- *Corresponding Author:
- J. Gong
Key Laboratory of High Altitude Hypoxia Environment and Life Health, School of Medicine, Xizang Minzu University, Xianyang, Shaanxi 712082, China
E-mail: gongjiang1975@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “249-258” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To study the autophagy and mechanism of phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in the directional differentiation of mesenchymal stem cells and to provide basic research results for its application in medical field is the main objective of the study. The lung cells and bone marrow mesenchymal stem cells of mice were used as experimental materials. The experimental materials were divided into five groups according to the settings of no-disturbance, induction of differentiation, promotion of autophagy and inhibition of autophagy. The characteristics and changes of phosphatidylinositol-3-kinase/ protein kinase B/mammalian target of rapamycin signaling pathway-related signaling molecules, autophagy activity and differentiation direction of mesenchymal stem cells in five groups were studied. According to the experimental results, the average value of phosphatidylinositol-3-kinase signaling molecules in the lithium chloride group was 6.21 on the 1st d of culture and the average value in the Dickkopf-1 group was 5.75. It can be seen that the activation and inhibition of autophagy activity are related to phosphatidylinositol-3-kinase/ protein kinase B/mammalian target of rapamycin signaling. There is a clear correlation between the activation of signaling molecules in the pathway. The expression of signaling molecules in the phosphatidylinositol-3- kinase/protein kinase B/mammalian target of rapamycin signaling pathway can affect the autophagy behavior of mesenchymal stem cells. This shows that mesenchymal stem cells have great application potential.
Keywords
Autophagy, phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway, mesenchymal stem cells, lung cells
Mesenchymal Stem Cells (MSCs) are adult stem cells derived from biological mesoderm, which retain a high degree of self-renewal and multidirectional differentiation potential[1]. These cells have been used in regenerative medicine and tissue engineering as a possible source of cells with the ability of differentiation in different tissues[2]. The cells themselves can differentiate into osteogenic mesenchymal cells and adipogenic mesenchymal cells, and under appropriate induction operations, MSCs can also differentiate into cells of other lineages[3,4]. Other common lineage cells include nerve cells, cardiomyocytes and epithelial cells[5]. Since the discovery of MSCs, these have been studied in the field of treatment of various diseases. One representative area is acute respiratory distress syndrome, a disease that causes diffuse damage to the alveolar epithelium and the ability of MSCs to differentiate into other lineages has a targeted effect on this diffuse damage to the epithelial tissue.
However, MSCs have obvious defects in the medical treatment of other tissues, mainly because the MSCs do not stay in the tissue for a long time and have limited differentiation ability. Therefore, it is necessary to further study the directional differentiation of MSCs[6]. The differentiation of stem cells is highly related to its autophagy mechanism. Some studies have shown that the proliferation and directional differentiation of stem cells can be promoted or inhibited by regulating the autophagy activity of stem cells[7]. Autophagy is an important mechanism for ensuring the turnover of intracellular substances, a process that allows damaged proteins and organelles to be degraded and recycled. At the same time, autophagy is also considered to be related to some degenerative diseases and it is also closely related to the mechanism of tumorigenesis[8]. As an example, Liu et al. showed the protein involvement in cell autophagy in primary bone tumors [9].
Phosphatidylinositol-3-Kinase (PI3K)/Protein kinase B (Akt)/mammalian Target of Rapamycin (mTOR) pathway is one of the most signal transduction pathways and a vital regulator of cell proliferation, flourishing, apoptosis and autophagy[10-12]. According to the previous studies, this signaling pathway totally exists in follicles with the ability of follicular atresia and apoptosis regulation[11].
There is a theory that the autophagy of MSCs is affected by the PI3K/Akt/mTOR signaling pathway, so regulating the expression of the PI3K/Akt/mTOR signaling pathway and its related signaling molecules can affect the autophagy of MSCs, further affecting its directional differentiation ability[13].
This study aims to explore the role and mechanism of PI3K/Akt/mTOR signaling pathway in autophagy during MSCs directional differentiation and provide basic results for the application of MSCs in the medical field.
Materials and Methods
General information:
In this study, MSCs were treated by in vitro co-culture and lung cells were used as in vitro co-culture materials to experimentally regulate the effect of PI3K/Akt/ mTOR signaling pathway on autophagy during the differentiation of MSCs. In the experiment, mice were used as the cell source and the MSCs and lung cells were extracted for operation. All animals were handled in accordance with animal ethics standards during the experiment.
Research methods:
Endotoxin-injured lung cells and normal lung cells were extracted from mouse lungs and spinal cord and MSCs were extracted from mouse spinal cords as research materials. The reason for using bone marrow MSCs is that the cells have strong proliferation and differentiation ability and are easy for gene transfection[14]. At the same time, it’s easy extraction and low immunogenic properties are also beneficial for experiments. The current methods for isolating MSCs from bone marrow include density gradient centrifugation, immunomagnetic bead method and whole bone marrow adherence method, among which the density gradient centrifugation method will inevitably damage the micro-organisms of the original MSCs during the operation process, environment and growth factors, while the immunomagnetic bead method, although highly accurate, can cause excessive cell death during the operation, and the equipment cost is very high. Whole bone marrow adherence method has the characteristics of small cell damage, high purity and high activity[15]. In terms of operation method and cost, the whole bone marrow adherence method is also a relatively simple and low-cost method among the mainstream methods. Therefore, the whole bone marrow adherence method is used here for the isolation of MSCs. When extracting the spinal cord tissue of the mice, the mice were killed by dislocation and the hind limb bones, tendons and related connective tissues were isolated under sterile conditions, and the bone marrow cavity was exposed from the back. The extracted bone marrow suspension was centrifuged at low speed and transferred for culture. During this period, the morphology and growth of the cells were observed under a microscope and the cells cultured through the Passage 3 (P3) were used as seed cells for the experiment.
