- *Corresponding Author:
- Xiaoli Jia
Reproductive Medicine Center,
Shaoguan Maternal and Child Health Hospital,
Shaoguan, Guangdong 512026,
China
E-mail: 1263288390@qq.com
| This article was originally published in a special issue, “Novel Therapeutic Approaches in Biomedicine and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:83(6) Spl Issue “61-66” |
Abstract
To investigate how estrogen level on the endometrial transformation day for hormone replacement therapy-frozen-thawed embryo transfer cycle influences pregnancy outcome. A retrospective analysis was conducted on clinical data from 772 patients who underwent hormone replacement therapy-frozenthawed embryo transfer between January 2016 and January 2020 in the Reproductive Medicine Center, Maternal and Child Health Hospital of Shaoguan City. The patients were divided into 5 groups according to their estradiol level on the endometrial transformation day as follows: Group A (estradiol<150 pg/ ml, 126 cycles). Group B (150≤estradiol<300 pg/ml, 318 cycles); Group C (300≤estradiol<450 pg/ml, 154 cycles); Group D (450≤estradiol<900 pg/ml, 115 cycles); Group E (estradiol>900 pg/ml, 59 cycles). There were no statistically significant differences in the general conditions among the 5 groups (p>0.05). Further comparative analysis on pregnancy outcomes among the 5 groups showed that there were no statistically significant differences in clinical pregnancy rate, embryo implantation rate and ectopic pregnancy rate among the 5 groups (p>0.05), but the first trimester abortion rate of Group E (30.3 %) was higher than that of Groups B to D (13.0 %, 11.4 %, 9.0 %) obviously and the difference was statistically significant (p<0.05). A further comparison of live birth rate among the 5 groups showed that the rates in Group A (35.7 %) and Group E (35.6 %) were lower than in Groups B to D (45.3 %, 46.8 %, 44.3 %), but there was no statistically significant difference (p>0.05). During the hormone replacement therapy-frozen-thawed embryo transfer cycle, high levels of estrogen may be associated with first trimester abortion. Moreover, when the estrogen level on the endometrial transformation day was within the range of 150-900 pg/ml, the live birth rate was relatively high.
Keywords
Estrogen, hormone replacement therapy, frozen-thawed embryo transfer
In recent years, with the rapid development of Assisted Reproductive Technology (ART), Frozen- Thawed Embryo Transfer (FET) has become mature gradually, with a success rate increased year by year, even exceeding that of fresh embryo transfer and furthermore. FET significantly reduced the incidence of Ovarian Hyperstimulation Syndrome (OHSS) and improved the cumulative pregnancy rate, for which the safety of patients was guaranteed[1]. In China, the Hormone Replacement Therapy (HRT) is widely used in FET presently, as the administration is simple and the monitoring frequency, as well as the cycle cancellation rate is low. Usually, the HRT relies on the use of exogenous estrogen for inhibiting the Hypothalamic- Pituitary-Ovarian Axis (HPOA) and the supplement of exogenous estrogen may cause a positive feedback to the pituitary gland and increase the release of Follicle- Stimulating Hormone (FSH), thus resulting in follicular development. Therefore, there should be sufficient exogenous estrogen in the follicular stage[2]. It is also very important to monitor serum estrogen in the whole process for implementation of the plan. However, in the cycle monitoring, whether the estrogen level on the endometrial transformation day may influence the pregnancy outcome of patients seeking assisted reproduction is still controversial[3-6]. Therefore, appropriate estrogen and progesterone levels are one of the key factors for the success of FET pregnancy. This study retrospectively analyzed the estrogen level on the endometrial transformation day and pregnancy outcome for 772 cycles of FET, aiming to observe the relationship between the estrogen level on the endometrial transformation day and the pregnancy outcome in patients who underwent HRT-FET and to further evaluate relevant clinical effects.
Materials and Methods
Subjects:
The patients who received HRT-FET between January 2016 and January 2020 in the Reproductive Medicine Center, Maternal and Child Health Hospital of Shaoguan city and total 772 cycles were included.
