- *Corresponding Author:
- Juling Xu
School of Medicine, Huzhou Normal College, China
E-mail: xu_juling@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences |
| Indian J Pharm Sci 2022:84(4) Spl Issue “84-91” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study was based on the evaluation of the positive effect of M2c macrophages on liver tissue inflammation, pathological changes and tissue damage in lipopolysaccharide/D-galactosamine induced acute hepatitis. Meanwhile, whether M2c macrophages induced anti-inflammatory effects through Janus kinase/signal transducer and activator of transcription signaling pathway was also investigated. Forty institute of cancer research female mice in the same batch with no significant difference in body weight were distributed randomly into 5 different groups namely the control group, solvent control group, lipopolysaccharide/D-galactosamine, lipopolysaccharide/D-galactosamine+M2c and lipopolysaccharide/ D-galactosamine+M2c+ruxolitinib, 8 in each group. 6 h after the last treatment of mice in each group, serum was collected to detect the content of alanine aminotransferase and aspartate transferase. The pathology of liver tissue was determined using hematoxylineosin staining. The determination of the levels of expression of anti-inflammatory factors, pro-inflammatory factors, related apoptosis proteins and Janus kinase/signal transducer and activator of transcription signaling pathway related proteins in liver tissue were carried out by Western blot. After 6 h of induction with lipopolysaccharide/D-galactosamine, the liver function (alanine aminotransferase, aspartate aminotransferase), pathological score of liver slices, liver pro-inflammatory factors and anti-inflammatory factors (interleukin-6, tumor necrosis factor-alpha, interleukin-1beta and interleukin-10) were significantly improved in the mice blood. Comparative to the normal control group, apoptosis-related proteins (B-cell lymphoma-extra-large, B-cell lymphoma 2, Bcl-2- associated X protein) and other indicators were higher with a significant difference (p<0.05) under the acute hepatitis model induced lipopolysaccharide/D-galactosamine, after infusion of M2c macrophages from the tail vein of mice. The above indexes of mice were improved and exhibited a statistically significant difference (p<0.05). After adding ruxolitinib to intervene Janus kinase/signal transducer and activator of transcription signaling pathway on the basis of lipopolysaccharide/D-galactosamine+M2c group, the liver function, liver slice pathological score, pro and anti-inflammatory factor and apoptosis-related protein expressions were significantly higher as compared to the lipopolysaccharide/D-galactosamine+M2c group with significant difference (p<0.05), while the observed differences were not significant (p>0.05) in the lipopolysaccharide/ D-galactosamine group. Lipopolysaccharide/D-galactosamine induces the occurrence of acute hepatitis, which can well mimic the virus the pathogenic process of hepatitis. M2c macrophages exhibited a critical anti-inflammatory role in acute hepatitis via activating the Janus kinase/signal transducer and activator of transcription signaling pathway.
Keywords
Lipopolysaccharide/D-galactosamine hydrochloride, alanine aminotransferase, interleukin-6, tumor necrosis factor-alpha
The main pathogenic factor of acute hepatitis injury is related to the infection of the hepatitis virus and viral infections (such as Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV)) are the primary cause of endstage liver disease (liver cancer or cirrhosis)[1]. However, viruses are not the direct cause of cytopathic, hepatocyte death and associated inflammatory responses. The direct cause is the recognition of infected hepatocytes by activating virus-specific T cells[2]. Inflammation is ubiquitous in the human body, which can not only reduce and eliminate the damage caused by harmful factors to the human body, but also promote tissue repair in the later phase[3], serious adverse reactions, even life-threatening.