After the MSCs were extracted, the lung cells of the mice were also extracted. The mice were divided into two groups and 5 μg/g of endotoxin was injected into the trachea of one group to induce respiratory distress syndrome. After the mice of each group were quickly sacrificed by cardiac puncture and blood drawing, the lung tissue was excised, washed and digested in culture medium. The treated lung cells also need to undergo three processing steps: Density gradient centrifugation, red blood cell lysis and ethylenediaminetetraacetic acid rinsing. Among them, density gradient centrifugation can effectively separate lung cells and extract lung cells at different levels. This operation is achieved by using lymphocyte separation liquid and a horizontal centrifuge. After adding the separation liquid to the short tube, the digested and filtered lung tissue mixture is superimposed on the liquid separation layer and centrifuged. After centrifugation, the tube should be clearly divided into three layers. The upper layer is mainly plasma, the middle layer is lymphocyte separation fluid and the lower layer is red blood cells, granulocytes and platelets. Erythrocyte lysis is a method of removing erythrocytes in the stock solution with erythrocyte lysing solution without damaging nucleated cells. The specific antigens present on the surface of the red blood cells, the enzymes in the lysate will attack these antigens, causing the red blood cells to deform and swell until they are lysed. At the same time, non-enzymatic lysate can also be used to treat erythrocytes. Due to the special electronegativity, osmotic fragility and suspension stability of erythrocytes compared with cells, non-enzymatic lysate can identify and lyse erythrocytes through these characteristics. A small amount of other cell types are lysed during this process, which is normal.
After the required cells are extracted, an in vitro co-culture model for the experiment needs to be established. This model is mainly done using insert cell culture dishes. The device is a two-layer cell culture device that can be used to detect the invasive ability and related characteristics of cells[16]. The principle is that the cells to be tested are placed in a low-nutrient medium, another layer is provided with a high-nutrient medium and a matrigel that mimics the extracellular matrix is placed in the middle of the two layers. The ability of the cells to invade can be seen by observing the entry of the cells into the high nutrient solution layer. Here, lung cells are placed in the upper layer and MSCs are placed in the lower layer to create an environment in which stem cells differentiate into lung cells. There are five groups of in vitro culture models.
Blank group: This group cultured MSCs alone without adding other cells or substances.
Normal cell group: This group cultured MSCs and normal lung cells.
Damaged cell group: The group cultured MSCs and endotoxin-induced injured lung cells.
Lithium chloride group: The group cultured MSCs and endotoxin-induced injured lung cells, and lithium chloride was added at a concentration of 4 mmol/l to activate the Wingless-related integration site (Wnt) pathway, and affect the expression of PI3K/Akt/mTORrelated signaling molecules.
Dickkopf-1 (DKK-1) group: The group cultured MSCs and endotoxin-induced injured lung cells, and added 20 ng/ml of DKK-1 to inhibit the Wnt pathway and affect the expression of PI3K/Akt/mTOR-related signaling molecules.
Observation indicators:
Morphological observation: Using an inverted microscope to observe the cultured MSCs and their differentiation process, the bone marrow MSCs used in the experiment should be spindle-shaped and similar in shape to fibroblasts. In addition, a large number of bone MSCs should show a spiral growth under the microscope field[17].
Detection of surfactant markers: The expression of surfactant in MSCs was detected by flow cytometry. The detection method performs multi-parameter quantitative analysis on a single sample and its principle is to measure cell size, morphology, internal structure, internal spatial distribution, etc. through the scattering of fluorescent signals. Sorting by flow cytometry is faster and more accurate among current methods. In the assay, MSCs should express Cluster of Differentiation (CD) 20 and CD90, but not CD45.
Osteogenic differentiation ability of cultured MSCs: The osteogenic differentiation ability of bone marrow MSCs is one of its important research values. In the experiment, the MSCs were divided into an induction group and a control group. The osteogenic differentiation induction medium was used in the induction group and the medium was changed normally in the control group, and the morphological changes of the cells in the two groups were observed. Afterwards, it was stained with alkaline phosphatase and mineralized nodules, and its changes were observed under the microscope. Finally, the alkaline phosphatase concentration and enzyme activity of the lysate were determined.
Adipogenic differentiation ability of cultured MSCs: The adipogenic differentiation ability of bone marrow MSCs is one of its important research values. The cells were seeded on culture plates and divided into control group and induction group. The normal medium exchange operation was carried out in the cells of the control group and the adipogenic differentiation induction medium was added in the induction group. The medium was changed every 3 d and the cell morphology changes were observed under the microscope. After 12 d of induction, oil red O was used for staining and the cell morphology after staining was observed.