Inclusion criteria: Age ≤38 y, with normal ovarian reserve, intrauterine membrane thickness ≥7 mm on transfer day.
Exclusion criteria: Serious uterine malformation, intrauterine adhesions, hydrosalpinx, endometriosis, repeated implantation failure or recurrent abortion, serious complications for medically related diseases etc. All the patients were required to sign an informed consent form.
Methods:
Grouping: The patients were grouped according to their Estradiol (E2) levels. Relevant data showed the average E2 level was 428.1 pg/ml, with median 270.3 pg/ml. The patients were divided into 5 groups: Group A (E2<150 pg/ml, 126 cycles); Group B (150≤E2<300 pg/ml, 318 cycles); Group C (300≤E2<450 pg/ml, 154 cycles); Group D (450≤E2<900 pg/ml, 115 cycles) and Group E (E2>900 pg/ml, 59 cycles). Age, Body Mass Index (BMI), infertility time, Anti-Mullerian Hormone (AMH) level, intrauterine membrane thickness, number of transplanted embryos, proportion of transplanted embryos and proportion of transplanted blastocysts on the day of embryo transfer and pregnancy outcome were compared among the groups.
Pregnancy outcome: Clinical pregnancy ratenumber of clinical pregnancies/cycles; Embryo implantation rate-the proportion of intrauterine pregnancy sacs in transplanted embryos as shown in color ultrasonography; First trimester abortion ratethe proportion of miscarriages without obvious causes before 13 w of pregnancy in clinical pregnancies.
Endometrium preparation plan for HRT cycle: In this study, an estrogen incremental method was used in the endometrium preparation for HRT cycle is as follows: d 2-4 of menstrual bleeding, estradiol valerate tablets were given orally (strength: 1 mg/tablet, Guangzhou Branch, Bayer Healthcare Co. Ltd., registration number: H20160679), 4 mg/d, 6 mg/d after 4-6 d. Serum E2 concentration monitoring and ultrasonographic endometrial thickness monitoring were performed. If the intimal thickness ≥7 cm, with duration of ≥9 d, intramuscular injection of progesterone 40 mg/d, is used for endometrial transformation. If the intimal thickness <7 cm, with duration of more than 21 d, the cycle would be given up. On d 3 after progesterone injection, blastocyst transfer was performed. For pregnancy, the hormone was supplemented continuously and reduced gradually from w 6 until w 8 when the medication was stopped.
Determination of serum E2 mass concentration: It is done by daily determination of endometrial thickness by ultrasonography and additional daily determination for injection of progesterone. For the patients, venous blood was taken at 8:00-9:00 pm and the hormone was determined by the fluorescence magnetic particle enzyme immunoassay method. For the serum E2 level, the myoglobin detection kit (ST AIA-PACK Myoglobin, Tosoh Corporation, Japan) E2 method was used. The intra-assay coefficients of variation were 5.18 % and 3.99 % respectively. The lower limit for sensitivity was E2>25 pg/ml.
Determination of pregnancy outcome: The embryo transfer was conducted under the guidance of transvaginal B-ultrasonography and 1 to 2 embryos were routinely transplanted (the embryo scores were based on Istanbul consensus)[7], followed by luteal support (progesterone, 60 mg/d, by intramuscular injection, or progesterone sustained-release vaginal gel, 90 mg/d, applied to vagina+dydrogesterone, 20 mg/d, orally). If the blood Beta-Human Chorionic Gonadotropin (β-hCG) on d 14 after the transfer exceeded 10.0 milliinternational units (mIU)/ml, the result was positive. The blood β-hCG was rechecked after 2 d. If it was doubled, a B-ultrasonography was performed on the vagina after 12 d and if the B-ultrasonography found pregnancy sac, clinical pregnancy occurred. The abortion that occurred before w 13 of pregnancy was called as first trimester abortion. If the pregnancy sac or even cardiac pulsation was observed outside the uterus, it was judged as ectopic pregnancy.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 23.0 software was used for statistical analysis. Qualitative data were expressed as percentage (%) and the chisquare test was used for comparison between groups. Quantitative data were expressed as mean±Standard Deviation (SD) (x̅±s) and the comparisons between groups were performed by independent sample t test. If p<0.05, the relevant difference was statistically significant.
