- *Corresponding Author:
- Yu Chen
Traditional Chinese Medicine Clinic, Tianjin Hospital of ITCWM Nankai Hospital, Nankai, Tianjin 300100, China
E-mail: chenyutmr@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “233-238” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Programmed cell death protein-1 and programmed death ligand-1 pathway activation play a vital role that can be modulated by tumor cells to blunt host immune surveillance. Periphery programmed cell death protein-1 expression level on cluster of differentiation 4+ and cluster of differentiation 8+ T lymphocytes of 185 female patients first diagnosed with triple negative breast cancer in Tianjin Medical University Cancer Institute and Hospital were analyzed by multicolor flow cytometry prior to any anti-tumor treatment. Patients who received chemotherapy alone for 2-8 cycles and received sequential samples were assessed with response evaluation criteria in solid tumors 1.1. Peripherally, the rate of cluster of differentiation 4+ programmed cell death protein-1+ and cluster of differentiation 8+ programmed cell death protein-1+ lymphocytes in general group were significantly higher than in the control group (p<0.0001). Post treatment programmed cell death protein-1 expression level on cluster of differentiation 4+ T lymphocytes and cluster of differentiation 8+ T lymphocytes was significantly lower than the baseline level in better response to neoadjuvant chemotherapy group (all p<0.05). In the contrary, programmed cell death protein-1 expression on both cluster of differentiation 4+ T lymphocytes and cluster of differentiation 8+ T lymphocytes showed no difference between pretreatment and post treatment in triple negative breast cancer patients with poor response to neoadjuvant treatment (all p>0.05). Peripheral cluster of differentiation 4+ programmed cell death protein-1+ and cluster of differentiation 8+ programmed cell death protein-1+ T lymphocytes of patients with triple negative breast cancer are associated with clinical response to neoadjuvant treatment.
Keywords
Triple negative breast cancer, T lymphocytes, programmed cell death protein-1, programmed death ligand-1
Triple Negative Breast Cancer (TNBC) which is a specific subtype of breast cancer with poor prognosis negatively express Estrogen Receptor (ER), Progesterone Receptor (PR) or Human Epidermal Growth Factor Receptor 2 (HER2) that constitutes 12 %-18 % of breast cancer patient[1]. Neoadjuvant Chemotherapy (NAC) is the standard treatment for early stage TNBC. However TNBC patients still have poor progression-free survival and overall survival due to chemotherapy resistance[2]. The interaction of Programmed Cell Death Protein-1 (PD-1)/Programmed Death Ligand-1 (PD-L1) results in activating the tumor immune escape, enhance tumor invasion and progression[3]. Anti-PD-1 agents and anti-PD-L1 agents activated the antitumor immune response which are associated with a better response rate and improved survival rate in different studies[4].
The anti-tumor effects of immune system is composed of T and B lymphocytes and Natural Killer (NK) cells[5,6]. Several studies reveal that higher level of Tumor-Infiltrating Lymphocytes (TILs) in the host microenvironment is closely related with better long-term prognosis and better response to antitumor treatment in different types of carcinomas[7,8]. While Cluster of Differentiation (CD) 4-positive (+) T lymphocytes act as T helper cells (Th cells) and CD8-positive (+) T lymphocytes shows cytotoxic function[9,10]. TNBC is the most immunogenic breast cancer subtype, compared with other subtypes. The rate of PD-1 and PD-L1 expression was absolutely higher in TNBC subtype, while T cells with PD-1 expressed character by “exhausted T cells”. In fact, the expression level of immune checkpoints like PD-1/PD-L1 on T lymphocytes in peripheral blood patients is also described here.
The purpose of this study was to investigate the character of PD-1 and PD-L1 expression level on T lymphocyte subsets in peripheral blood of patients with TNBC who received NAC to elucidate clinical significance.
