- *Corresponding Author:
- J. Wu
Department of Infectious Diseases, Cangzhou Central Hospital, Cangzhou, Hebei Province 061001, China
E-mail: 18504176016@163.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “133-139” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To examine the therapeutic efficacy of Lianhua Qingwen granule combined with peramivir sodium chloride injection in treating influenza and level changes of serum inflammatory factors. The clinical data of 100 influenza sufferers enrolled in our infirmary from January 2018 to January 2020 retrospectively analyzed and they were randomized in the contrast group (n=50) and the experience group (n=50). The former was provided with peramivir sodium chloride injection, while the latter was medicated with Lianhua Qingwen granule after peramivir sodium chloride injection. The clinical efficacy, serum inflammatory factor, C-reactive protein, interleukin-6 level and procalcitonin level of both groups of sufferers were compared. The overall response rate of the injected group was 80 %, lower compared with 96 % of the injected and medicated group (p<0.05). The antipyretic time and sore throat disappearance time, cough disappearance time and general ache disappearance duration in the medicated group were remarkably shorter than those without Lianhua Qingwen powder (p<0.05). The differences in interleukin-6, C-reactive protein and procalcitonin levels before treatment between both groups were not statistically remarkable (p>0.05) and the levels of interleukin-6, C-reactive protein and procalcitonin of both groups of sufferers decreased after treatment. The levels of interleukin-6, C-reactive protein and procalcitonin of patients got injection and took medicine were remarkably lower than those who had no granule (p<0.05). Lianhua Qingwen granule combined with peramivir sodium chloride injection shows a remarkable potency in the treatment of influenza. It can shorten the treatment time and reduce the level of serum inflammatory factors. It is worthy of clinical promotion and application.
Keywords
Lianhua Qingwen granule, peramivir sodium chloride injection, influenza, serum inflammatory factors
Influenza is a branchy chronic respiratory infectious disease caused by influenza virus, which is seasonal epidemic, has a high incidence, develops rapidly, and easily causes spread and epidemic[1]. Influenza often occurs in groups with low immune function, such as children and the elderly. If the disease was found, it should be treated in time. If it was not treated and controlled in time, it would cause acute sinusitis, otitis media, myocarditis, nephritis and so on. Influenza virus hosts are widely distributed, sufferers and recessive infected persons are the main sources of infection and droplet transmission and contact transmission are the main transmission routes[2,3]. The main clinical symptoms are high fever, fatigue, headache, body ache, etc., and some topical symptoms such as nasal congestion, rhinorrhea, dry cough, discomfort behind sternum, facial flushing, conjunctival congestion, etc.,[4]. The therapeutic efficacy of Lianhua Qingwen granule combined with peramivir sodium chloride injection on influenza and level changes of serum inflammatory factors was researched in this paper.
Materials and Methods
General data:
The clinical data of 100 sufferers with influenza treated in our medical center from January 2018 to January 2020 were analyzed retrospectively and they were split into the contrast group (n=50) and the experience group (n=50). Among them, there were 21 males and 29 females in the first group, aged from (18-63) y, with a mean age of (45.2±5.7) y, a course of disease of 3 h to 42 h and an average course of disease of (23.5±3.2) h. In the second group, there were 24 males and 26 females, aged from (19-60) y, with a mean age of (45.4±5.6) y, with a course of 2 h to 40 h and an average course of (24.3±3.8) h. There was no remarkable difference in general data between both groups (p>0.05), with comparability.
Inclusion criteria:
Sufferers fitting in the diagnostic criteria of influenza; there are fever, cough, sore throat, fatigue and other symptoms; the onset time is within 48 h. Sufferers have certain cognitive and communication skills; this research is approved by the hospital ethics committee, sufferers and their families know the purpose and process of this experimental research and sign an informed consent form.
Exclusion criteria:
Sufferers complicated with lower respiratory tract infection; sufferers with severe heart, kidney, liver and other organs of severe dysfunction; sufferers with malignant tumors; sufferers with drug aller gy.