Results and Discussion
For the comparison of general conditions among the 5 groups, there were no statistically significant differences in age, AMH, intrauterine membrane thickness, number of transplanted embryos, proportion of transplanted embryos and proportion of transplanted blastocysts on the day of embryo transfer (p>0.05) (Table 1).
| Parameters | Groups A | Groups B | Groups C | Groups D | Groups E | p value |
|---|---|---|---|---|---|---|
| Numbers | 126 | 318 | 154 | 115 | 59 | |
| Peak E2 value (pg/ml) | 115.32±25.07 | 224.57±40.29 | 357.33±42.41 | 604.64±127.97 | 1357.63±373.28 | <0.01 |
| Age (y) | 30.7±4.3 | 31.7±4.4 | 31.9±4.4 | 32.4±4.3 | 32.4±4.8 | NS |
| BMI (kg/m2) | 22.6±3.0 | 22.0±3.4 | 21.9±2.8 | 22.4±3.1 | 22.8±3.3 | NS |
| AMH | 3.3±2.5 | 3.7±2.6 | 3.6±2.7 | 3.4±2.5 | 3.4±2.7 | NS |
| Endometrial thickness on transformation day (mm) | 9.8±1.3 | 9.7±1.4 | 9.3±1.3 | 8.8±1.3 | 8.7±1.3 | NS |
| Number of embryos transferred | 1.47±0.53 | 1.50±0.53 | 1.55±0.51 | 1.61±0.56 | 1.63±0.55 | NS |
| Day of embryo transfer (%) | ||||||
| 5 | 40.0 % (74/185) | 35.1 % (167/476) | 38.2 % (91/238) | 36.8 % (68/185) | 38.5 % (37/96) | NS |
| 3 | 79.3 % (88/111) | 74.6 % (232/311) | 76.9 % (113/147) | 71.8 % (84/117) | 72.9 % (43/59) | NS |
Note: NS: Not Significant
Table 1: Comparison of General Conditions among Patients in Groups A to E
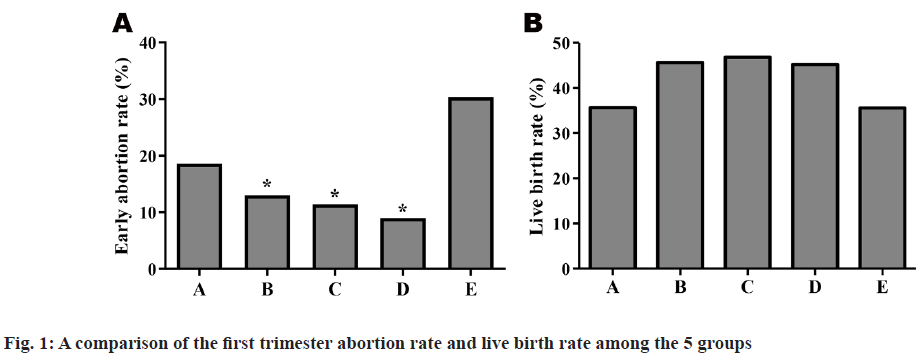
A pairwise comparison was conducted among the 5 groups. There were no statistically significant differences in clinical pregnancy rate, embryo implantation rate and ectopic pregnancy rate among the 5 groups (p>0.05), but the first trimester abortion rate of Group E (30.3 %) was higher than that of Groups B to D (12.98 %, 11.67 %, 8.21 %) obviously and the difference was statistically significant (p<0.05). The abortion rate of Group A (18.08 %) was higher than that of Groups B to D slightly and the difference was not statistically significant (p>0.05). A further comparison of live birth rate among the 5 groups showed that the rates in Group A (35.33 %) and Group E (35 %) were lower than in Groups B to D (45.46 %, 46 %, 47 %), but there was no statistically significant difference (p>0.05) shown in Table 2 and fig. 1.