Materials and Methods
Subjects:
A total of 185 patients diagnosed with TNBC, who primarily received NAC from 2020-2021 and without any previous treatment in Tianjin Hospital of ITCWM Nankai Hospital were obtained in this study. All patients received endoscopic tests and confirmed with TNBC histologically were included in the study. The exclusion criteria were as follows. Age<18 y or age>75 y; patients with Eastern Cooperative Oncology Group (ECOG) performance status scale>2; patients who received antitumor treatment previously; patients who has undergone blood transfusion; patients who were diagnosed with current or past history of other carcinomas; patients with autoimmune diseases (e.g. rheumatoid arthritis, systemic lupus erythematosus, hyperthyroidism or chronic liver disease); patients with infectious diseases (e.g. tuberculosis, bacterial, fungal or viral infections and hepatitis B or hepatitis C). The studies involving human participants were reviewed and approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital.
Tumor response to NAC:
All the patients received Magnetic Resonance Imaging (MRI) scan and ultrasonography to evaluate tumor response to NAC before and after every two cycles of NAC. Tumor response to NAC was analyzed by two clinical doctors independently by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). The end point of our study is pathological response to neoadjuvant chemotherapy in patients with TNBC. According to histopathological criteria for the assessment of therapeutic response in breast cancer (2007 version), the tumor response was classified in accordance with the RECIST v1.1. Complete Response (CR), Partial Response (PR), Stable Disease (SD), Progressive Disease (PD) were gathered and assessed based on modified European Group for Blood and Marrow Transplantation (EBMT) criteria.
Blood sample collection:
5 ml of heparinized peripheral blood samples of each patient with TNBC and healthy people were collected at the appointed time before treatment. Plasma samples of each person were obtained via separation through standard density gradient centrifugation within 30 min.
Flow cytometry:
Flow cytometry was used to analyze the phenotype of T lymphocytes which was performed using a Becton-Dickinson FACSCaliburTM flow cytometer. To identify the PD-1 expression of CD4+ and CD8+ subsets, cells were phenotypically characterized via incubation with relevant Fluorescein Isothiocyanate (FITC)-Phycoerythrin (PE) and monoclonal Antibodies (mAbs). Subsets were performed using the following markers, mouse anti-human CD3-FITC, mouse antihuman CD4-FITC, mouse anti-human CD8-FITC, PE mouse anti-human CD279 (PD-1), PE mouse antihuman CD274 (PD-L1).
Statistical analysis:
CD4+ and CD8+ lymphocytes were then analyzed for the percentage of PD-1 expression via comparison with the control. Data were obtained and analyzed using the Prism software. Two or more intergroup variable comparisons were performed using Statistical Package for the Social Sciences (SPSS) software (version 16.0) by student’s t-test and one-way Analysis of Variance (ANOVA). A 2-tailed p-value less than 0.05 was considered as statistically significant.
Results and Discussion
185 patients were involved in our study and all the patients received modified radical mastectomy or breast-conserving surgery. 185 patients lies within the range of 26-71 y, while 149 patients (80 %) were under 60 y old. 18 patients received 2 cycles of NAC, 135 patients received 4-6 cycles and 32 patients completed more than 6 cycles. 182 patients received taxane and anthracycline-based regimen sequentially or in combination and 28 patients received vinorelbine plus platinum based regimen when first line of treatment failed. Of 185 patients, 145 (78 %) neutropenia or neuropathy patients were of grade 1 or 2, while 64 thrombocytopenia patients were of grade 1 or 2. The rate of chemotherapy-induced grade 3 or 4, nausea and vomiting patients were 19 (10 %) of 182 patients. 5 patients required regimen dose reduction of 20 %, due to circumstances including grade 3 or 4, neutropenia and thrombocytopenia, no discontinuation of treatment due to adverse effects were observed (Table 1).