As the most important immune defense cells in inflammatory diseases, macrophages can release proinflammatory mediators to promote the formation of inflammatory factors[4]. The plasticity and functional polarization of macrophages exhibited a critical role in the inflammatory response[5]. In a specific microenvironment, macrophages can undergo the transformation of M1 and M2 phenotypes. M1 macrophages are capable to secrete various proinflammatory factors in the early stages of an inflammatory response, M1 macrophages can secrete a variety of pro-inflammatory factors including Interleukin-6 (IL-6), and Tumor Necrosis Factor-Alpha (TNF-α), etc. It has the ability to activate type I immune response, causing tissue cell damage and preventing the process of inflammatory response. In contrast, M2 macrophages are capable to secrete various inflammatory inhibitory factors. M2 macrophages are divided into different subtypes such as alternate activation (M2a), type II alternate activation (M2b) or acquired inactivation (M2c). The three have special surface markers and are capable to secrete various antiinflammatory factors and chemokine’s including IL- 10, IL-13 and CC Chemokine Ligand 17 (CCL-17)[6]. The results of multiple polarizations of macrophages can affect the outcome of many diseases. M2c macrophages are considered to be the main subtype that determines the initiation of inflammation[7] and its antiinflammatory factor IL-10 can inhibit inflammation through signaling pathways. The development of the response further promotes the self-healing process of tissue damage[6,8]. Various researches has described the possibility of the Janus Kinase (JAK)/Signal Transducer and Activator of Transcription 3 (STAT3) signaling pathway participating in certain physiological processes including cell differentiation, proliferation and apoptosis after liver injury by mediating the signal transduction of various anti-inflammatory and proinflammatory factors[9,10]. Ruxolitinib is a novel targeted drug that selectively inhibits JAK. It has been clinically used for targeted therapy of hematological diseases such as myelofibrosis[11]. Activation of the JAK2/ STAT3 pathway has a significant inhibitory effect[12].
The animal model of Lipopolysaccharide/DGalactosamine (LPS/D-gal) induced acute hepatitis is a representative model of viral acute hepatitis and its pathogenic mechanism is related to oxidative stress, stimulation, hepatocyte necrosis and inflammatory response. LPS is an endotoxin that activates inflammatory cytokines and can cause liver tissue damage[13]. D-gal is a hepatotoxic substance that mainly induces liver toxicity by inhibiting the hepatocytes and protein synthesis. D-gal increases the sensitization effect of LPS more than 1000 times[13]. Therefore, the liver injury model induced LPS/D-gal has been used excessively to better understand the underlying acute liver injury mechanism and discover new hepatoprotective agents. The combined application of LPS and D-gal increased the Reactive Oxygen Species (ROS) production. Excessive ROS can stimulate macrophages to produce pro-inflammatory cytokines, cause hepatocyte apoptosis and aggravate hepatic inflammatory damage[14].
This study intends to investigate whether the increase of M2c macrophages has a positive effect on the inflammation, pathological changes and tissue damage of acute hepatitis liver tissue in a mouse acute hepatitis model induced by LPS/D-gal combination. In the meanwhile, via inhibit JAK/STAT3 by ruxolitinib pathway; this study further verified whether M2c macrophages exert anti-inflammatory effects via the JAK/STAT3 signaling pathway.
Materials and Methods
Research objects:
The study was conducted on the Specific Pathogen-Free (SPF), Institute of Cancer Research (ICR) female mice. The mice aged 6-8 w, weighing 18-25 g and randomly divided into 8 mice in each group. The approval of this experiment was obtained by the hospital ethics committee.
Grouping, model preparation and drug intervention: Forty ICR female mice with no significant difference in body weight in the same batch were divided into 6 different groups (8 mice/group).
Normal control group: Without any treatment, injected with exactly the same proportion of normal saline as the LPS/D-gal group.
Solvent control group: Injected with the same proportion of solvent in the LPS/D-gal group without adding solute.
LPS/D-gal: Model group received intraperitoneal injection of LPS/ D-gal (LPS 40 μg/kg+D-gal 800 mg/ kg).
LPS/D-gal+M2c: In the model group intravenous infusion of 1×10-6 M2c macrophages.
LPS/D-gal+M2c+ruxolitinib: After the model group was established, 1×10-6 M2c macrophages were infused from the tail vein and ruxolitinib was administered orally after modeling (INCB018424) 180 mg/kg.
M2c macrophage preparation in this study: Macrophage-Colony Stimulating Factor (M-CSF) (50 ng/ml)+IL-10 (50 ng/ml) was added to macrophages (RAW 264.7).