The related molecules of PI3K/Akt/mTOR signaling pathway was detected on the 1st, 3rd and 7th d after cell culture. Western blotting was used for detection and the signal molecules as detection objects included PI3K, AKT and mTOR. The expression of these signaling molecules can be used to understand the activation of PI3K/Akt/mTOR signaling pathway during the differentiation of MSCs to other cells[18].
The autophagy marker proteins of MSCs were detected on the 1st, 3rd and 7th d after culture in the laboratory. The detection method uses Western blotting, which can detect a specific target protein from a mixture of multiple proteins with high accuracy. The stem cell autophagy marker proteins used for observation here are microtubule-associated protein 1 alpha (α)/1 beta (β)-Light Chain 3 (LC3) I and LC3-II and Beclin-1 proteins.
LC3 dual fluorescent autophagy virus (monomeric Red Fluorescent Protein (mRFP)-Green Fluorescent Protein (GFP)-LC3) autophagy indicator marker and tracking autophagy protein changes. Changes in LC3 protein were followed on the 1st, 3rd and 7th d after culture using mRFP-GFP-LC3. The autophagy activity of cells can be judged according to the intensity and co-localization of red and green fluorescence.
Cell proliferation index: The changes in the number of cells were roughly evaluated by inverted phase contrast microscopy at daily intervals and the tetrazolium salt colorimetric experiment was performed on each experimental group on the 1st, 3rd and 7th d, and the cells in each group were calculated based on the results, growth and proliferation.
Index of cell invasion rate: For the 5 experimental groups, bone marrow MSCs were placed in the upper layer of the insert cell culture dish and lung cells were placed in the lower layer, and the interstitial and basement membrane structures were reorganized. After culturing in a cell incubator, the cell invasion rate was observed. The method for judging this index is the ratio of the sum of the number of cells migrated to the lower layer and attached to the lower surface of the terminal to the total number of cells before culture.
Statistical analysis:
The analysis software used in this study was Statistical Package for the Social Sciences (SPSS) 13.0 and (x̄±s) was used to indicate the measurement data involved in the study. The test method was t-test. The test result was p<0.05 to indicate statistical significance and p<0.01 to indicate the result of significant statistical significance.
Results and Discussion
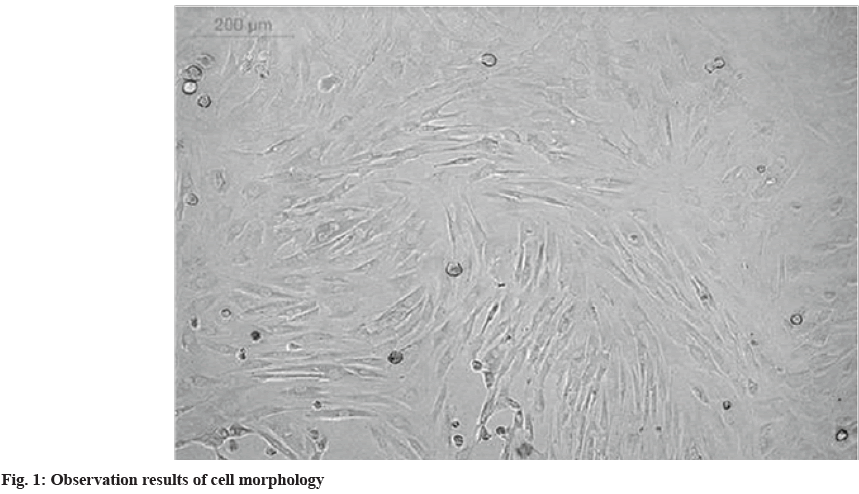
Identification results of the culture of MSCs were explained here. In the experiment, the whole bone marrow adherence method was used for the isolation of primary cell culture. Fig. 1 shows the situation when the cultured cells were digested and passaged to the 3rd passage. The cells in the figure were distributed evenly and the overall growth trend was swirling.
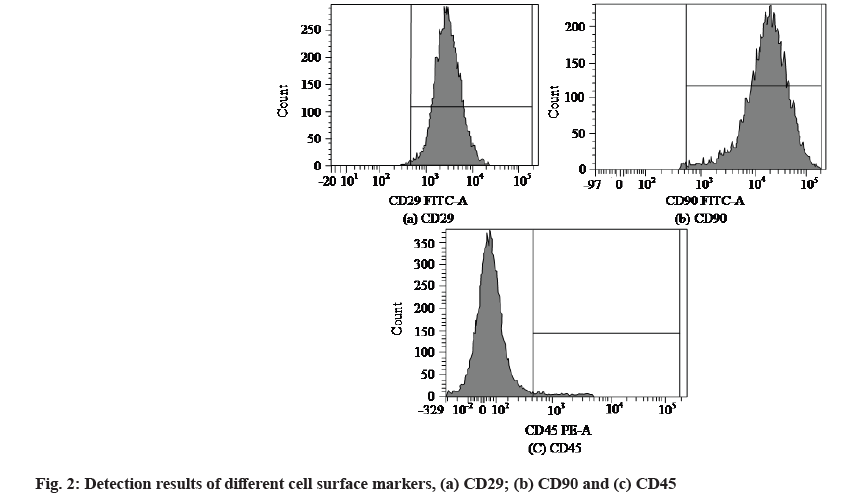
After direct observation of cell growth, cell surface markers were detected by flow cytometry and the detection results were shown in fig. 2. Fig. 2a is the detection result of CD29+, fig. 2b is the detection result of CD90+ and fig. 2c is the detection result of CD45- negative.
