db mice. Biol Pharm Bull. 2009;32:1734-40.
- Li M, Wang W, Wang P, Yang K, Sun H, Wang X.The pharmacological effects of morroniside and loganin isolated from Liuweidihuang Wan, on MC3T3-E1 cells. Molecules2010;15:7403-14.
- Wang W, Huang W, Li L, Ai H, Sun F, Liu C, et al. Morroniside prevents peroxide-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Cell MolNeurobiol 2008;28:293-305.
- Wang W, Xu J, Li L, Wang P, Ji X, Ai H, et al. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull 2010;83:196-201.
- Wang W, Sun F, An Y, Ai H, Zhang L, Huang W, et al.Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol 2009;613:19-23.
- Li X, Wang Q, Zhang X, Sheng X, Zhou Y, Li M, et al. HPLC study of pharmacokinetics and tissue distribution of morroniside in rats. J Pharm Biomed Anal 2007;45:349-55.
- Xiong S, Li J, Zhu X, Wang X, Lü G, Zhang Z. Determination of morroniside concentration in beagle plasma and its pharmacokinetics by high performance liquid chromatography-tandem mass spectrometry. Se Pu 2014;32:290-3.
- Shanmugasundaram S, Manjunatha N, Vijayan R, Khatwal RB, Samanta MK. Determination and estimation of pharmacokinetic profile of caffeine in form of extract of green tea leaves and its analogy with synthetic form. Indian J Pharm Sci 2011;73:649-55.
- Xu H, Zhang T, Yang H, Xiao X, Bian Y, Si D, et al. Preparation of evodiamine solid dispersions and its pharmacokinetics. Indian J Pharm Sci 2011;73:276-81.
- Katayama M, Kawakami Y, Katayama R, Shimamura S, Okamura Y, Uzuka Y. Preliminary study of effects of multiple oral dosing of clarithromycin on the pharmacokinetics of cyclosporine in dogs. J Vet Med Sci 2014;76:431-3.
- Mahmoud MA, Ebid AH, Shouman SA, Ebid EN. Pharmacokinetics of vancomycin in oncology Egyptian paediatrics: a dosage adjustment trial. Indian J Pharm Sci 2014;76:82-6.
 and female
and female  beagle dogs
beagle dogs