Method:
Sufferers in both groups underwent conventional examination. Professional medical staff used nasopharyngeal swabs to obtain sufferer’s nasopharyngeal secretions and professional examiners performed rapid influenza antigen detection. According to sufferer’s conditions, they were given cough-relieving, phlegm-reducing drugs and atomization, and cooperated with physical cooling. Sufferers in the contrasted group were treated with peramivir sodium chloride injection (Guangzhou Nanxin Pharmaceutical Co., Ltd., Bibliography of National Drugs: H20130029, specification: 100 ml/ bottle). The specific usage every day, 10 mg/kg each time, intravenous drip, continuous treatment for 5 d. In addition to peramivir sodium chloride injection the other group had Lianhua Qingwen granule (Beijing Yiling Pharmaceutical Co., Ltd., Bibliography of National Drugs as supplementary: Z20100040, specification: 6 g×10 bags), 6 g each time, three times in a day (tid), continuous treatment for 5 d.
Observation indexes and evaluation criteria:
The overall effectiveness, antipyretic time, sore throat disappearance time, cough disappearance time and general ache disappearance time between both groups were compared. The adverse drug reactions of both groups were compared. Enzyme- Linked Immunosorbent Assay (ELISA) was applied to detect the levels of serum inflammatory factors Interleukin (IL)-6 and Procalcitonin (PCT), and automatic biochemical analyzer was used to measure the content of serum C-Reactive Protein (CRP).
Evaluation criteria:
The sufferers were judged according to their symptoms, signs, recovery time of body temperature and improvement degree of syndrome scores. Syndrome scores mainly include cough, sore throat, fever through whole body, nasal congestion and other symptoms, with scores of 0, 1, 2 and 3 respectively according to severity. The more severe the symptoms, the higher the score.
Cured: After 48 h of treatment, the body temperature returns to normal, the symptoms and signs disappear completely and the condition is not repeated.
Remarkably effective: After 48 h of treatment, the body temperature returns to normal and the total scores of other symptoms and signs decrease by more than 2/3.
Effective: After 72 h of treatment, the body temperature returns to normal, but there will be repeated cases; the total scores of other symptoms and signs decreased by 1/3-2/3.
Ineffective: After 72 h of treatment, the total scores of symptoms and signs decrease by less than 1/3, and the condition does not improve and deteriorates.
Overall effective rate (%)=(Cured cases+remarkable effective+effective cases)/Total cases×100 %
Statistical methods:
In this research, Statistical Package for the Social Sciences (SPSS) 20.0 was selected as the data processing software and GraphPad Prism 7 (Graph pad Software, San Diego, United States of America (USA)) was used to draw this data. Chi-square (χ2) test is used for counting data and [n (%)] is used, while t test is used for measuring data and (x̄ ±s) is used. When p<0.05, the difference is statistically remarkable.
Results and Discussion
The granule increased the overall response rate from 80 % resulting from mere peramivir sodium chloride injection by 16 % in the combined treated group (p<0.05) as shown in Table 1.
| Groups | Cases | Cured | Remarkably effective | Effective | Ineffective | Overall effective rate |
|---|---|---|---|---|---|---|
| Contrast group | 50 | 30 | 12 | 6 | 2 | 48 (96.0 %) |
| Experience group | 50 | 21 | 9 | 10 | 10 | 40 (80 %) |
| χ2 | 6.0606 | |||||
| p | 0.014 |
Table 1: Comparison of the Overall Efficacy of Two Groups [n (%)]
The antipyretic time, sore throat disappearance time, cough disappearance time and general ache disappearance duration showed in the combined treated patients were remarkably shortened due to the medicine in the group (p<0.05) as shown in Table 2.