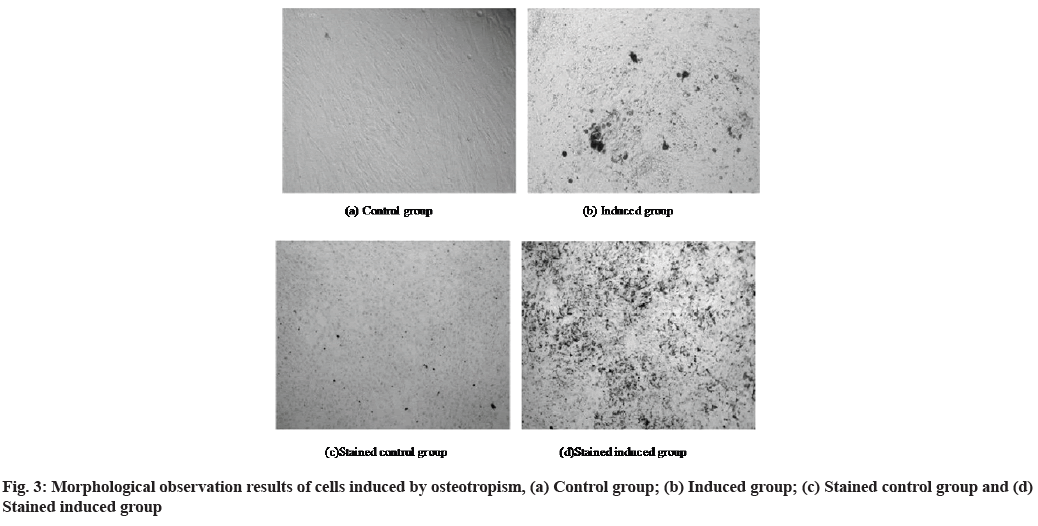
Identification results of directional differentiation ability of MSCs are as follows. Since the bone marrow MSCs were used in the experiment, the differentiation directions of these cells included osteogenic differentiation and adipogenic differentiation. The morphological observation results of the osteogenic differentiation process of bone marrow MSCs are shown in fig. 3. Fig. 3a shows the morphology of the cells in the control group at 12 d after the induction of osteotropism and fig. 3b shows the morphology of the cells in the induction group at 12 d after the induction of the osteopathy. Fig. 3c is the morphology of the cells in the control group after staining and fig. 3d is the morphology of the cells in the induction group after staining.
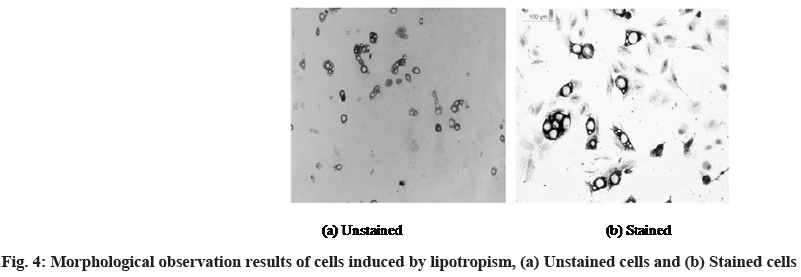
At the same time, the morphology of adipogenic differentiation of bone marrow MSCs was observed and the results are shown in fig. 4. Fig. 4a shows the shape of the cells in the induction group when they are not stained and fig. 4b shows the shape of the cells in this group after being stained with oil red O.
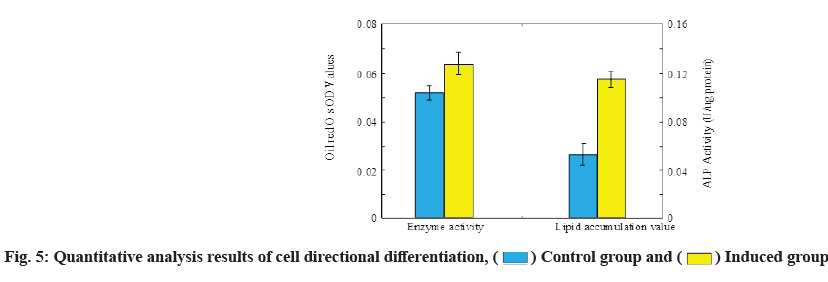
After the morphological observation of the two differentiation directions of MSCs, the cell differentiation was analyzed from a quantitative point of view. The analysis results are shown in fig. 5. During the analysis, mineralized nodule staining was used for bone orientation induction and a semi-quantitative detection method of residual dye solution was used for fat orientation induction.