| Category | Number (N) | Percentage (%) |
|---|---|---|
| Age | ||
| <60 y | 149 | 80.54 |
| ≥60 y | 36 | 19.46 |
| Pre-NAT tumor stage | ||
| cT1 | 21 | 11.35 |
| cT2 | 130 | 70.27 |
| cT3 | 31 | 17.30 |
| cT4 | 2 | 0.01 |
| Unknown | 1 | 0.01 |
| Pre-NAT nodal status | ||
| Node positive | 108 | 58.38 |
| Node negative | 76 | 41.08 |
| Unknown | 1 | 0.01 |
| Tumor grading | ||
| G1 | 2 | 0.01 |
| G2 | 148 | 80.00 |
| G3 | 35 | 18.91 |
| Post-NAT tumor stage | ||
| ypT0-T1 | 72 | 38.92 |
| ≥ypT2 | 91 | 49.19 |
| Unknown | 22 | 11.89 |
| Post-NAT nodal status | ||
| ypN0-N1 | 121 | 65.41 |
| ≥N2 | 63 | 34.05 |
| Unknown | 1 | 0.01 |
| Ki-67 index | ||
| <14 % | 76 | 41.08 |
| ≥14 % | 109 | 58.92 |
Note: T0: No evidence of cancer; T1: Tumor is smaller than 20 mm; T2: Tumor is larger than 20 mm; T3: Tumor is larger than 50 mm; T4: Tumor has grown into the chest wall; skin and inflammatory breast cancer; G1: Tumor cells are well-differentiated; G2: Moderately differentiated; G3: Poorly differentiated; N0: No regional lymph node metastasis; N1: Cancer has spread to 1 to 3 regional lymph nodes and N2: Cancer has spread to 4 or more regional lymph nodes and yp: Neoadjuvant pathologic stage groups
Table 1: Clinicopathological Characteristics of Patients with TNBC
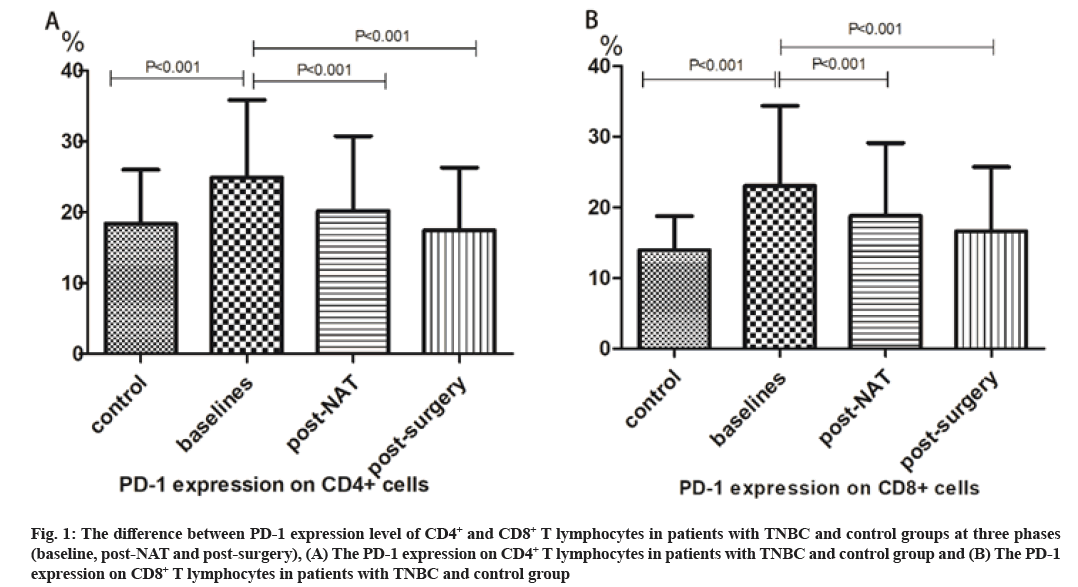
Predictive value of the CD4+PD-1+ and CD8+PD-1+ T lymphocytes count in peripheral blood was explained here. The peripheral percentage of PD-1+CD4+ T lymphocytes was 24.9 %±10.9 % at baseline in general TNBC group was significantly higher compared with control group (18.4 %±7.6 %). Similarly, PD-1+CD8+ T lymphocytes level in peripheral blood at baseline was 23.0 %±11.3 % which were higher compared with control group (14.0 %±4.8 %). PD-1 expression level on CD4+ lymphocytes (at baseline vs. post-surgery: 24.9 %±10.9 % vs. 17.5 %±8.9 %) and CD8+ lymphocytes (at baseline vs. post-surgery: 23.0 %±11.3 % vs. 16.6 %±9.1 %) decreased significantly after receiving surgery (fig. 1A and fig. 1B).
