- *Corresponding Author:
- Hanaa Mohammed Tashkandi
Department of General Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah 21589, Saudi Arabia
E-mail: atashkndee@kau.edu.sa
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “156-166” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Breast cancer ranks as the second major cause for cancer-related female mortalities worldwide. The features such as family history, old age, late-aged menopause, early-aged menarche, estrogen-replacement treatments for longer periods and first childbirth at later-age are common breast cancer related risk factors. Studies have shown that pomegranate (Punica granatum Linn.) and its constituents can efficiently affect multiple signaling pathways involved in inflammation, cellular transformation, hyperproliferation, angiogenesis, initiation of tumorigenesis and eventually suppressing the final steps of tumorigenesis and metastasis. Pomegranate extracts have been reported to significantly downregulate the proliferation and expression of transcription factors or proteins which are involved in breast cancer cells. These activities may lead to the up-regulation of protective factors or suppression of the gene expression, receptors as well as regulatory factors and may be associated with the suppression of the development of new blood vessels as well. In this review we discussed the therapeutic role of pomegranate against breast cancer. In addition, we revised the bioactive constituents of pomegranate, nanoencapsulation of these compounds and its safety on health.
Keywords
Breast cancer, Punica granatum, nanoencapsulation, bioactive compounds
Cancer was the second leading cause of death, after heart disease, in the United States in 2020. In 2020, there were 602 350 cancer deaths; 284 619 were among females and 317 731 among males[1]. Breast cancer ranks as the second major reason for cancerrelated female mortalities[2]. The features such as family history, old age, late-aged menopause, earlyaged menarche, estrogen-replacement treatments for longer period and first childbirth at later-age are common breast cancer related risk factors. Steroid hormones, especially estrogens are considered to facilitate the occurrence of breast cancer[3,4]. Pomegranate (Punica granatum Linn.) is obtained from a deciduous tree belonging to the family Lythraceae[5,6]. Pomegranate has been used in various medicinal systems of medicine for the treatment and therapy of a multitude of diseases and ailments[7]. Studies have shown that pomegranate and its constituents can efficiently affect multiple signaling pathways involved in inflammation, cellular transformation, hyperproliferation, angiogenesis, initiation of tumorigenesis and eventually suppressing the final steps of tumorigenesis and metastasis [7,8].
Pomegranate extracts have been reported to significantly decrease the Specificity protein (Sp) (Sp1, Sp3, Sp4) and micro Ribonucleic acid (miR)- 27a and miR-155 in breast cancer cells. Sp1, Sp3, Sp4 transcription factors are widely overexpressed in multiple types of cancers[9] and regulate survival, angiogenesis and proliferation-related genes. Sp1 regulates Nuclear Factor kappa B (NF-κB) in the NF-κB p65 subunit’s promoter region through a Guanine-Cytosine (GC)-rich binding site[10].
The downregulation of Sp transcription factors could further mediate NF-κB downregulation by pomegranate extracts. The miR-27a based suppression of Sp-repressor Zinc Finger and BTB Domain containing 10 (ZBTB10) results in higher expression of Sp transcription factors in breast cancer cells. Several anticancer agents are known to down- regulate miR-27a while up-regulating the ZBTB10[11]. Similarly, pomegranate extracts increased the transcriptional repressor ZBTB10 expression[10] along with the Src Homology-2 Domain-containing Inositol 5-Phosphatase 1 (SHIP1) expression, which is a target gene of miR155. This lipid phosphatase negatively regulates the Phosphatidylinositol- 3,4,5-Trisphosphate (PI3K)/Protein Kinase B (Akt) signaling pathway, which is commonly activated in different tumors and promotes cell survival and proliferation[10,11]. These findings reveal that the decreased levels of miR-27a and miR-155 could partially be responsible for the anti-cancer impact of pomegranate extract on breast cancer cells[10].
This review provides a comprehensive review on the anti-breast cancer activities of pomegranate in the treatment and prevention of breast cancer.
Pomegranate and its Constituents
Pomegranate belongs to the genus Punica and family Lythraceae. Pomegranate is native in Asian countries including Iran to Northern India. It grows in many of the Middle Eastern countries and was originally introduced from Syria and Israel since around 1600 Before the Common Era (BCE)[12].