| Groups | Cases | Antipyretic time | Sore throat disappearance time | Cough disappearance time | General ache disappearance time |
|---|---|---|---|---|---|
| Contrast group | 50 | 35±6 | 67±5 | 64±4 | 38±6 |
| Experience group | 50 | 23±5 | 47±4 | 46±3 | 26±3 |
| T | 10.8643 | 22.0863 | 25.4558 | 12.6491 | |
| p | 0 | 0 | 0 | 0 |
Table 2: Comparison of The Disappearance Time of Symptoms between both Groups (x̄±s)
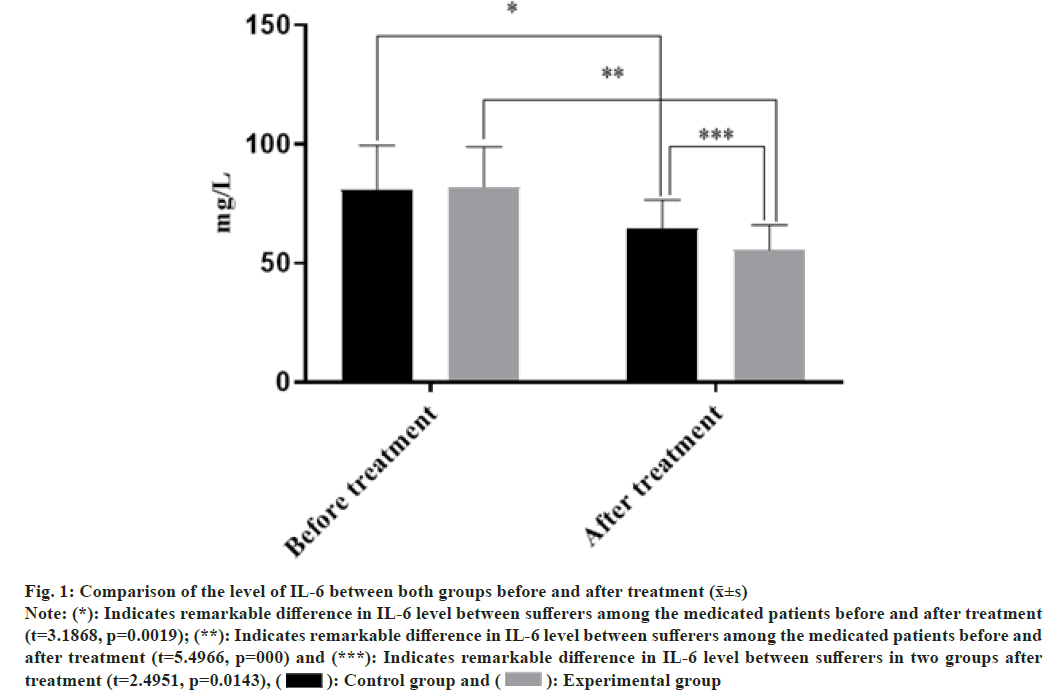
There was no statistically remarkable difference in IL-6 level between both groups before treatment (p>0.05), while after discriminatory treatment, the IL-6 level in both groups decreased and the combined treated cases were detected lower level of IL-6 than merely injected patients (p<0.05) as shown in fig. 1. The abscissa indicates the level of IL-6 in mg/l before and after treatment. The levels of IL-6 in the contrast group were (68±26) mg/l and (56±17) mg/l before and after treatment, respectively. The levels of IL-6 in the experience group were (70±24) mg/l and (48±15) mg/l before and after treatment, respectively.
Fig. 1: Comparison of the level of IL-6 between both groups before and after treatment (x̄±s)
Note: (*): Indicates remarkable difference in IL-6 level between sufferers among the medicated patients before and after treatment
(t=3.1868, p=0.0019); (**): Indicates remarkable difference in IL-6 level between sufferers among the medicated patients before and
after treatment (t=5.4966, p=000) and (***): Indicates remarkable difference in IL-6 level between sufferers in two groups after
treatment (t=2.4951, p=0.0143), ( ): Control group and (
): Control group and ( ): Experimental group
): Experimental group
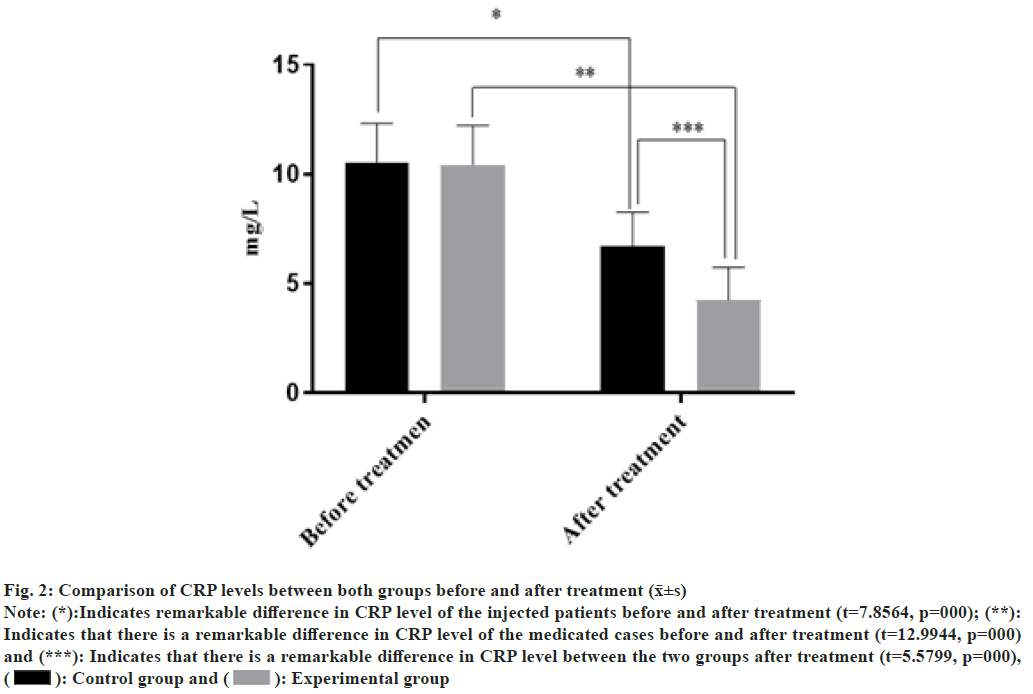