Liver histopathological section preparation:
6 h after the last treatment of the mice in each group, the experimental mice were dissected, and about 30 mg of liver tissue (including the middle and edge parts) was taken, and then transferred to the embedding box with its the original structure not damaged as much as possible, and fixed in 10 % formaldehyde solution. Paraffin embedding, sectioning, Hematoxylin and Eosin (HE) staining, observation under the microscope, photographing and recording were performed. Tissue sections were scoring according to the severity of the pathological. A score range of 0-4 points is set and 3 fields of view are selected for each pathological section. The average value was calculated and the degree of inflammatory cell infiltration was the standard. More than 80 % were scored as 4 points; 60 % as 3 points; 40 % as 2 points; 20 % as 1 point; if there is no inflammatory cell infiltration it is counted as 0 points. The specific scores are calculated according to the actual test results.
Serological index detection:
6 h after the last treatment, the serum of each mouse in each group were collected by centrifuging blood sample of each mouse at 4°, 3000 r/min and 15°. The separated serum was tested in strict accordance with the instructions of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) detection kits.
Western blot detection of protein expression levels:
After 6 h of the last treatment, the experimental mice were dissected in each group and 30 mg of mouse liver tissue was taken in duplicate and labeled. Then, the tissue protein was extracted followed by the determination of the protein concentration via the Bicinchoninic Acid (BCA) method using the BCA protein quantitative analysis kit. Finally, the protein expression was detected by Western blot. For specific operation steps, please refer to the corresponding testing manual.
Research methods:
Test method: In order to study the mechanism and role of M2c macrophages in LPS/D-gal-induced acute hepatitis, we analyzed the following indicators, pathological evaluation includes mouse liver histopathological score; liver function evaluation includes serum ALT and AST levels; inflammation assessment includes detection of IL-6, IL-1β, TNF-α, IL-10 related inflammatory proteins in liver tissue; apoptosis assessment includes detection of B-Cell Lymphoma 2 (BCL-2), B-Cell Lymphoma-Extra- Large (BCL-xL), Bcl-2-Associated X Protein (BAX) related proteins in liver tissue and detection of JAK/ STAT3 signaling pathway includes detection of JAK and STAT3 proteins.
Statistical analysis:
For statistical analysis of the data, Statistical Package for the Social Sciences (SPSS) 20.0 software was used and the results were represented as mean±standard deviation (x̅±s). In different treatment groups, independent samples t test was used, while the comparison of the differences among multiple groups was carried out using a one-way analysis of variance, p<0.05 suggested a statistically significant difference.
Results and Discussion
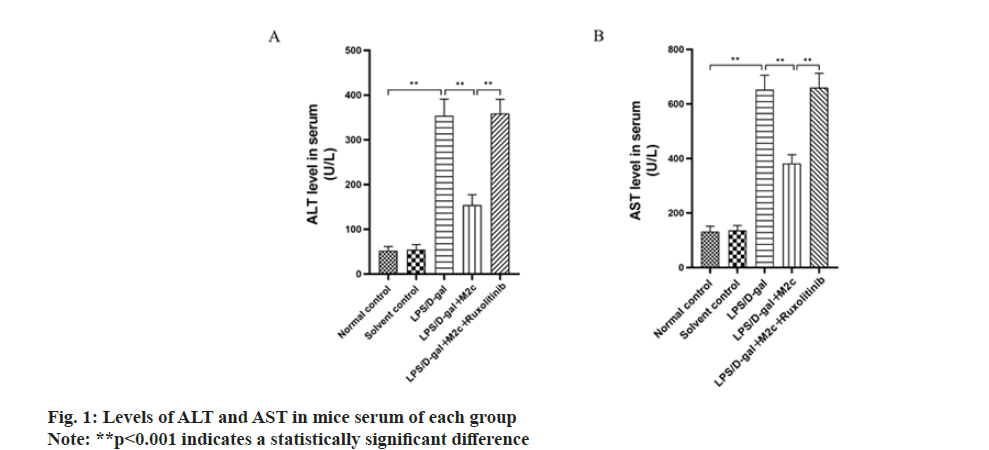
Each group was investigated for the serum levels of AST and ALT in mice. The study found that under the combined effect of LPS/D-gal, a significantly higher serum level of ALT and AST was observed in the LPS/Dgal group (354.2±36.7) U/l and (652.5±53.2) U/l, than that of normal control (52.3±9.2) U/l and (132.5±19.3) U/l, respectively (p<0.001). ALT and AST between the normal control and the solvent control groups did not exhibit a significant difference (p>0.05). As compared to the LPS/D-gal group, the ALT and AST levels of mice serum in the LPS/D-gal+M2c group were lower with a statistically significant difference (p<0.001). Further analysis revealed the increased level of ALT and AST of the mice after oral administration of ruxolitinib (LPS/D-gal+M2c+ruxolitinib group) upon comparison with the LPS/D-gal+M2c group, with a statistically significant difference (p<0.001) as shown in fig.1.