Detection results of signal molecule activation of PI3K/Akt/mTOR signaling pathway is explained here. Table 1 shows the detection results of the activation of several pathways in different cell states and induction modes. It can be seen that both induction methods significantly affect the expression of MSCs, showing obvious inhibitory and promoting effects.
| Time (d) | Access | Blank group | Normal cell group | Injured cell group | Lithium chloride group | DKK-1 group |
|---|---|---|---|---|---|---|
| 1 | PI3K | 6.51±0.05 | 7.31±0.04 | 6.42±0.04 | 6.21±0.03 | 5.75±0.07 |
| AKT | 4.80±0.04 | 4.24±0.08 | 4.10±0.05 | 4.12±0.04 | 4.93±0.02 | |
| mTOR | 5.49±0.08 | 4.11±0.02 | 5.03±0.03 | 4.89±0.08 | 5.73±0.06 | |
| 3 | PI3K | 5.97±0.04 | 6.08±0.05 | 2.05±0.07 | 3.09±0.05 | 8.02±0.02 |
| AKT | 4.64±0.04 | 4.20±0.07 | 4.13±0.02 | 3.08±0.04 | 5.10±0.02 | |
| mTOR | 4.72±0.04 | 5.15±0.08 | 3.25±0.03 | 4.82±0.07 | 5.62±0.04 | |
| 7 | PI3K | 3.89±0.02 | 4.08±0.08 | 3.84±0.05 | 3.47±0.06 | 8.04±0.08 |
| AKT | 3.41±0.07 | 3.19±0.05 | 3.41±0.02 | 2.84±0.03 | 6.71±0.03 | |
| mTOR | 2.89±0.03 | 3.04±0.08 | 2.88±0.05 | 2.83±0.04 | 7.44±0.03 |
Table 1: Detection Results of Activation of Relevant New Signal Analysis of Several Channels
The changes of autophagy in MSCs under the influence of PI3K/Akt/mTOR signaling pathway is necessary to detect the expression of autophagy marker proteins at different times. The autophagy marker proteins involved in the detection include LC3-I, LC3-II and Beclin-1, and the detection results are shown in Table 2.
| Time (d) | MSCs autophagy marker protein | Blank group | Normal cell group | Injured cell group | Lithium chloride group | DKK-1 group |
|---|---|---|---|---|---|---|
| 1 | LC3-I | 0.29±0.07 | 0.40±0.07 | 0.45±0.02 | 0.46±0.06 | 0.20±0.04 |
| LC3-II | 0.28±0.03 | 0.34±0.06 | 0.39±0.01 | 0.40±0.07 | 0.22±0.04 | |
| Beclin-I | 0.29±0.05 | 0.32±0.03 | 0.41±0.02 | 0.51±0.05 | 0.19±0.06 | |
| 3 | LC3-I | 0.39±0.05 | 0.41±0.02 | 0.58±0.06 | 0.69±0.08 | 0.21±0.04 |
| LC3-II | 0.33±0.04 | 0.39±0.08 | 0.53±0.06 | 0.63±0.02 | 0.29±0.05 | |
| Beclin-I | 0.41±0.06 | 0.45±0.04 | 0.52±0.03 | 0.73±0.06 | 0.25±0.07 | |
| 7 | LC3-I | 0.50±0.04 | 0.45±0.06 | 0.69±0.03 | 0.98±0.24 | 0.38±0.04 |
| LC3-II | 0.59±0.05 | 0.62±0.03 | 0.72±0.05 | 1.11±0.03 | 0.39±0.13 | |
| Beclin-I | 0.54±0.03 | 0.60±0.07 | 0.71±0.06. | 1.01±0.02 | 0.41±0.02 |
Table 2: Expression of Autophagy Marker Proteins of MSCs
Tracking results of LC3 changes was shown here. In order to accurately track and visualize the changes of autophagy in MSCs, the changes of LC-3 protein were tracked by the mRFP-GFP-LC3 dual fluorescent autophagy indicator system marker, and the results are shown in fig. 6. In fig. 6, the 1st and 2nd rows are the detection results of GFP and RFP on the 3rd d, respectively, and the 3rd and 4th rows are the detection results of GFP and RFP on the 7th d, respectively. From left to right were blank group, normal cell group, DKK-1 group, damaged cell group and lithium chloride group. On the 1st d, there was little difference in the brightness of the groups, but on the 3rd d, the brightness of the DKK-1 group increased significantly.
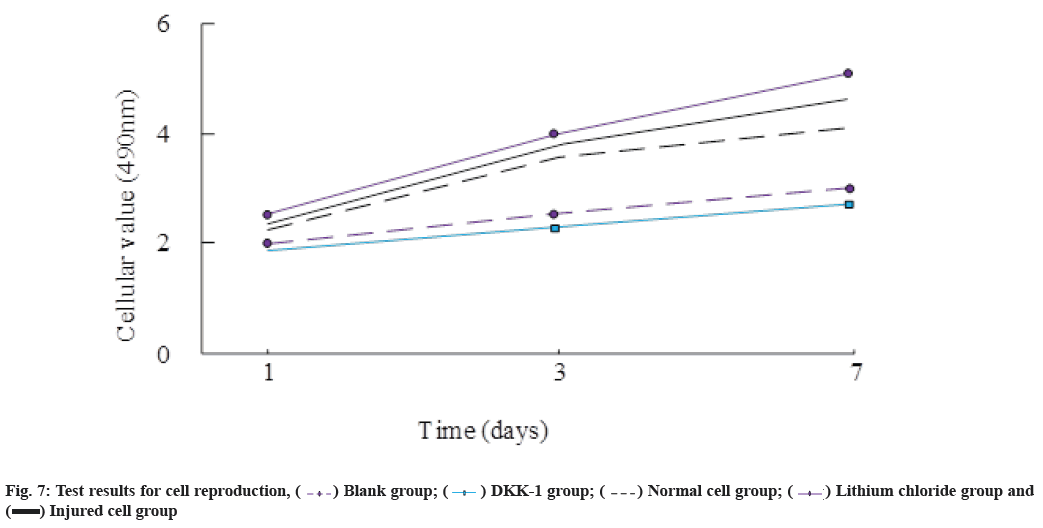
Test results of cellular reproduction are as follows. The experiment also monitored the changes in cell reproduction and recorded the test results on the 1st, 3rd and 7th d of the experiment for comparison. The results are shown in fig. 7. It can be seen that the cell value of the lithium chloride group is the highest, and the DKK-1 group is the lowest.