Studies have demonstrated that pomegranates may be used as natural remedy due to their capability against a wide variety of diseases. Almost all the parts of pomegranate, including its flowers, peel, juice, arils and bark has shown a good biological activity. There are wide ranges of phytochemical properties which have demonstrated antimicrobial activities in pomegranate. The most active ingredients in pomegranate are Ellagic Acid (EA) and hydrolysable tannins, like Punicalagin (PU)[13].
Anticancer and anti-inflammatory activity has been found in the juice of the pomegranate as well as in its peel and oil resulting in antiproliferative effect, cell cycle arrest, reduction in invasion and angiogenesis. The wide variety of clinical administration of pomegranate to treat and prevent cancer and inflammation is due its phytochemistry and pharmacological properties[14].
Published papers have revealed that pomegranate may be beneficial in the treatment and prevention of many types of cancer like breast cancer, lung cancer, prostate cancer and skin cancer. It is thought that drinking Pomegranate Juice (PJ) will provide protection from cancer because it contains high levels of antioxidants as well as other nutrients[15].
Pomegranate reduces the proliferation of cancerous cells and induces programmed cell death, also slows down the blood supply to tumors, resulting in their starvation and shrinking[16]. Pomegranate grows as shrub reaching in height between 1.5 to 5 m, with glossy leaves and thorny and irregular branches appearing as evergreen in frigid regions and as a deciduous shrub in temperate ones[5].
The Punicaceae family has only one genus and two species, including: The edible Punica granatum and the inedible one Punica protopunica[5]. The different local names given to pomegranate are presented in fig. 1[5].
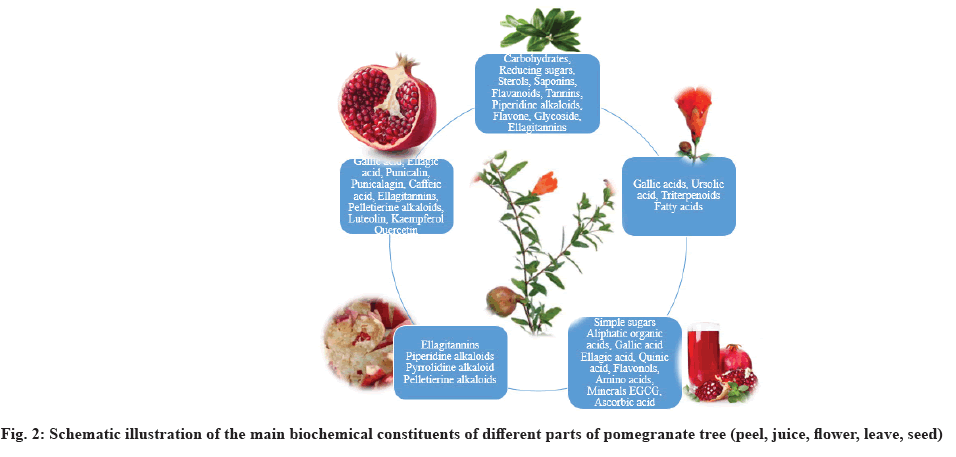
Pomegranate contains a variety of phenolic compounds like Ellagitannins (ETs) (punicalin, pedunculagin, PU, EA, ET, gallagic acid and gallotannins), anthocyanins (delphinidin, cyanidin and pelargonidin glycosides), flavonoids (quercetin, kaempferol and luteolin glycosides) and phenolic acids like chlorogenic acid, caffeic, o-coumaric acid, rutin, p-coumaric acid, ferulic acid, vanillic, phloridzin and syringic acid. The most abundant polyphenol with high molecular weight is PU. A huge amount of this polyphenol is found in arils with a substantial number of polyphenols like gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, ferulic acid, coumaric acids and catechin[17].
The pomegranate fruit itself without the peel is not toxic, while its bark and roots are toxic. A hydroalcoholic extract may induce genotoxicity at various levels of expression like clastogenic, mutagenic and recombinogenic[18] (fig. 2 and Table 1[19-27]).