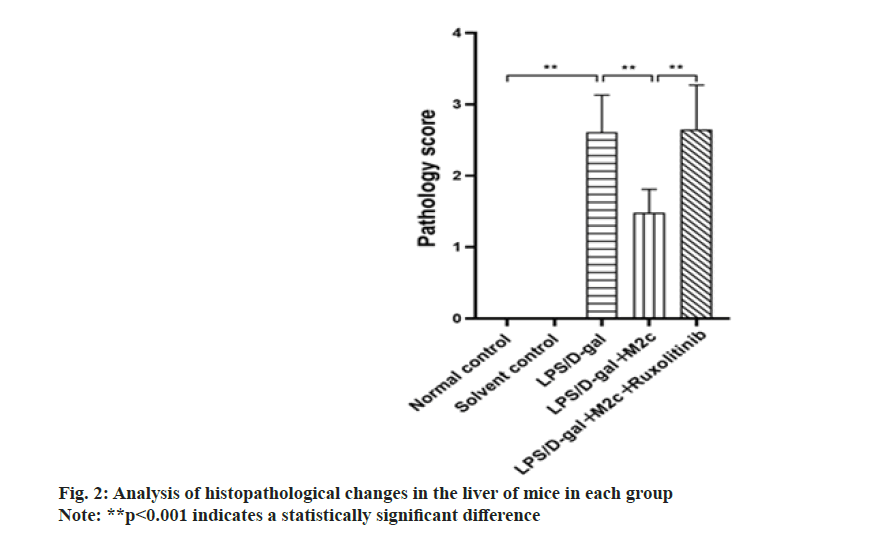
The pathological sections were performed on the mice liver tissues of each group, and the results exhibited no abnormal changes in the normal control group and the solvent control group. After 6 h of LPS/D-gal induction, the pathological sections of the liver in the LPS/D-gal model group showed, massive hemorrhage, partial liquefaction and necrosis of hepatocytes, partial vacuolar degeneration, infiltration of a huge extent of inflammatory cells in the local liver parenchyma, increased phagocytic cells in the hepatic sinus and histopathological changes such as individual vascular embolism. Upon comparison with the LPS/D-gal group, the mice in the LPS/D-gal+M2c group showed partial hemorrhage in the liver, decreased hepatocyte necrosis, decreased phagocytic cells in the hepatic sinus, and some histopathological changes such as vascular embolism. The histopathological sections of the liver in the LPS/D-gal+M2c+ruxolitinib group were similar to the LPS/D-gal group. Each group’s pathological sections of liver tissue were scored and then those scores were compared across groups. The observed average score of the LPS/D-gal group was (2.6±0.5) points, while the scores of the normal control and the solvent control groups were both 0 points. LPS/ D-gal group exhibited a significant difference upon comparison with control groups (normal control group and solvent control group) (p<0.001). The observed findings exhibited that the treatment of LPS/D-gal would cause severe case damage to liver tissue. As compared to the LPS/D-gal group, the scores of the liver histopathological sections of the mice in the LPS/ D-gal+M2c group were reduced. Statistical analysis showed that only the LPS/D-gal+M2c and the LPS/Dgal groups had significant differences in the scores of liver pathological sections (p<0.001). As compared to the LPS/D-gal+M2c group, the pathological section score of the LPS/D-gal+M2c+ruxolitinib group increased to (2.65±0.62) points with a significant difference (p<0.001), as illustrated in fig. 2. However, in the scores of liver pathological sections between the LPS/D-gal and LPS/D-gal+M2c+ruxolitinib groups, no significant difference was observed (p<0.05), indicating that the addition of M2c improved the liver damage of the mice, but the addition of ruxolitinib will inhibited JAK/STAT3 pathway. Thus, it was speculated that M2c no longer played an anti-inflammatory role after JAK/STAT3 pathway was inhibited. The results of liver pathological damage in the LPS/Dgal+ M2c+Ruxolitinib group were consistent with the LPS/D-gal group.