Fig. 1: The difference between PD-1 expression level of CD4+ and CD8+ T lymphocytes in patients with TNBC and control groups at three phases (baseline, post-NAT and post-surgery), (A) The PD-1 expression on CD4+ T lymphocytes in patients with TNBC and control group and (B) The PD-1 expression on CD8+ T lymphocytes in patients with TNBC and control group
Predictive value of the CD4+PD-1+ and CD8+PD-1+ T lymphocytes count for early tumor response was explained here. 26 patients were confirmed with pathologic CR after surgery. 81 patients achieved clinically PR with no pathologic CR after surgery. 69 patients experienced SD and 9 patients had PD. In the subgroup analysis, assessed in response to Neoadjuvant Therapy (NAT), the percentage of PD-1+CD4+ and PD-1+CD8+ T lymphocytes decreased after NAC in CR group and PR group (both p value<0.001). The percentage of CD4+PD-1+ and CD8+PD-1+ T lymphocytes show no significant difference between baseline and post-NAT in SD and PD subgroups (p>0.05) as shown in Table 2.
| Group | N | PD-1+CD4+ | p | PD-1+CD8+ | p |
|---|---|---|---|---|---|
| CR group | 26 | ||||
| Baseline | 26.3±11.3 | 23.7±11.2 | |||
| Post-NAT | 14.8±9.0 | <0.001 | 12.9±7.5 | <0.001 | |
| Post-surgery | 13.0±6.8 | 0.07 | 12.1±6.9 | <0.001 | |
| PR group | 81 | ||||
| Baseline | 24.2±11.3 | 22.4±11.5 | |||
| Post-NAT | 18.0±9.4 | <0.001 | 16.9±9.5 | <0.001 | |
| Post-surgery | 15.6±8.0 | <0.001 | 15.1±8.1 | <0.001 | |
| SD group | 69 | ||||
| Baseline | 25.7±10.9 | 24.2±11.3 | |||
| Post-NAT | 24.7±10.9 | 0.19 | 23.1±10.9 | 0.12 | |
| Post-surgery | 21.1±9.5 | <0.001 | 20.1±9.9 | <0.001 | |
| PD group | 9 | ||||
| Baseline | 20.8±7.8 | 18.6±10.2 | |||
| Post-NAT | 21.1±9.0 | 0.80 | 20.4±7.7 | 0.22 | |
| Post-surgery | 19.3±6.1 | 0.42 | 17.2±7.5 | 0.56 |
Table 2: The Percentage of PD-1+CD4+ and PD-1+CD8+ T Lymphocytes Associated with Clinical Response to NAC
Several immune checkpoint proteins expressed in most solid tumors, including breast cancer, lung cancer and renal cell cancer may be associated in inhibiting selfimmune responses[11,12].
In TNBC, PD-1 and PD-L1 expression has been reported frequently than other subtypes of breast cancer, while PD-1 inhibitor like pembrolizumab already has begun to show anti-tumor activity in this phase I trial[13]. In this famous phase III, randomized IMpassion130 trial, TNBC patients treated with atezolizumab combined with nab-paclitaxel had better progression-free survival, compared with placebo plus nab-paclitaxel in PDL1 positive subgroup[2]. A new era with breast cancer immunotherapy was opened using anti-PD-1 and anti- PD-L1 immunotherapies to treat TNBC patients.