| Category | Phytochemical | Chemical structure | Main anti-breast cancer activities | References |
|---|---|---|---|---|
| Anthocyanins and anthocyanidins | Gallagic acid | 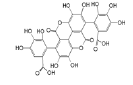 |
Induces apoptosis and ferroptosis resulting in programmed cell death | [19] |
| EA derivatives | EA | 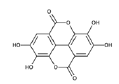 |
Direct inhibition of Alpha-Actinin 4 (ACTN4) | [20] |
| Anthocyanins and anthocyanidins | Punicic acid (Omega-5 fatty acid) | 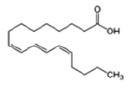 |
Apoptosis induction via PKC pathway and based on lipid peroxidation | [21] |
| ETs and gallotannins | Pedunculagin | 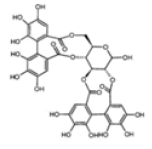 |
Inhibition of aromatase activity, proapoptic effect, inhibition of migration, invasion and metastasis | [22] |
| Anthocyanins and anthocyanidins | Delphinidin 3-O-beta-D- glucoside (Myrtillin) | 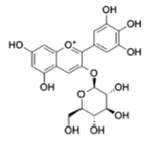 |
Inhibition via Akt/ HOX Antisense Intergenic RNA (HOTAIR) signaling pathway inactivation | [23] |
| Anthocyanins and anthocyanidins | Cyanidin 3-glucoside | 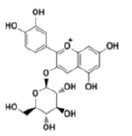 |
Attenuates the angiogenesis of breast cancer via inhibiting Signal Transducer and Activator of Transcription 3 (STAT3)/VEGF pathway |
[24] |
| Anthocyanins and anthocyanidins | Pelargonidin 3-glucoside | 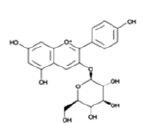 |
Affect the production of selected pro and anti-inflammatory cytokines | [25] |
| Anthocyanins and anthocyanidins | Pelargonidin 3,5-diglucoside | 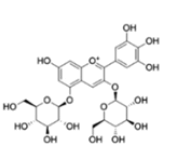 |
Affect the production of selected pro and anti-inflammatory cytokines | [25] |
| Anthocyanins and anthocyanidins | Cyanidin 3,5-diglucoside | 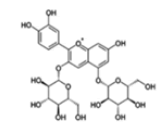 |
Inhibition of the STAT3/ VEGF pathway and angiogenesis | [26] |
| Anthocyanins and anthocyanidins | Delphinidin 3,5-diglucoside | 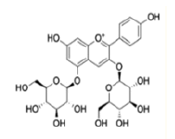 |
Mediated via inhibition of Fyn kinase activity and the TNF-α-induced Cyclooxygenase-2 (COX-2) expression | [27] |
Table 1: Biochemical Components of Pomegranate and Its Main Anti-Breast Cancer Activities
Effects of Pomegranate Components on Breast Cancer
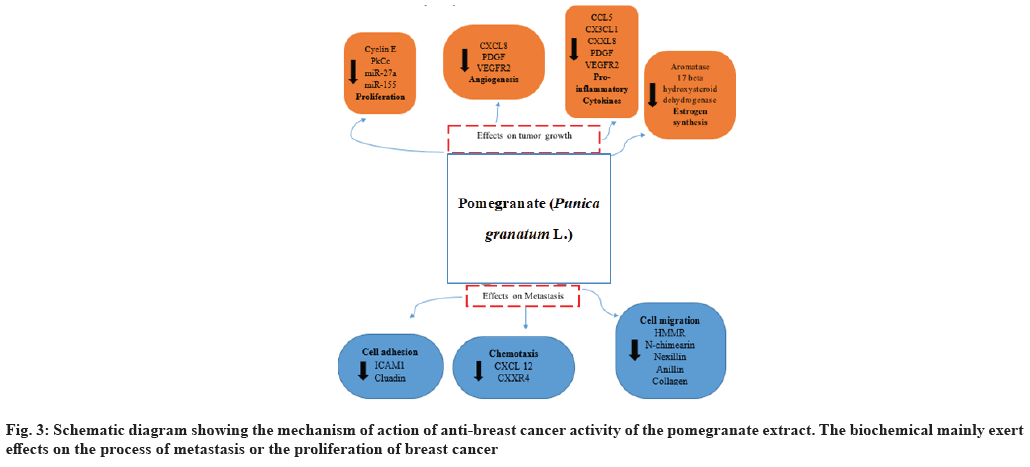
As explained above, pomegranate comprised several biochemicals which have different biomedical activities. The effects of these constituents on breast cancer mostly investigated by the applications of extracts from different parts of pomegranate fruit or chromatographically purified juice fractions[21]. However, recent studies have employed either as single or a combination of components targeting breast and other cancer types. In this line, one study deduced the promising anti-cancer potential of three pomegranate components (Punicic acid (P), Luteolin (L) and Ellagic acid (E)) against breast cancer and prostate cancer cells (fig. 3).