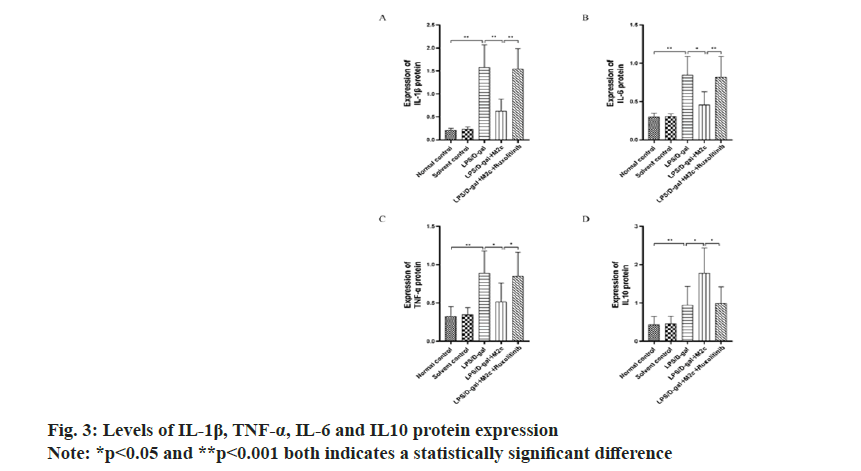
IL-6, IL-1β and TNF-α are the main inflammatory factors, and IL-10 is an anti-inflammatory factor. The results of the Western blot exhibited the increased expression of IL-1β, IL-6, TNF-α and IL10 in the LPS/ D-gal group upon comparison with the normal control group and the solvent control group with significant difference (p<0.001). Upon comparison with the LPS/ D-gal group, the decreased protein expressions of IL-1β, TNF-α and IL-6 were observed in the LPS/D-gal+M2c group (p<0.05), with a significant decrease (p<0.05) in IL-10 expression (anti-inflammatory factor). The result also showed the increased IL-1β, IL-6 and TNF-α expressions in the LPS/D-gal+M2c+ruxolitinib group as compared to the LPS/D-gal+M2c group, while the expression of IL10 was decreased with statistically significant difference (p<0.05). The results were described in fig. 3.
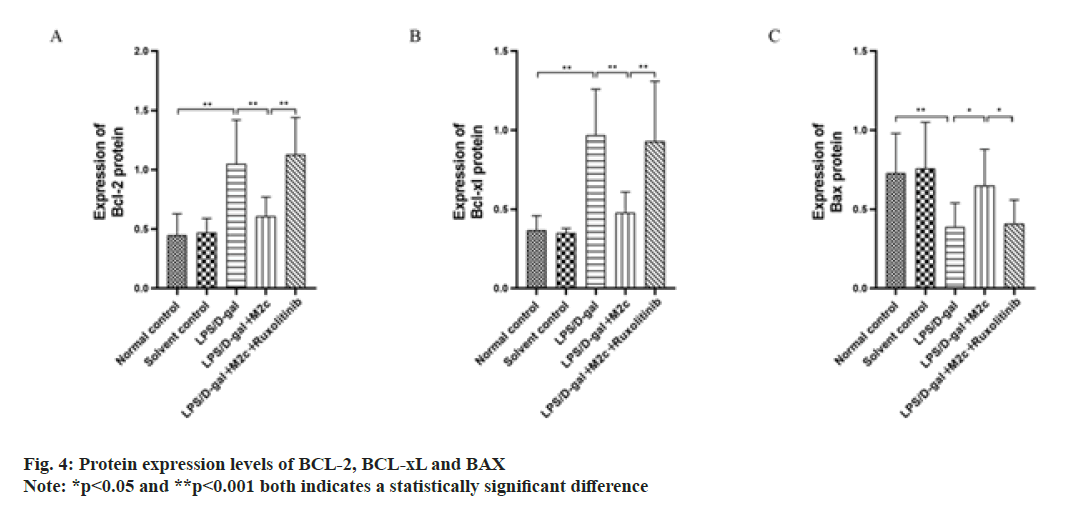
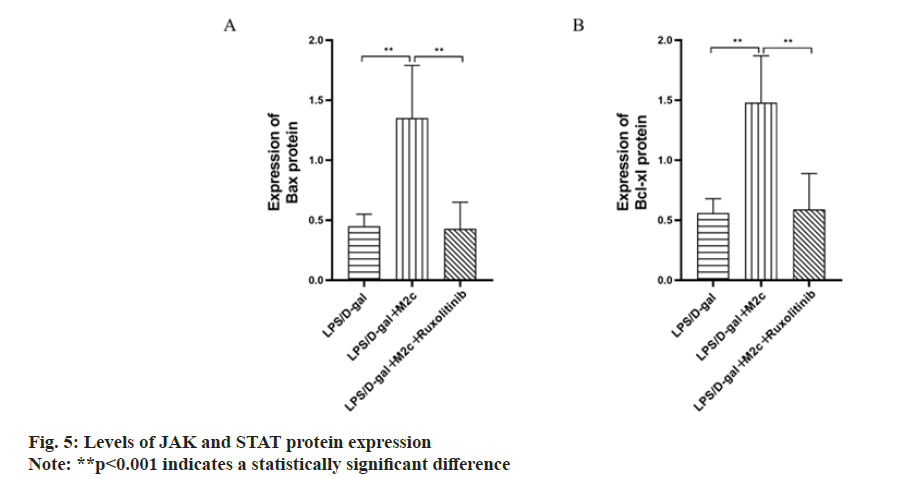
BCL-2, BAX and BCL-xL are the main proteins associated with apoptosis, among which BCL-xL and BCL-2 are anti-apoptotic proteins and can promote cancer cell proliferation, while BAX is a pro-apoptotic protein and can promote cancer cell apoptosis. The results of this study showed the increased Bcl-2 and BCL-xL protein expressions in the LPS/D-gal group upon comparison with the normal control group, while BAX expression was decreased with a statistically significant difference (p<0.001). As compared to the LPS/D-gal group, the BCL-2 and BCL-xL expressions in the LPS/D-gal+M2c group were both decreased (p<0.001) with increased protein expression of BAX (p<0.05). Upon comparison with the LPS/D-gal+M2c group, the protein expressions of BCL-2 and BCL-xL in the LPS/D-gal+M2c+ruxolitinib group increased with decreased BAX expression and exhibited statistically significant differences in the expression of the apoptosis-related proteins between the two groups (p<0.05) as described in fig. 4.