| Parameters | Groups A | Groups B | Groups C | Groups D | Groups E |
|---|---|---|---|---|---|
| Numbers | 126 | 318 | 154 | 115 | 59 |
| Clinical pregnancy rate | 55.5 % (70/126) | 58.1 % (185/318) | 57.1 % (88/154) | 58.2 (67/115) | 55.9 % (33/59) |
| Implantation rate | 44.3 % (82/185) | 47.3 % (225/476) | 46.2 % (110/238) | 44.3 % (82/185) | 39.6 % (38/96) |
| Early abortion rate | 18.6 % (13/70) | 13.0 % (24/185) | 11.4 % (10/88) | 9.0 % (6/67) | 30.3 % (10/33) |
| Ectopic pregnancy rate | 2.9 % (2/70) | 1.6 % (3/185) | 3.4 % (3/88) | 1.5 % (1/67) | 3.0 % (1/33) |
| Live birth rate | 35.7 % (45/126) | 45.6 % (145/318) | 46.8 % (72/154) | 45.2 % (52/115) | 35.6 % (21/59) |
Note: Compared with E group, *p<0.05
Table 2: Comparison of Pregnancy Outcome Among Groups A to E
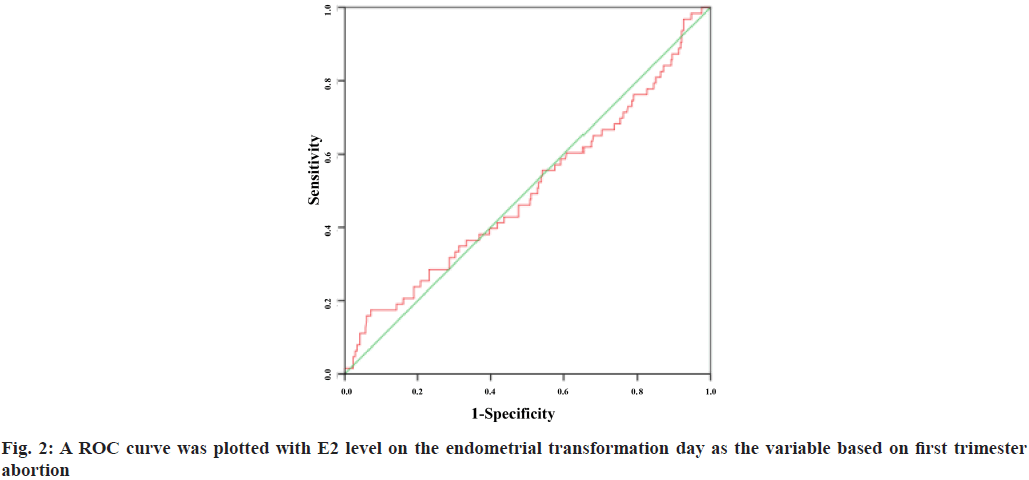
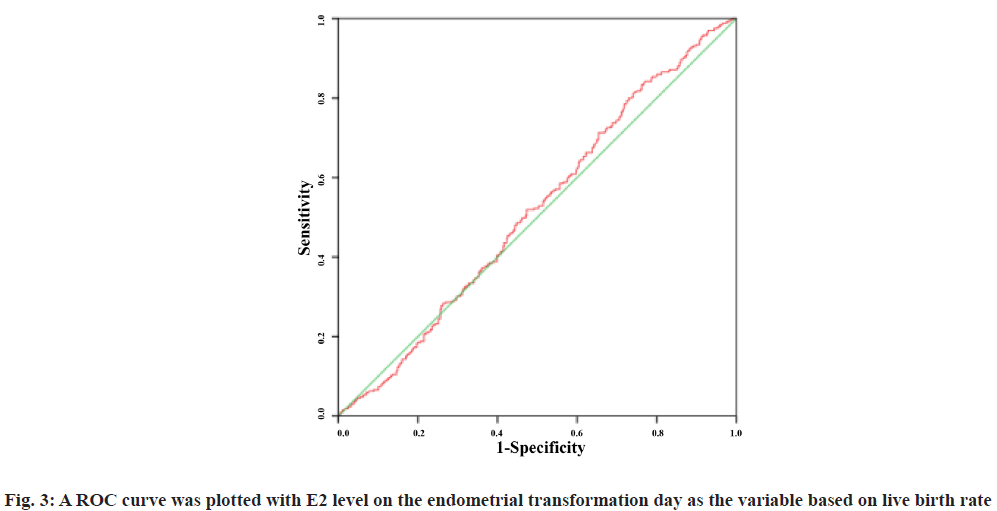
A Receiver Operating Characteristic (ROC) curve was plotted with E2 level on the endometrial transformation day as the variable based on first trimester abortion and live birth rate. The Area under the Curve (AUC) was 0.498 (95 % Confidence Interval (CI): 0.416-0.580) and 0.517 (95 % CI: 0.476-0.558) respectively. The results showed that E2 level failed to predict the first trimester abortion rate and live birth rate and there was no significant correlation between the two (fig. 2 and fig. 3).
The implantation of embryos is a process of interaction between maternal and fetal tissues, in which estrogen plays a key role. In the follicular stage, estrogen ripens the endometrium through inducing the progesterone receptor in the endometrium[8]. Through an experiment on ovariectomized rodents, Dey et al.[9] found that preconditioning with progesterone alone could not guarantee the successful implantation of blastocysts, but the implantation could succeed if a small amount of estrogen was used in advance[10]. However, does excessive estrogen affect the process of embryo implantation? Some studies have shown that excessive estrogen does have adverse effects. High estrogen level may cause trophoblast cell death and inhibit trophoblast cell reproduction in first trimester