Higher rate of TILs has been demonstrated to be associated with better response to anti-tumor therapy and better survival outcomes in breast cancer. Recently, some studies demonstrated that the tumor immune environment have been correlated with the effectiveness and prognosis of both immunotherapy and traditional anti-tumor therapy[14]. Although tumor biopsy samples are directly related with the tumor microenvironment, samples are difficult to obtain each two cycles of NAC. Peripheral blood biomarkers were detected in more investigates, which may be applied in the clinic. Absolute lymphocyte count may not predict the clinical response and effect in patients who received NAC accurately in various researches[15,16].
Periphery proliferating Kiel 67+ (Ki67+) CD8+ effectorlike T cells may be another biomarker associated with improved clinical outcomes of patients with Non-Small Cell Lung Cancer (NSCLC) who received anti-PD-1 immunotherapy[17]. In patients with prostate cancer, Kwek et al. demonstrated that improved overall survival was correlated with PD-1 on CD4 effector T cells (Teff) expression level at baseline[18]. Zgodzinski et al. revealed that PD-1 and its legend PD-L1 were expressed on T lymphocytes on tumor cells, peripheral blood and lymph nodes in gastric cancer[19]. In advanced breast cancer, 15 patients enrolled in one single arm phase II clinical trial received albumin bound paclitaxel intravenous and imiquimod cream topically to investigate the modulation of TILs[20].
The level of T lymphocytes in peripheral blood is intrinsically dynamic in response to NAT. The aim of this study was to detect the frequencies of PD-1+ expression on CD4+ and CD8+ T lymphocyte in TNBC patients who received NAC. Several studies prove that up-regulation of PD-1 expressed in T lymphocytes were activated by PD-1/PD-L1 pathway which may inhibit T cell proliferation during the interaction of T cells, which may lead to immune functional defect in TNBC patients[21].
In our study, the first objective is to assess the difference between pretreatment PD-1 expression level and postsurgery PD-1 expression level. The second objective is to assess the association of PD-1 expression level with Polymerase Chain Reaction (PCR) rate. In result, this trial also revealed that peripheral baseline PD-1 expression level on CD4+ and CD8+ T lymphocytes was highly significant than post-NAT in CR subgroup. To the contrary, there is no difference between baseline level and post-NAT levels of peripheral PD-1 expression on CD4+ and CD8+ T cells in non-CR group.
According to our results, the PD-1 expression level of CD4+ and CD8+ T lymphocytes of patients with TNBC are significantly higher than healthy control group. The periphery PD-1 expression level on CD4+ and CD8+ T lymphocytes can also be detected in CR group who have better response to NAT.
In our research, CD4+PD-1+ and CD8+PD-1+ T lymphocytes as immunological biomarkers are identified as predictors in response to NAC in TNBC. PD-1 abnormal expression in peripheral T lymphocytes may predict T lymphocytes inhibition which will lead to impaired immune reaction in TNBC patients. More investigations are needed to find the relationship between peripheral blood components and clinical outcomes like progression-free survival and overall survival.
Ethical approval:
The study protocol was reviewed and approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital.
Author’s contributions:
Conception and design was done by Yu Chen and Li Zhang; administrative support was given by Yu Chen; provision of study materials or patients was given by Yu Chen; collection and assembly of data was done by Yu Chen and Li Zhang; data analysis and interpretation was done by Yu Chen and Li Zhang; manuscript writing was done by all the authors and final approval of the manuscript was given by all the authors.
Acknowledgements:
This study was supported by National Natural Science Foundation of China (81700183); National Natural Science Foundation of China (81602020) and Tianjin “131” innovative talents training project-second level candidate (2018).
Conflict of interests:
The authors declared no conflict of interest.