To further investigate whether the anti-inflammatory effect of M2c macrophages in mice with acute hepatitis is through the intervention of JAK/STAT3 signaling pathway, ruxolitinib (INCB018424) was added to inhibit the JAK/STAT3 signaling pathway in the acute hepatitis model in mice. The study found that compared with the LPS/D-gal group, the expression levels of JAK and STAT3 proteins in the LPS/D-gal+M2c group were increased with a statistically significant difference (p<0.001). After the addition of ruxolitinib (INCB018424) inhibition, the protein expression levels of JAK and STAT3 decreased to the level of LPS/D-gal group. Analysis of the previous results showed that after inhibiting the activity of JAK/STAT3 signaling pathway, the serum ALT and AST levels, liver histopathological sections, protein expressions of inflammatory and antiinflammatory factors and apoptosis-related protein expressions were all similar to those in the LPS/D-gal group with no statistical significance (p>0.05) as shown in fig. 5.
In response to environmental signals, macrophages are capable of switching between functional phenotypes. Classically activated macrophages (M1) have pro inflammatory effects and are important cellular components in anti-inflammatory, antibacterial and antitumor immunity. While alternative activated macrophages (M2) are present in the presence of IL-13 or IL-4 cytokines, especially its subtype M2c macrophages perform a series of physiological processes by producing anti-inflammatory factors such as IL-10, including anti-inflammatory, wound repair and tissue remodeling[6]. Some studies have found that[15], LPS/D-gal induces the synthesis and secretion of early pro-inflammatory factors including IL-1β, IL-6 and TNF-α, thereby inducing the incidence and progress of inflammatory responses. While M2c inhibits the occurrence of immune response by activating the expression of anti-inflammatory factor IL-10 in later phase of inflammation, hinders the excessive activation of inflammatory response and promotes the body to maintain homeostasis.