Finally, the cell invasion rate of MSCs was detected. This link can be used to analyze the ability of MSCs to differentiate into other cells. The measurement results are shown in Table 3.
| Time-12 h | ||
|---|---|---|
| Groups | Results | |
| Blank group | Cell invasion rate | 0 |
| p | - | |
| p* | <0.05 | |
| Normal cell group | Cell invasion rate | 16.77±1.21 |
| p | <0.05 | |
| p* | - | |
| Injured cell group | Cell invasion rate | 18.01±0.04 |
| p | <0.05 | |
| p* | <0.05 | |
| Lithium chloride group | Cell invasion rate | 19.25±0.31 |
| p | <0.05 | |
| p* | <0.05 | |
| DKK-1 group | Cell invasion rate | 19.02±0.05 |
| p | <0.05 | |
| p* | <0.05 | |
Note: p represents the result of the comparison between the corresponding group and the blank group and p* represents the result of the comparison between the corresponding group and the normal cell group
Table 3: Cell Invasion Rate Detection Results
MSCs have strong self-renewal ability and have multi-directional differentiation potential. Such cells can differentiate into osteogenic mesenchymal cells and adipogenic mesenchymal cells, and experiments have shown that artificial induction of such cells can also differentiate into neural cells and epithelial cells[19]. In this experiment, by observing the morphology of MSCs and the identification results of flow cytometry, it can be seen that the cell culture results are relatively successful and the characteristics of MSCs are very obvious. The differentiation direction induction experiment was carried out on the cultured stem cells and it was found that according to the different induction methods, the MSCs showed a very obvious differentiation direction. The alkaline phosphatase activity of the cells reached 0.114 under the osteoinduction and the average lipid accumulation value of the cells under the adipogenic induction reached 0.058 and in both induction directions, the response of MSCs to the dye was very obvious. The experimental results on the differentiation ability of MSCs are consistent with the theory. In the activity of MSCs, the ability of autophagy is indispensable. Autophagy facilitates the recycling of cytoplasm and accelerates organelle synthesis[20]. Through the mechanism of autophagy, MSCs can ensure the normal progress of their proliferation and differentiation functions. However, studies have shown that in general, the basal autophagy activity of MSCs in vivo is not high and the autophagy activity will gradually decrease during the process of directional differentiation induction, which leads to the loss of proliferation and proliferation of MSCs differentiation ability[21].
The autophagy activity of cells can be effectively regulated which is an important factor for fully applying the differentiation potential of MSCs for the treatment of diseases. There is a view that the autophagy activity of MSCs may be regulated by the PI3K/Akt/mTOR signaling pathway. Therefore, this study focused on the differentiation of MSCs into alveolar epithelial cells as the research content to observe the PI3K/Akt/ mTOR signaling is the effect of the pathway on this process[22]. In the experiment, the MSCs were set under different induction conditions and it was found that the expression of PI3K/Akt/mTOR signaling molecules in the injured lung cell group and the lithium chloride group was significantly down-regulated compared with the normal lung cell group. In addition, the PI3K/ Akt/mTOR signaling molecules in the DDK-1 group also showed significant differences from those in the normal lung cell group with the expression of AKT and mTOR-related signaling molecules on the 1st d being 4.93 and 5.73, respectively. The experimental results show that the expression of PI3K/Akt/mTOR pathway related signaling molecules in MSCs will show great differences under different induction environments. At the same time, observing the detection of cell reproduction and invasion rate in the experiment, it was found that the DDK-1 group had the smallest cell reproduction value and the lithium chloride group had significantly higher values than other groups. Induction environment and its differentiation performance showed obvious consistency with the expression of PI3K/Akt/mTOR pathway-related signaling molecules. Other related studies have reached the same conclusion as this experiment. During the process of activating the Wnt pathway and accelerating the differentiation of other MSCs, the expression of Phosphatase and Tensin homolog (PTEN) proteins of lithium chloride will increase, significantly up-regulated, that is, when MSCs differentiate into other cells, their differentiation ability is greatly affected by the PTEN-PI3K/Akt/ mTOR signaling pathway.