Anti-breast cancer assessment was performed using estrogen-sensitive Michigan Cancer Foundation-7 (MCF7) cells of Estrogen Receptor positive (ER+), estrogen-insensitive Human Breast Cancer cell line (MDA-MB-231) cells of Estrogen Receptor negative (ER-) and a non-neoplastic cell lines like human Breast Epithelial cell line (MCF10A). The combination of L+E+P (equal amounts) not only inhibited breast cancer cell growth but also reduced cancer cell migration and enhanced cell adhesion without affecting the normal cells[28]. L+E+P also increased the cell adhesion-related genes expressions including Intercellular Adhesion Molecule 1 (ICAM1) and Claudin 1 (CLDN1) and decreased cell migration-related genes expression including Hyaluronan-Mediated Motility Receptor (HMMR). These components also inhibited cancer cells chemotaxis to C-X-C Motif Chemokine Ligand 12 (CXCL12), which is a chemokine involved in the metastasis of breast cancer[29]. Adhesion loss and rise in CXCL12 chemotaxis and cell migration are known to cause breast cancer metastasis. During this study, the combination of L+E+P enhanced the E-cadherin expression whereas it contrarily alleviated the two Twist-Related protein (TWIST) genes expressions, which participate in epithelial- to-mesenchymal transitions. Small interfering Ribonucleic Acid (siRNA)-based knockdown of E-cadherin or overexpression of TWIST partially reversed L+E+P inhibitory effect on the cancer cell migration. Moreover, cytokine/chemokine multiplex arrays demonstrated a significant reduction in the pro-inflammatory cytokines/chemokines (Regulated upon Activation, Normal T Cell Expressed and Secreted (RANTES and Interleukin-8 (IL-8)) levels in response to L+E+P application. This phenomenon confirms their potential of decreasing inflammation to ultimately affect cancer progression[30]. Multiple studies have confirmed the anti-cancer potential of luteolin, EA and punicic acid against breast cancer cells. Luteolin is known to induce dose-dependent suppression of MCF-7 cell proliferation and Insulin- like Growth Factor 1 (IGF-1)[31].
Luteolin-based significant reduction of Akt phosphorylation and IGF-1-dependent IGF-1 Receptor (IGF-1R) has also been reported. ER is supposed to directly participate in the luteolin- based inhibition of IGF-1-induced cell proliferation. Luteolin also significantly reduced ER expression. ER-specific siRNA-based ER knockdown in MCF- 7 cells reduced the luteolin inhibition efficacy on IGF-1-induced cell proliferation. Therefore, it can be deduced that ER is probably the molecular target of luteolin. The results further demonstrated that luteolin inhibitory effects on MCF-7 cell growth occur via IGF-1-mediated PI3K-Akt pathway inhibition. The antiangiogenesis impact of EA on breast cancer has been established[32]. EA could significantly inhibit the Vascular Endothelial Growth Factor (VEGF)- induced migration, cell proliferation and tube formation in human endothelial cells. Furthermore, the EA could also restrict the Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) tyrosine kinase activity and its downstream signaling pathways (PI3K/Akt and Mitogen-Activated Protein Kinase (MAPK)) in endothelial cells. The studies have also reported a reduction in phospho VEGFR2 levels and inhibition of MDA-MB-231 xenograft tumor growth by EA. Molecular docking simulation based on in silico analysis indicated the interaction of EA with the Adenosine Triphosphate (ATP) binding region of VEGFR-2. These findings depict that EA could target the VEGFR-2 signaling pathway in breast cancer to exert anti-angiogenesis effects[28].
Punicic acid is the most abundant seed oil ingredient, which is known to possess anti-cancer properties against breast cancer cells. Punicic acid could inhibit the proliferation of MDA-MB-231 and MDA-ER alpha (α) 7 cell lines, which are known as estrogen- insensitive and estrogen-sensitive breast cancer cell lines, respectively[21]. Punicic acid could further induce the MDA-MB-231 and MDA-ERα7 cell apoptosis and disrupt the cellular mitochondrial membrane potential. The investigations have also revealed the importance of lipid oxidation for the apoptosis and cell proliferation effects of punicic acid. However, Protein Kinase C (PKC) inhibitors could partially block these effects of punicic acid in MDA-MB-231 and MDA-ERα7 cells. These findings establish the PKC pathway and lipid oxidation-based anticancer effects of punicic acid.