The JAK-STAT and activator of transcription signal transduction pathway is an important pathway that mediates the signal transduction of a variety of cytokines, and is involved in many aspects of the body’s inflammatory response, immune response and immune cell differentiation. The STAT3 signaling pathway has been shown in recent studies to play a vital role in the recovery from liver injury, which can promote hepatocyte proliferation, regulate carbohydrate and lipid metabolism, and activate the expression of hepatoprotective acute-phase proteins[16].
The JAK2/STAT3 signaling pathway can widely participate in the pathological processes including cell differentiation, proliferation and apoptosis after liver injury by mediating the signal transduction of various inflammatory factors[9]. Under the stimulation of inflammatory factors, JAK2 receptors are prone to phosphorylation, which activates JAK2 and further activates STAT3 and then mediates inflammation such as Interferon Gamma (IFN-γ), IL-1β, TNF-α and IL-6 by regulating gene transcription. At the same time, the overexpressed inflammatory factors can accelerate the phosphorylation of JAK/STAT3, thereby causing the occurrence and expansion of the inflammatory cascade[17].
The mechanism of action between M2c macrophages and the JAK/STAT3 signaling pathway is still unclear. Komohara et al.[18] elucidated that M2c macrophages inhibited the proliferation of glioma cells by activating the transcription factor STAT3. There are studies indicate[19] that in glioblastoma patients, inhibiting STAT3 not only inhibits the growth of tumor cells but also reverses tumor immune tolerance by weakening the immunosuppressive influence of M2c macrophages. Some studies[20] have also shown that the inhibition of the JAK/STAT3 signaling pathway may further inhibit the M2-type polarization of macrophages, that is, inhibit the activation of M2-type macrophages, so that anti-inflammatory factors cannot be expressed and functioned. In acute hepatitis induced by LPS/ D-gal, the relationship between the JAK/STAT3 signaling pathway and M2c macrophages and the mechanism of action are still unclear. Therefore, in this study, ruxolitinib was added to inhibit the JAK/ STAT3 signaling pathway and the mechanism of action between the two was explored by comparing the changes of related indicators in mice with acute hepatitis before and after adding ruxolitinib.
The finding of this study revealed that after 6 h of induction with LPS/D-gal, the blood liver function (ALT, AST), liver slice pathological score, liver proinflammatory factors and anti-inflammatory factors (IL-1β, TNF-α, IL-6 and IL10) and its apoptosis-related proteins (BCL-2, BCL-xL, BAX) etc., were higher as compared to the normal control group, indicating that LPS/D-gal induces the occurrence of acute hepatitis, which can effectively mimics the pathogenic process of viral hepatitis. Under the LPS/D-gal-induced acute hepatitis model, after infusion of M2c macrophages from the tail vein of mice, all the above indicators in mice were improved, indicating that M2c macrophages have a positive impact on the inflammatory response of mice.
After adding ruxolitinib (INCB018424) on the basis of LPS/D-gal+M2c group to inhibit JAK/STAT3 signaling pathway, the liver function, liver slice pathological score, pro and anti-inflammatory factors and expression of apoptosis-related proteins were significantly different from those in LPS/D-gal+Mac group (p<0.05). However, there was no statistically significant difference compare to LPS/D-gal group (p>0.05), indicating that after inhibiting the activity of the JAK/STAT3 signaling pathway, even adding M2c macrophages did not improve the inflammatory response of the mice. Therefore, this study speculates that M2c macrophages play an anti-inflammatory role in acute hepatitis through the JAK/STAT3 signaling pathway. Once this pathway is inhibited, M2c macrophages cannot be activated.
In conclusion, this study established an acute hepatitis model by injecting LPS/D-gal into the tail vein of mice and successfully simulated the pathological process of clinical immune liver disease. To further explore the anti-inflammatory influence of M2c macrophages on mice with acute liver injury by regulating the JAK/ STAT3 signaling pathway, thereby inhibiting the inflammatory cascade.