There was no statistically notable difference in CRP levels between both groups before treatment (p>0.05), but after different treatment, the medicated group CRP level was remarkably lower (p<0.05) as shown in fig. 2. The abscissa indicates CRP level before and after treatment, and the ordinate indicates CRP level in mg/l; the CRP levels of sufferers among the injected patients before and after treatment were (9.3±2.5) mg/l and (5.6±2.2) mg/l, respectively. The levels of CRP among the injected patients before and after treatment were (9.2±2.5) mg/l and (3.2±2.1) mg/l, respectively.
Fig. 2: Comparison of CRP levels between both groups before and after treatment (x̄±s)
Note: (*):Indicates remarkable difference in CRP level of the injected patients before and after treatment (t=7.8564, p=000); (**):
Indicates that there is a remarkable difference in CRP level of the medicated cases before and after treatment (t=12.9944, p=000)
and (***): Indicates that there is a remarkable difference in CRP level between the two groups after treatment (t=5.5799, p=000),
( ): Control group and (
): Control group and ( ): Experimental group
): Experimental group
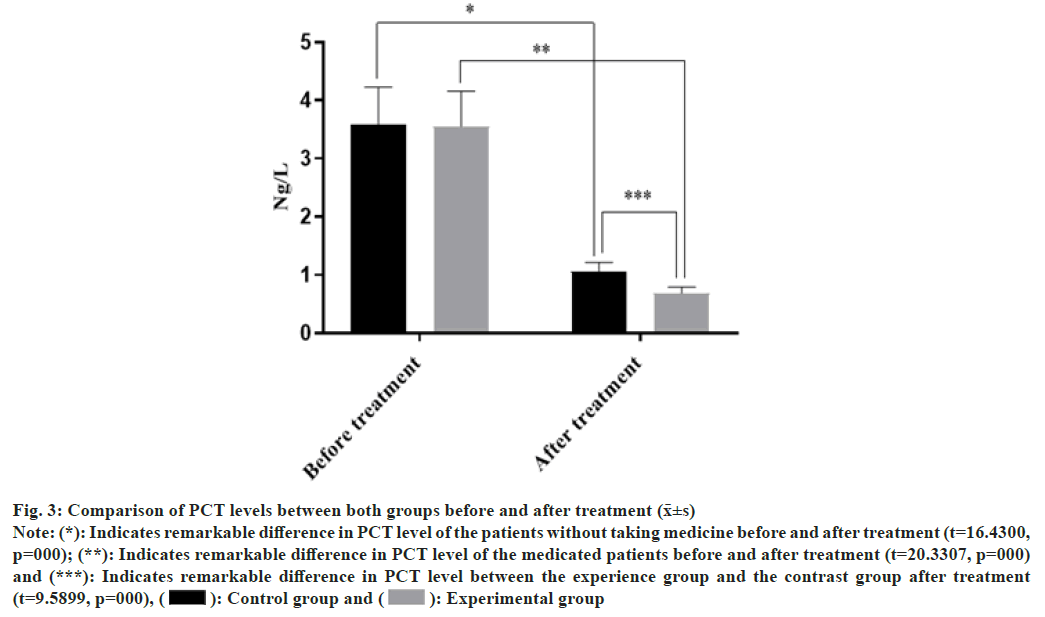
There was no statistically remarkable difference in PCT levels between both groups before treatment (p>0.05), but PCT levels in both groups decreased; and PCT levels of the medicated and injected patients dropped more than those without the medicine powder after treatment and cure measures (p<0.05) as shown in fig. 3. The abscissa indicates the PCT level before and after treatment, and the ordinate indicates the PCT level in ng/l; PCT levels before and after treatment among the injected patients were (3.13±0.91) ng/l and (0.96±0.21) ng/l, respectively; PCT levels before and after treatment among the medicated patients were (3.12±0.86) ng/l and (0.61±0.15) ng/l, respectively.