Pomegranate extract has generated cytotoxicity and anti-inflammatory properties in breast cancer cells during in vivo and in vitro investigations. The anti-breast cancer properties of pomegranate extract could partially depend upon the targeting of microRNAs[33,34]. Pomegranate Seed Oil (PSO) has prevented different types of tumor cell proliferation[34]. It has reduced mammary carcinogenesis in the mouse as well. Jeune et al.[35] have reported MCF-7 breast cancer cell growth inhibition by pomegranate extracts through the process called apoptosis. Angiogenesis is important for the nutrient and oxygen supply during tumor metastasis and growth whereas pomegranates could suppress the development of new blood vessels which supply the cancer cells[36].
Kim et al.[37] have reported the inhibitory effects of pomegranate components on various types of breast cancers. They further suggested a potential role of pomegranate in cancer suppression and treatment. During another study, Shirode et al. deduced that the encapsulation of pomegranate polyphenols could enhance their bio-efficacy in inhibiting the growth of breast cancer cells (Aneuploid Mammary Epithelial cells (Hs578T) and MCF-7)[34].
Recently, the link between breast cancer risk and dietary factors has been a major focus of investigations and several studies have revealed beneficial aspects of pomegranates in countering breast cancer[38]. Kim et al.[37] have reported aromatase inhibition by the polyphenols from the pericarp, PSO and fermented PJ. Aromatase is important for breast carcinogenesis as it converts androgen into estrogen[39]. The inhibition of estrogen biosynthetic enzyme (17-beta (β)-hydroxysteroid dehydrogenase) by pericarp, PSO and fermented PJ-derived polyphenols has been established[40]. In addition to the anti-estrogenic effects, pericarp, PSO and fermented PJ-derived polyphenols could also inhibit the growth of MB-MDA-231 and MCF-7 breast cancer cell lines. Fermented PJ-derived polyphenols have also been revealed to inhibit the formation of 7,12-Dimethylbenz[a]anthracene (DMBA)- induced cancerous lesions in the mammary gland organ culture of a murine[37]. Adams et al.[41] have demonstrated anti-aromatase and anti-proliferative activities in breast cancer cells in response to the application of pomegranate ETs-derived compounds. During a study, pomegranate ET-derived compounds including gallagic acid, EA and urolithins A and B (sulfated, methylated and acetylated analogs) were subjected to live cell-based assay and placental microsome aromatase assay to investigate their anti- aromatase activity. Methylated urolithin A, urolithin A, methylated urolithin B, urolithin B, urolithin B sulfate, acetylated urolithin B and gallagic acid caused a significant inhibition of aromatase activity in placental microsomes. Further comparison of these compounds using aromatase over-expressing cell line (MCF-7aro) presented urolithin B as the most potent inhibitor of aromatase. Aromatase activity was significantly inhibited at urolithin B doses of 2.35 μM (p≤0.05) and 4.7 μM (p≤0.01). Gallagic acid also exerted significant anti-aromatase activity at a dose of 4.7 μM (p≤0.01). Urolithins were further tested to assess their efficacy against testosterone- induced cell proliferation. Urolithin B was noted to successfully inhibit testosterone-induced cell proliferation followed by gallagic acid[41]. These data suggest that pomegranate intake could be beneficial for the chemoprevention of breast cancer.
Pomegranate Pericarp Methanolic Extract (PME) is known to exhibit Selective Estrogen Receptor Modulator (SERM) activity in in vivo estrogen deprivation models and human breast cancer cell lines[42]. SERMs are the ligands for ER and could exhibit antagonist or agonist functions depending upon the tissue type. SERMs are commonly employed in estrogen-dependent breast cancer therapies. PME treatment caused a significant dose- dependent cell growth inhibition in the ER+ MCF- 7 cell line. However, PME was unable to affect the ER− MDA MB-231 cell proliferation. 17β-estradiol- induced MCF-7 cell proliferation was also inhibited by PME treatment and furthermore, PME down- regulated the expression of estrogen-responsive genes (Progesterone Receptor (PR), pS2 and ERα) in MCF-7 cells. The lack of PME estrogenicity was finally confirmed in Ovariectomized (OVX) mice by assessing the epithelial heights and uterine wet weights as the estrogenicity markers. 17β-estradiol increased the normalized and absolute uterine wet weight by almost two times in OVX animals. However, uterus weights were not significantly different among PME-treated and vehicle-treated OVX control groups. Thus, these findings confirmed the lack of PME estrogenicity in the uterine endometrium. Similarly, uterine histology depicted that even though the 17β-estradiol induced uterine epithelium proliferation but luminal epithelial proliferation was not observed in PME-treated OVX mice[43]. Rocha et al.[44] further tested PJ and its components to assess their efficacy against breast cancer metastasis-related various processes. They used two breast cancer cell lines (MCF-7 (ER+) and MDA-MB-231 cells (ER−)) and a non-neoplastic cell line (MCF10A). The results demonstrated that PJ or a combination of its components (punicic acid, luteolin and EA) decreased cancer cell migration and growth, and increased the adhesion of cancer cells, without affecting normal cells. PJ and its three components also restricted pro-inflammatory cytokines/ chemokines production in cancer cells. Interestingly, PJ and its components were found to promote the cancer cell adhesion-related gene expressions, inhibit the genes related to cell migration and prevent cancer cell chemotaxis to stromal cell-derived factor 1α.