Funding:
The study was supported by grants from public welfare application research program of Huzhou (2020GY39).
Conflict of interests:
The authors declared no conflict of interest.
References
- Doycheva I, Zhang T, Amjad W, Thuluvath PJ. Diabetes and hepatocellular carcinoma: Incidence trends and impact of liver disease etiology. J Clin Exp Hepatol 2020;10(4):296-303.
[Crossref] [Google Scholar] [PubMed]
- Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharm Res 2019;139:126-40.
[Crossref] [Google Scholar] [PubMed]
- Jaggi U, Matundan HH, Yu J, Hirose S, Mueller M, Wormley Jr FL, et al. Essential role of M1 macrophages in blocking cytokine storm and pathology associated with murine HSV-1 infection. PLoS Pathog 2021;17(10):e1009999.
[Crossref] [Google Scholar] [PubMed]
- Yi YS. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw 2016;16(6):337-43.
[Crossref] [Google Scholar] [PubMed]
- Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol 2019;106(2):345-58.
[Crossref] [Google Scholar] [PubMed]
- Daniel B, Nagy G, Czimmerer Z, Horvath A, Hammers DW, Cuaranta-Monroy I, et al. The nuclear receptor PPARγ controls progressive macrophage polarization as a ligand-insensitive epigenomic ratchet of transcriptional memory. Immunity 2018;49(4):615-26.
[Crossref] [Google Scholar] [PubMed]
- Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy 2016;9:101.
- Hajighasemlou S, Nikbakht M, Pakzad S, Azadbakht A, Muhammadnejad S, Mirmoghtadaei M, et al. Anti-inflammatory effect of mesenchymal stem cells on hepatocellular carcinoma in the xenograft mice model. Vet Med Sci 2022.
[Crossref] [Google Scholar] [PubMed]
- Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatol Treat 2020;31(1):33-40.
[Crossref] [Google Scholar] [PubMed]
- Owen KL, Brockwell NK, Parker BS. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers 2019;11(12):2002.
[Crossref] [Google Scholar] [PubMed]
- Griveau A, Wiel C, Ziegler DV, Bergo MO, Bernard D. The JAK1/2 inhibitor ruxolitinib delays premature aging phenotypes. Aging Cell 2020;19(4):e13122.
[Crossref] [Google Scholar] [PubMed]
- Song HT, Cui Y, Zhang LL. JAK2/STAT3 signaling pathway is involved in Ang II-induced proliferation of mouse vascular smooth muscle cells and the intervention effect of ruxolitinib. Med Animal Cont 2022;38(7):680-7.
- Fang H, Liu A, Chen X, Cheng W, Dirsch O, Dahmen U. The severity of LPS induced inflammatory injury is negatively associated with the functional liver mass after LPS injection in rat model. J Inflamm 2018;15(1):21.
[Crossref] [Google Scholar] [PubMed]
- Hu J, Ying H, Zheng Y, Ma H, Li L, Zhao Y. Alanyl-glutamine protects against lipopolysaccharide-induced liver injury in mice via alleviating oxidative stress, inhibiting inflammation and regulating autophagy. Antioxidants 2022;11(6):1070.
[Crossref] [Google Scholar] [PubMed]
- Wang DX, Wang H, Zhou Y. Research progress on the role of macrophage polarization in inflammatory diseases. Chin Med J 2017;12(9):1427-30.
- Chen XL, Meng Qi, Liu KX. The role of STAT3 signaling pathway in liver injury protection. World Chin J Digest 2014;22(8):1051-7.
- Levy DE, Darnell JE. Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3(9):651-62.
[Crossref] [Google Scholar] [PubMed]
- Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T, et al. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci 2012;103(12):2165-72.
[Crossref] [Google Scholar] [PubMed]
- Liu JT, Zhang SY, Wang KX. Infiltration of M2c tumor-associated macrophages in glioma and its clinical significance. Chin J Microinvasive Neurosurg 2021;26(5):201-4.
- Ma CF, Zhan XL, Sun ZC. JAK/STAT-based gardenia side inhibits M2 polarization of macrophages in vitro. Chin Pharm 2020;23(10):1899-904.