pregnancy[11]. In a test of baboons, elevated E2 had adverse effects on trophoblast reproduction and endometrial receptivity in first trimester pregnancy[12]. However, the influence of estrogen level on the endometrial transformation day on pregnancy outcome is still controversial clinically. Some studies have shown that high E2 level can cause a low pregnancy rate in In vitro Fertilisation (IVF) cycle[6]. Subsequently, Fritz et al.[3] grouped people according to their estrogen levels for study and the results showed that the live birth rate was 6 times lower in the first 10 percentiles with the highest E2 peak value than in the last 10 percentiles with the lowest E2 peak value. This suggests that elevated E2 level may adversely affect the synchronization of endometrium and embryo before progesterone transforms the endometrium. However, does excessively low E2 level on the endometrial transformation day have an impact? Remohi et al.[13] showed that low E2 level (<100 pg/ml) was not associated with adverse pregnancy rate in the oocyte cycle of artificial FET donor.
Salumets et al.[14] found that in the HRT-FET cycle, if the intimal thickness was 7-15 mm, the serum E2 level had no correlation with pregnancy outcome. Therefore, the patients with intimal thickness <7 mm, 15 d after medication were excluded in this study. Finally, the results of this study showed that in the HRT-FET cycle, high level of estrogen might be associated with the first trimester abortion and the first trimester abortion rate in Group E (E2>900 pg/ml) was significantly higher than that in Groups B to D (150≤E2<900 pg/ml), i.e. the difference was statistically significant. In addition, on the endometrial transformation day, if the estrogen level was in the range of 150-900 pg/ml and the live birth rate was relatively high; when the estrogen level <150 pg/ml or >900 pg/ml, the live birth rate showed a downtrend. Niu et al.[15]. evaluated the predictive value of E2 level in the HRT-FET cycle for pregnancy outcome and the results showed that elevated E2 level failed to predict pregnancy rate, which was consistent with the results of this study[16].