Fig. 3: Comparison of PCT levels between both groups before and after treatment (x̄±s)
Note: (*): Indicates remarkable difference in PCT level of the patients without taking medicine before and after treatment (t=16.4300,
p=000); (**): Indicates remarkable difference in PCT level of the medicated patients before and after treatment (t=20.3307, p=000)
and (***): Indicates remarkable difference in PCT level between the experience group and the contrast group after treatment
(t=9.5899, p=000), ( ): Control group and (
): Control group and ( ): Experimental group
): Experimental group
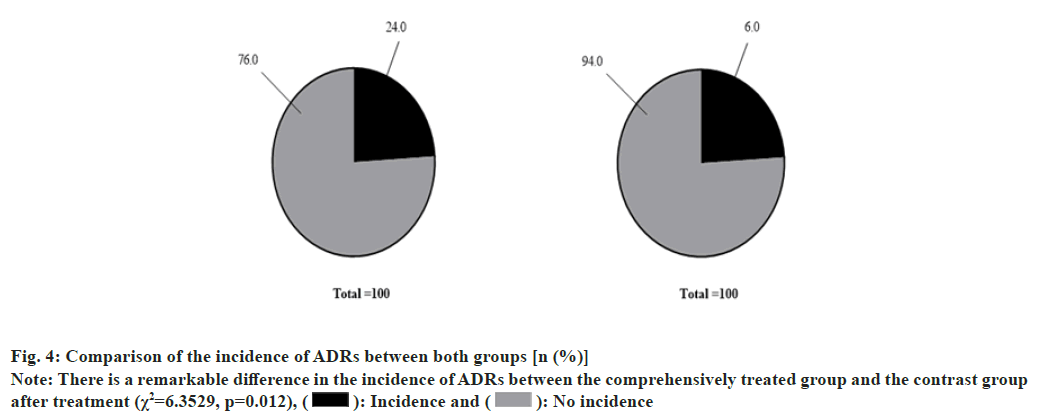
Among the 50 sufferers in the injected group, there were 2 cases of nausea, 1 case of vomiting, 3 cases of diarrhea, 4 cases of abdominal pain and 2 cases of dizziness, and the incidence of Adverse Drug Reactions (ADRs) was 12 (24 %). Among the 50 sufferers in the medicated group, there were 1 case of nausea, 1 case of vomiting, 0 case of diarrhea, 1 case of abdominal pain and 0 case of dizziness and the incidence of ADRs was 3 (6 %) as shown in fig. 4. fig. 4A show the incidence of ADRs among the injected cases; fig. 4B shows the incidence of ADRs among the medicated patients; the incidence of ADRs among the injected cases was 12 (24 %) and the nonoccurrence rate was 38 (76 %). The incidence of ADRs among the medicated patients was 3 (6 %), and the non-occurrence rate was 47 (94 %).
Influenza is highly contagious, which can be spread through air droplets, people-to-people contact, etc., and the transmission speed is fast, so it is easy to diffuse and spread widely[1]. We should pay more attention to influenza and actively treat it to prevent serious adverse consequences[5]. Influenza is a selflimiting disease, but some people will have pneumonia and other complications, which will develop into severe influenza. Because of its rapid development, sufferers may die of branchy chronic respiratory distress syndrome or multiple organ failure[6]. It is believed by traditional Chinese medicine that influenza is a category of diseases such as "epidemic colds", "anemofrigid syndrome", "epidemic disease" and "infectious damp heat" and its etiology is mainly exogenous pathogenic qi, which is grumpy, strong in pathogenic qi, and unable to be resisted by healthy qi, so pathogenic qi invades the muscle surface and enters from the nose and mouth, resulting in various symptoms[7,8].