Several chemo-preventative investigations have highlighted the antioxidant and pro-apoptotic properties of Pomegranate Fruit Extract (PFE) and its components against breast cancer[45]. Punicic acid, which is a polyunsaturated fatty acid of PSO has been reported to initiate the apoptosis in estrogen-insensitive and sensitive cell lines (MDA- ER-7 and MDA-MB-231) along with significant inhibition of cell growth[46]. Caruso et al.[47] have described PFE methanolic extract-based reduction in MCF-7 cell proliferation and a dose-dependent rise in cell apoptosis. These PFE properties could be related to the enhanced pro-apoptotic gene (Bcl- 2 Associated X-Protein (BAX)) expression and a reduced anti-apoptotic gene (B-cell lymphoma-2 (Bcl-2)) expression. Costantini et al.[48] found a high abundance of punicic acid and its components in the hydrophilic PSO fraction (80 % aqueous methanol extract). They further evaluated their anti-inflammatory potential by employing breast cancer lines (MDA-MB-231 and MCF-7). The results demonstrated significantly, decreased cell viability of both cell lines after hydrophilic extract treatment with an increase in G0/G1 cell cycle phase as compared to untreated cells without significantly increased apoptosis in both cell lines. The results further revealed a decrease in the levels of pro- inflammatory cytokines (IL-17, IL-12, IL-6, IL- 2, Monocyte Chemoattractant Protein-1 (MCP-1), CXCL10, Tumor Necrosis Factor alpha (TNF-α), Macrophage Inflammatory Protein (MIP)-1α and MIP-1β) and VEGF at the higher doses of PSO hydrophilic extracts. Shirode et al.[49] have studied PFE anti-breast cancer potential using MCF-7 cells by investigating the changes in gene expression at the whole genome level. PFE treatment reduced the MCF-7 cell proliferation, which altered the expressions of 903 genes (up-regulation of 505 genes and down-regulation of 398 genes). Most of the up- regulated genes were related to apoptosis regulation whereas the down-regulated genes were involved in the chromosomal organization, mitosis, RNA processing, Deoxyribonucleic Acid (DNA) repair and DNA damage response. Genes including RAD50 Double Strand Break Repair Protein (RAD50), MRE11 Homolog, Double Strand Break Repair Nuclease (MRE11), Nibrin 1 (NBS1), MutS Homolog 6 (MSH6), RAD51 Recombinase (RAD51), BRCA1/BRCA2-containing Complex Subunit 3 (BRCC3), Breast Cancer gene 1 (BRCA1) and Breast Cancer gene 2 (BRCA2) related to DNA repair and damage response were noted to be down-regulated[49]. Chen et al.[50] conducted a complementary DNA (cDNA) microarray-based investigation to understand the underlying molecular mechanisms of EA-induced MCF-7 cell growth inhibition. The results revealed that EA involves cell cycle arrest-based inhibition of breast cancer cell growth and proliferation. The alterations in genes belonging to the Transforming Growth Factor-beta (TGF-β)/Suppressor of Mothers against Decapentaplegic (SMAD) signaling pathway were found to regulate the EA-based cell cycle arrest in MCF-7 cells. TGF-β is considered as a highly potent tumor suppressor that acts by promoting apoptosis, differentiation and cell growth inhibition[51].