Other studies in this field have shown that various receptors, ligands and related components of the Wnt signaling pathway can therefore be expressed on MSCs[23]. In the lithium chloride-induced environment, the expression of autophagy-related proteins significantly increased during the differentiation of MSCs into alveolar epithelial cells[24]. At the same time, autophagy inhibitors can significantly reduce the differentiation of MSCs[25]. The results of this study indicate that the differentiation process of MSCs is affected by its autophagy mechanism. The higher the autophagy activity of MSCs within a certain range, the stronger the cell differentiation ability and the lower the autophagy activity, the weaker the cell differentiation ability. In this experiment, several autophagy marker proteins in MSCs were detected by Western blotting. The test results showed that the expressions of LC3-I, LC3-II and Beclin-1 proteins in the injured cell group and the lithium chloride group were significantly up-regulated compared with the normal cell group. On the 7th d, the Beclin-1 expression in the lithium chloride combined injury cell group was 1.0 and 0.71, respectively, while the Beclin-1 expression in the blank group was only 0.54. In contrast, in the DKK-1 group, the expression of the MSCs in this group was significantly inhibited on all three autophagy marker proteins. The DKK-1 group had a LC3-I expression of 0.2 on the 1st d and at the same time the blank group and normal cell group were 0.31 and 0.4, significantly higher than the DKK-1 group. At the same time, by observing the tracking effect of the dual fluorescent autophagy indicator labeling system on LC-3 protein, it is not difficult to see the changes of LC-3 protein in each group of subjects in different environments. On the 1st d, there was little difference in the brightness of each group. However, on the 3rd d, the brightness of the DKK-1 group was greatly improved and the damaged cells in the lithium chloride group were both dimmed.
On the 7th d, the indicator brightness of the five groups of research subjects has shown obvious difference, from bright to dark, they are DKK-1 group, blank group, normal cell group, damaged cell group and lithium chloride group. The DKK-1 group in which the autophagy marker protein was inhibited had the highest brightness and the lithium chloride group in which the expression of this protein was upregulated showed the lowest brightness. The experimental results show that the autophagy activity of MSCs has a direct impact on their differentiation behavior.
The research results were summarized and it was found that regulating the PTEN-PI3K/Akt/mTOR signaling pathway would significantly affect the autophagy activity of cells. The autophagy activity of MSCs affects their differentiation ability. Therefore, the PI3K/Akt/mTOR signaling pathway plays an important regulatory role in the directional differentiation and autophagy of MSCs. By up-regulating or down-regulating the expression of PI3K/Akt/mTOR signaling pathway-related molecules, the autophagy of MSCs shows differentiation.
Conflict of interests:
The authors declared no conflict of interest.
References
- Song Z, Lili W, Yangming O, Sining X, Huiying Y. Effects of mesenchymal stem cells-conditioned medium on senescence phenotype of polyploid lung cancer cells induced by docetaxel. China Clin Pract Med 2022;13(2):23-30.
- Haddadi M, Mousavi MJ. Regenerative medicine: Highlight on the significance of therapeutics with novel strategies. Int J Adv Biol Biomed Res 2020;8(4):370-87.
- Alasady MS, Kanj AEH, Kanj A. Periodontitis therapy with periodontal-derived stem cells (PDSCs) for dental tissue regeneration. J Med Chem Sci 2023;6(1):160-7.
[Crossref]
- Mingyue O, Lili W, Sining X, Al E. Effects of human umbilical cord mesenchymal stem cells and their conditioned medium on the proliferation, migration and apoptosis of non-small cell lung cancer polyploid A549 cells. Tumor Res Clin 2022;34:8-14.
- Deng L, Wu X, Zhu X, Yu Z, Liu Z, Wang J, et al. Combination effect of curcumin with docetaxel on the PI3K/AKT/mTOR pathway to induce autophagy and apoptosis in esophageal squamous cell carcinoma. Am J Transl Res 2021;13(1):57.
[Google Scholar] [PubMed]
- Xiping L, Guoning C, Peiqing L, Al E. Effects of Shenling Baizhu Powder and Tongxie Yaofang on the growth and proliferation of bone marrow and peripheral blood-derived mesenchymal stem cells in rats with ulcerative colitis. Zhonghua Chin Med J 2020;35:368-72.
- Tianyi L, Yang Y, Shanshan W, Suping Y, Jiaqi L, Jiaping W. Effect of bone marrow mesenchymal stem cells transplantation via renal artery on renal inflammation in experimental rats with adriamycin-induced chronic kidney disease. J Interv Radiol 2021;30(9):915-9.
- Jingxia L, Zhiqing L. Serum response factor promotes differentiation of rat bone marrow mesenchymal stem cells into smooth muscle cells via histone acetylation. J Third Mil Med Univ 2021;43(4):303-10.
- Liu W, Feng Z, Qu N, Li R, Niu Y. NUPR1 contribution to autophagy in primary bone tumor cells by regulating the Akt/mTOR signaling pathway. Acta Med Mediterr 2022;38(2):1223-8.
- Hamidipour N, Fazeli M, Hedayati M, Dehghani M, Gerami R. PI3K/Akt/mTOR and CDK4 combined inhibition enhanced apoptosis of thyroid cancer cell lines. Int J Adv Biol Biomed Res 2020;8(2):214-24.
- Bi HL, Miao CX. Effects of the combination of oestradiol valerate and medroxyprogesterone acetate in repairing premature ovarian failure by regulating PI3K/Akt/mTOR signalling pathway-related proteins. Acta Med Mediterr 2022;38:2269-73.
[Crossref]
- Deng CY, Mao WJ, Zhang PL. Oxiracetam exerts neuroprotective effect by regulating neuronal apoptosis and autophagy. Acta Med Mediterr 2022;38:275-80.