Paramivir is a new inhibitor, which can effectively inhibit the activity of neuraminidase, and then inhibit the bond breakage between viral hemagglutinin and sialic acid of infected cells, thus preventing the release of virus and reducing clinical symptoms[9,10].
Traditional Chinese medicine has obvious advantages in treating influenza, and it is also widely used in clinic. Lianhua Qingwen granule is a kind of Chinese patent medicine. Its main components are Forsythia suspensa, honeysuckle, Isatis root, Houttuynia cordata, gypsum, ephedra, basket fern rhizome, Rhodiola rosea, patchouli, bitter almond, rhubarb, menthol and licorice. It has the effects of clearing away plague and detoxification, dispersing lung heat. Lianhua Qingwen granule is based on classic famous prescriptions such as Yinqiao powder and Maxing Shigan decoction, which are modified and refined[11-13]. Forsythia suspensa and honeysuckle are used as the chief drugs in the prescription, which are light and transparent, clear away heat and toxic materials, and dissipate stagnation and reduce swelling; it is matched with menthol, Isatis root, Houttuynia cordata and basket fern rhizome to dispel wind and heat, cool blood and reduce swelling, and eliminate phlegm and detoxification; gypsum and rhubarb can clear away heat and purge fire, detoxify, relieve cough and asthma, ephedra can clear away lung heat, relieve cough and asthma and gypsum and ephedra can be treated with each other to prevent ephedra from spreading too much heat, which can obviously enhance the efficacy of dispersing lung heat; Rhodiola rosea and bitter almond can clear away heat and phlegm, relieve cough and asthma and breathe smoothly; patchouli can clear away turbidity and relieve summer heat; licorice can clear away heat and toxic materials, relieve cough and asthma, and harmonize various drugs[14-17]. This medicine embodies the characteristics of traditional Chinese medicine, which gives consideration to both health and qi, and has both exterior and interior solutions.
In this research, the overall effective rate was 96 % in the experience group, remarkably higher than 80 % in the contrast group (p<0.05). The antipyretic time, sore throat disappearance time, cough disappearance time and general ache disappearance time in the experience group were remarkably shorter than those in the contrast group (p<0.05). It shows that Lianhua Qingwen granule combined with paramivir sodium chloride injection has obvious effect on influenza. Similar to the research results of Mitchell et al.[18], it is suggested that Lianhua Qingwen granule can effectively improve the symptoms of fever, cough, sore throat and so on. The level changes of IL-6, CRP and PCT in two groups were not remarkably different at baseline (p>0.05). After treatment, the levels of IL-6, CRP and PCT in the experience group were remarkably lower than those in the contrast group (p<0.05), which can effectively reduce the levels of inflammatory factors as CRP and PCT, and regulate IL-6 immunity and promote the recovery of immune function. Research by Fedson et al.[19] shows that Lianhua Qingwen granule combined with paramivir sodium chloride injection can regulate immune function, reduce immune damage and inhibit immune imbalance. During the treatment period, the incidence of ADRs in the contrast group was 24 %. The incidence of ADRs in the experience group was 6 %, which was remarkably lower than that in the contrast group (p<0.05).
In general, Lianhua Qingwen granule combined with paramivir sodium chloride injection has remarkable therapeutic efficacy on influenza, which can shorten the treatment time and decrease the level of serum inflammatory factors, and is worthy of clinical promotion and application.
Funding:
This study was supported by Scientific Research Program of Hebei Administration of Traditional Chinese Medicine, No. 2021281
Conflict of interests:
The authors declared no conflict of interests.
References
- Ichikawa H, Hiratani M, Yasuhara A, Tsujii N, Oshimo T, Ono H, et al. An open-label extension long-term study of the safety and efficacy of aripiprazole for irritability in children and adolescents with autistic disorder in Japan. Psychiatry and Clin Neurosci 2018;72(2):84-94.
[Crossref] [Google Scholar] [PubMed]
- Sambala EZ, Wiyeh AB, Ngcobo N, Machingaidze S, Wiysonge CS. New vaccine introductions in Africa before and during the decade of vaccines?are we making progress? Vaccine 2019;37(25):3290-5.
[Crossref] [Google Scholar] [PubMed]
- Larson Williams A, Mitrovich R, Mwananyanda L, Gill C. Maternal vaccine knowledge in low-and middle-income countries?and why it matters. Hum Vaccin Immunother 2019;15(2):283-6.