Bishayee et al.[52] performed a study to evaluate the chemopreventive efficacy of Pomegranate Emulsion (PE) oral administration DMBA-induced mammary tumorigenesis in female Sprague-Dawley rats. Reduced tumor incidence and cumulative tumor burden were noted in PE-administered rats in comparison to control rats. PE-treated tumors presented almost normal alveolar and ductal structures having uniform epithelial cells without hyperplasia whereas the tumors in control rats were characterized by extensive histological epithelial proliferation. The chemopreventive impact against DMBA-initiated mammary tumors mainly depended upon the PE-based induction of apoptosis and reduction in cell proliferation[52]. The mechanism of PE chemopreventive potential was further evaluated during another study[52,53]. The results depicted reduced ER-α and ER-β expressions, nuclear translocation of β-catenin and cytoplasmic accumulation in PE- treated tumors. These data deduced a PE-induced disruption of ER and Wingless-Related Integration Site (Wnt)/β-catenin signaling pathways as the molecular basis of its chemopreventive impact against DMBA-inflicted rat mammary tumors.
Nanoencapsulation of Pomegranate Bioactive Compounds
Despite the known beneficial aspects, the issues such as short retention time, low systemic bioavailability and poor absorption of ETs and their metabolites might undermine their full chemo-preventive potential. For example, ETs such as PU could not be absorbed by the human body in intact form. They are hydrolyzed in the human intestinal tract to EA moieties, which are further converted to urolithins by colonic microbiota before absorption[54]. EA mainly accumulates in the epithelial cells of the intestine and their absorption into the systemic circulation is limited[55]. Therefore, after PJ consumption only low nanomolar range concentrations of free EA and urolithins are detected in human blood[56]. The absorbed EA and urolithins also have short half- life due to rapid liver metabolism and urinary excretion[57].
ETs encapsulation into biodegradable and biocompatible Nanoparticles (NPs) could overcome the drawbacks such as short half-life, gastrointestinal hydrolysis, low systemic bioavailability and poor absorption. Nanotechnology approaches have been applied in cancer therapies to reduce toxicity, promote selective tumor uptake and increase bioavailability and stability[58,59]. Recently, these approaches are being applied to prevent cancer through dietary phytochemicals[60]. These investigations have led to the development of a promising nanoencapsulation- based nano chemoprevention approach with better efficacy of bioactive food compounds. Paliwal et al.[61] have reported better efficacy of Epigallocatechin-3- Gallate (EGCG) nano prototypes from green tea, resveratrol from table grapes and curcumin from turmeric as compared to their free counterparts.
Poly Lactic-co-Glycolic Acid (PLGA) NPs are biodegradable, biocompatible and stable in biological fluids. They could ensure the sustainable release of loaded compounds by preventing their early degradation[62]. Cells uptake PLGA NPs via clathrin- mediated endocytosis and fluid-phase pinocytosis and they could rapidly exit the endo-lysosomes to enter the cytoplasm[63,64]. Ester linkages of PLGA undergo enzymatic and spontaneous hydrolysis to generate glycolic acid and lactic acid. Glycolic acid and lactic acid are endogenous molecules, which can be easily metabolized into carbon dioxide and water through the Krebs cycle. PLGA polymer applications in humans are considered safe[64].
The European Medicine Agency and United States (US) Food and Drug Administration have approved PLGA NPs applications through the parenteral route and the usage of PLGA microparticles as implants. Rapid opsonization of PLGA NPs by immunoglobulins and complement proteins is the main drawback. The reticuloendothelial system may clear PLGA NPs to restrict their approach to the target tissues. The modification of PLGA NPs surfaces with biocompatible polymers (Polyethylene Glycol (PEG)) could help in reducing opsonization to prolong their blood circulation time[64].