[Crossref]
- Warmink K, van Valkengoed DR, Versteeg S, Eijkelkamp N, Weinans H, Korthagen NM, et al. Mesenchymal stem cell derived extracellular vesicles as treatment for osteoarthritis in a rat high fat diet groove model. Osteoarthritis Cartilage 2021;29:S410-1.
- Liu XH, Zhao ZH, Zhou Y, Wang CL, Han YJ, Zhou W. Effect of ginsenoside Rg_1 in delaying premature ovarian failure induced by D-gal in mice through PI3K/Akt/mTOR autophagy pathway. Zhongguo Zhong Yao Za Zhi 2020;45(24):6036-42.
[Crossref] [Google Scholar] [PubMed]
- Chunlu Y, Xia P, Fangyu A, Al E. Effects of selenium-enriched astragaloside on mTOR signaling pathway in IL-1β-induced degenerated chondrocytes in rats. Chin J Osteoporos Bone Miner Res 2021;14:382-8.
- Junlong F, Haisong L, Jisheng W. Based on PI3K/Akt/mTOR signaling pathway to explore the mechanism of leech centipede medicine on improving diabetic erectile dysfunction in rats. J Beijing Univ Tradit Chin Med 2021;44:1118-25.
- Dong R, Bai Y, Xu J, Dong S. Mesenchymal stem cell/endothelial progenitor cell extracellular matrix-based tissue engineering bone for the repair of rat femoral defects. Chin J Trauma 2020;36(9):837-46.
- Lemos VP, Rossi AM, Granjeiro JM, Calasans-Maia MD, Sartoretto SC, Sesterheim P, et al. Osteogenic potential of nanostructured carbonated hydroxiapatite microspheres associated with mesenchymal stem cell in vitro and in vivo. Cytotherapy 2021;23(4):29.
- Xiaowei B, Cuiping Z, Bingmin L, Kui M, Jie Y, Xiaobing F. Effects of exosomes derived from placental mesenchymal stem cells on high glucose-induced senescence in human dermal fibroblasts. J Third Mil Med Univ 2020;42(12):1163-73.
- Meiao T, Xuejun F, Min C. Experimental study of sinomenine combined with bone marrow mesenchymal stem cells to prevent Con A-induced immune liver injury in rats. China J Integr Med 2020;40:575-81.
- Bai TS, Li ZR, Huang P, Xu Y, Liu WH. The ameliorating effect of venlafaxine on the depressive symptoms of depression model rats and its effect on PI3K/Akt/mTORC1 signaling pathway in hippocampus. Chin J Integ Med Cardio Cerebrovasc Dis 2021;19(14):2348-52.
- Yan W, Rong L, Xiangdong Z. Immunohistochemical effects of Sishen Pills on PI3K/Akt/mTOR signaling pathway in colon tissue of rats with ulcerative colitis due to spleen-kidney yang deficiency. Chin J Lab Anim Sci 2021; 29:42-8.
- Minjing L, Li G, Ye C. Effects of Xuefu Zhuyu decoction-containing serum on hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells and PI3K/AKT/mTOR signaling pathway. Chin Med Bind J 2020;40:836-41.
- Zhao Z, Wang M, Shao F, Liu G, Li J, Wei X, et al. Porous tantalum-composited gelatin nanoparticles hydrogel integrated with mesenchymal stem cell-derived endothelial cells to construct vascularized tissue in vivo. Regen Biomater 2021;8(6):rbab051.
[Crossref] [Google Scholar] [PubMed]
- Jianan L, Mo K, Xiaoran L. The mechanism of ginsenoside CK inhibiting Aβ-induced microglial activation and inflammatory response based on PI3K/AKT/mTOR signaling pathway. Zhonghua J Tradit Chin Med 2021;36:4652-7.
 Induced group
Induced group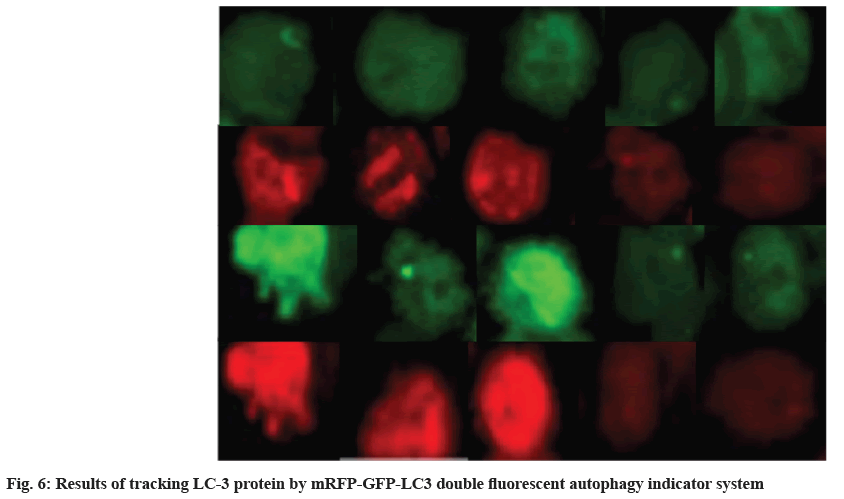
 DKK-1 group;
DKK-1 group;  Lithium chloride group and
Lithium chloride group and  Injured cell group
Injured cell group