[Crossref] [Google Scholar] [PubMed]
- Higashiguchi M, Matsumoto T, Fujii T. A meta-analysis of laninamivir octanoate for treatment and prophylaxis of influenza. Antivir Ther 2018;23(2):157-65.
[Crossref] [Google Scholar] [PubMed]
- Wei W, Wan H, Peng X, Zhou H, Lu Y, He Y. Antiviral effects of Ma Huang Tang against H1N1 influenza virus infection in vitro and in an ICR pneumonia mouse model. Biomed Pharmacother 2018;102:1161-75.
[Crossref] [Google Scholar] [PubMed]
- Smee DF, Jung KH, Westover J, Gowen BB. 2?-Fluoro-2?-deoxycytidine is a broad-spectrum inhibitor of bunyaviruses in vitro and in phleboviral disease mouse models. Antivir Res 2018;160:48-54.
[Crossref] [Google Scholar] [PubMed]
- Newland JG, Laurich VM, Rosenquist AW, Heydon K, Licht DJ, Keren R, et al. Neurologic complications in children hospitalized with influenza: Characteristics, incidence and risk factors. J Pediatr 2007;150(3):306-10.
[Crossref] [Google Scholar] [PubMed]
- Botwina P, Owczarek K, Rajfur Z, Ochman M, Urlik M, Nowakowska M, et al. Berberine hampers influenza a replication through inhibition of MAPK/ERK pathway. Viruses 2020;12(3):344.
- Chang CY, Chung JB, Perera D. Influenza myocarditis complicated with atrioventricular block in a pregnant woman. Nigerian J Cardiol 2020;17(1):79.
- Tubiana S, Launay O, Galtier F, Tattevin P, Postil D, Vanhems P, et al. Attitudes, knowledge and willingness to be vaccinated against seasonal influenza among patients hospitalized with influenza-like-illness: Impact of diagnostic testing. Hum Vaccin Immunother 2020;16(4):851-7.
[Crossref] [Google Scholar] [PubMed]
- de Hoog ML, Venekamp RP, Damoiseaux RA, Schilder AG, Sanders EA, Smit HA, et al. Impact of repeated influenza immunization on respiratory illness in children with preexisting medical conditions. Ann Family Med 2019;17(1):7-13.
- Corson S, Robertson C, Reynolds A, McMenamin J. Modelling the population effectiveness of the national seasonal influenza vaccination programme in Scotland: The impact of targeting all individuals aged 65 y and over. Influenza Other Respir Viruses 2019;13(4):354-63.
- Chu EC, Yip AS. A rare presentation of benign acute childhood myositis. Clin Case Rep 2019;7(3):461-4.
[Crossref] [Google Scholar] [PubMed]
- Han Y, Yin J, Zeng Y, Chu CI, Chiang YC, Fang Y. Determinants of parental intentions to vaccinate kindergarten children against seasonal influenza in Xiamen, China. J Prim Prev 2019;40(3):325-42.
[Crossref] [Google Scholar] [PubMed]
- Havers FP, Fry AM, Goswami D, Nahar K, Sharmin AT, Rahman M, et al. Population-based incidence of childhood pneumonia associated with viral infections in Bangladesh. Pediatr Infect Dis J 2019;38(4):344-50.
[Crossref] [Google Scholar] [PubMed]
- Salto-Quintana JN, Rivera-Alfaro G, Sánchez-Ramos EL, Gómez-Gómez A, Noyola DE. Post-pandemic influenza-associated mortality in Mexico. Pathogens Global Health 2019;113(2):67-74.
[Crossref] [Google Scholar] [PubMed]
- Harris SR. Psychogenic movement disorders in children and adolescents: An update. Eur J Pediatr 2019;178(4):581-5.
[Crossref] [Google Scholar] [PubMed]
- Mitchell J, Chabrelie A Rose J, Charbonneau D, Ishida Y. Evaluation of the influenza risk reduction from antimicrobial spray application on porous surfaces. Risk Anal 2018;38(7):1502-17.
[Crossref] [Google Scholar] [PubMed]
- Fedson DS. Clinician-initiated research on treating the host response to pandemic influenza. Hum Vaccin Immunother 2018;14(3):790-5.
[Crossref] [Google Scholar] [PubMed]