The causes of first trimester abortion after ART treatment are complicated and generally, people believe that more than 50 % of spontaneous abortions have different degrees and different types of chromosomal abnormalities of embryos[17,18]. Schieve et al.[19] showed that the In vitro Fertilization and Embryo Transfer (IVF-ET) abortion rate among women aged 20-29 y was 10.1 %, but increased by three times among those aged above 40 y. In this study, patients of elderly age, as well as patients with chromosomal abnormalities, past abortion history and repeated implantation failure were also excluded. In conclusion, in the HRT-FET, the serum estrogen level reflects the endometrial receptivity to a certain extent[20] and high level of estrogen may increase the risk of first trimester abortion, which should be noted in future clinical practice.
Acknowledgements:
Keyan Miao and XiaoLi Jia contirbute same to this work.
Conflicts of interest:
The authors declare that they have no conflict of interest.
Reference
- Zech J, Brandao A, Zech M, Lugger K, Neururer S, Ulmer H, et al. Elective frozen-thawed embryo transfer (FET) in women at risk for ovarian hyperstimulation syndrome. Reprod Biol 2018;18(1):46-52.
- Niu ZH, Feng Y, Sun YJ, Zhang HQ, Zhang AJ. Significance of serum estradiol monitoring in hormone replacement-frozen embryo transfer cycles. J Shanghai Jiaotong Univ Sci 2009;8:963-6.
- Fritz R, Jindal S, Feil H, Buyuk E. Elevated serum estradiol levels in artificial autologous frozen embryo transfer cycles negatively impact ongoing pregnancy and live birth rates. J Assist Reprod Genet 2017;34(12):1633-8.
- Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod 1995;10(9):2432-7.
- Forman R, Belaisch-Allart J, Fries N, Hazout A, Testart J, Frydman R. Evidence for an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertil Steril 1988;49(1):118-22.
- Arslan M, Bocca S, Arslan EO, Duran HE, Stadtmauer L, Oehninger S. Cumulative exposure to high estradiol levels during the follicular phase of IVF cycles negatively affects implantation. J Assist Reprod Genet 2007;24(4):111-7.
- "The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum Reprod 2011;26:1270-83.
- Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril 2011;96(3):530-5.
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 1994;120(5):1071-83.
- Yoshinaga K. Endocrinology of implantation. Maternal/fetal endocrinology. WB Saunders Company: Philadelphia 1994;20:336.
- Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biol Reprod 2015;93(3):74-1.
- Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 2006;27(4):483-90.
- Remohi J, Ardiles G, Garcia-Velasco JA, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod 1997;12(10):2271-6.
- Zenkert S, Schubert B, Fassnacht M, Beuschlein F, Allolio B, Reincke M. Steroidogenic acute regulatory protein mRNA expression in adrenal tumours. Eur J Endocrinol 2000;142(3):294-9.
- Niu Z, Feng Y, Sun Y, Zhang A, Zhang H. Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary?. J Exp Clin Assist Reprod 2008;5(1):1-5.
- Dong M, Qiu JH, Tan JC. Predictive value of serum estrogen level at HCG day to live birth rate in different age groups. J Reprod Med 2019;28(3):249-57.
- Carp H, Guetta E, Dorf H, Soriano D, Barkai G, Schiff E. Embryonic karyotype in recurrent miscarriage with parental karyotypic aberrations. Fertil Steril 2006;85(2):446-50.
- Huang Y, Su Y, Sun Yz, Guo Y. Clinical analysis of spontaneous abortion after assisted reproductive technology treatment. J Reprod Med 2008;17(4):299-300.
- Schieve LA, Tatham L, Peterson HB, Toner J, Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol 2003;101(5):959-67.
- Lee VC, Li RH, Ng EH, Yeung WS, Ho PC. Luteal phase support does not improve the clinical pregnancy rate of natural cycle frozen-thawed embryo transfer: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol 2013;169(1):50-3.