References
- Tella SH, Kommalapati A, Singhi RK. Cost-effectiveness in managing skeletal related events in breast cancer: A strategy of less-intense dosing schedule of bone modifying agents. Transl Cancer Res 2018;7(1):S81-4.
- Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med 2019;17(1):1-9.
[Crossref] [Google scholar] [PubMed]
- Barroso-Sousa R, Tolaney SM. Clinical development of PD-1/PD-L1 inhibitors in breast cancer: Still a long way to go. Curr Treat Options Oncol 2020;21(7):1-22.
[Crossref] [Google scholar] [PubMed]
- Wang J, Yang T, Xu J. Therapeutic development of immune checkpoint inhibitors. Adv Exp Med Biol 2020;1248:619-49.
[Crossref] [Google scholar] [PubMed]
- Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther 2017;25(8):1769-81.
[Crossref] [Google scholar] [PubMed]
- Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol 2016;13(4):228-41.
[Crossref] [Google scholar] [PubMed]
- Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. The emerging role of CD8+ tissue resident memory T (TRM) cells in antitumor immunity: A unique functional contribution of the CD103 integrin. Front Immunol 2018;9:1904.
[Crossref] [Google scholar] [PubMed]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17(12):e542-51.
[Crossref] [Google scholar] [PubMed]
- Caserta S, Borger JG, Zamoyska R. Central and effector memory CD4 and CD8 T-cell responses to tumor-associated antigens. Crit Rev Immunol 2012;32(2):97-126.
[Crossref] [Google scholar] [PubMed]
- Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+ CD28–T cells in immune disorders. Trends Mol Med 2012;18(8):446-53.
[Crossref] [Google scholar] [PubMed]
- Jin F, Wu Z, Hu X, Zhang J, Gao Z, Han X, et al. The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility. Cancer Biol Med 2019;16(1):38-54.
[Crossref] [Google scholar] [PubMed]
- Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: Opportunities and challenges. Immunotherapy 2016;8(7):821-37.
[Crossref] [Google scholar] [PubMed]
- Dawood S, Rugo HS. Targeting the host immune system: PD-1 and PD-L1 antibodies and breast cancer. Curr Opin Support Palliat Care 2016;10(4):336-42.
[Crossref] [Google scholar] [PubMed]
- Cassim S, Pouyssegur J. Tumor microenvironment: A metabolic player that shapes the immune response. Int J Mol Sci 2019;21(1):157.
[Crossref] [Google scholar] [PubMed]
- Music M, Prassas I, Diamandis EP. Optimizing cancer immunotherapy: Is it time for personalized predictive biomarkers? Crit Rev Clin Lab Sci 2018;55(7):466-79.
[Crossref] [Google scholar] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88(1):218-30.
[Crossref] [Google scholar] [PubMed]
- Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 2017;114(19):4993-8.
[Crossref] [Google scholar] [PubMed]
- Kwek SS, Lewis J, Zhang L, Weinberg V, Greaney SK, Harzstark AL, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab PD-1+ CD4 T cells and survival with ipilimumab treatment. Cancer Immunol Res 2015;3(9):1008-16.
[Crossref] [Google scholar] [PubMed]
- Zgodzinski W, Grywalska E, Zinkiewicz K, Surdacka A, Majewski M, Zakoscielny A, et al. Peripheral blood T lymphocytes are downregulated by the PD-1/PD-L1 axis in advanced gastric cancer. Arch Med Sci 2019;15(3):774-83.
[Crossref] [Google scholar] [PubMed]
- Salazar LG, Lu H, Reichow JL, Childs JS, Coveler AL, Higgins DM, et al. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: A phase 2 clinical trial. JAMA Oncol 2017;3(7):969-73.
[Crossref] [Google scholar] [PubMed]
- Katz H, Alsharedi M. Immunotherapy in triple-negative breast cancer. Med Oncol 2018;35(1):1-9.
[Crossref] [Google scholar] [PubMed]