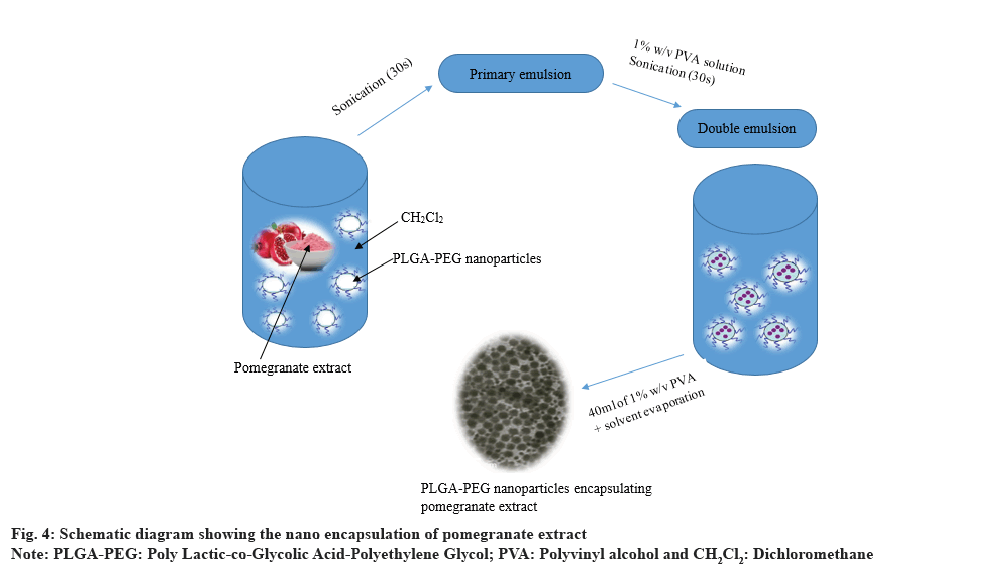
Shirode et al.[34] have studied the nanoencapsulation of pomegranate bioactive compounds using PLGA NPs for the chemoprevention of breast cancer (fig. 4). The data demonstrated that nanotechnology- enabled pomegranate polyphenols delivery enhanced the anti-breast cancer effects.
Safety of Pomegranate
Pomegranates are being used for centuries without adverse outcomes[65]. Animal studies have not indicated pomegranate toxicity at the commonly used concentrations in traditional medicine[66]. PJ extracts and oil could be used without risking the health of individuals. Pomegranate administrations (1420 mg/d extract tablets) have not produced adverse effects on the renal function or liver in humans[67].
A significant ratio of antioxidants is found in pomegranate extracts. Therefore, pomegranate- based natural supplements are a suitable alternative for bioactive polyphenols[68]. Bassiri-Jahromi et al.[69] have recently administered 3 different doses of pomegranate peel extract to Bagg and Albino (BALB)/c mice and no toxicity was observed regarding the weight gain, food intake and biochemical or behavioral factors. These administrations did not disturb the biochemical parameters such as cholesterol, glucose, Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT). Similarly, the inflammation was not observed at the supratherapeutic dose (7.5 mg/kg) as well.
Conclusion
In the current setting, cancer prevention via dietary agents is quite a promising arena of oncology that has drawn a significant amount of attention from both scientists in basic and clinical sciences and the general masses due to dietary agents have proven ability to prevent or suppress cancers, their low cost and easy availability.
However, current challenges relate to establish the key component of these dietetic agents which are responsible for the anticancer effects and the mechanisms through which they suppress cancer. Accumulating research provides extensive evidence related to biological activities of pomegranate- derived products particularly with respect to their anticancer properties. Anti-breast cancer activity of pomegranate, reduced cell migration and inhibited chemotaxis, pomegranate extract may reduce cancer cell invasion and motility, both of which are required for metastasis. One study demonstrated that PFE resulted in dose-dependent inhibition of estrogen and PR-negative human breast cancer cell proliferation, invasion and motility, and inhibited breast cancer cell growth. The anti-cancer activity of pomegranate have been enhanced by using different types of carriers like nanoparticles, however, intensive research is needed in this area for better therapeutic effects.
Conflict of interests:
The authors declared no conflict of interest.
References
- CDC. An update on cancer deaths in the United States. Centers for Disease Control and Prevention; 2022.
- Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun 2021;41(11):1183-94.
[Crossref] [Google Scholar] [PubMed]
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ 2020;371:3873.
[Crossref] [Google Scholar] [PubMed]
- Alqahtani WS, Almufareh NA, Domiaty DM, Albasher G, Alduwish MA, Alkhalaf H, et al. Epidemiology of cancer in Saudi Arabia thru 2010-2019: A systematic review with constrained meta-analysis. AIMS Public Health 2020;7(3):679-96.
[Crossref] [Google Scholar] [PubMed]
- Shaygannia E, Bahmani M, Zamanzad B, Rafieian-Kopaei M. A review study on Punica granatum L. J Evid Based Complementary Altern Med 2016;21(3):221-7.
[Crossref] [Google Scholar] [PubMed]
- Guerrero-Solano JA, Jaramillo-Morales OA, Jiménez-Cabrera T, Urrutia-Hernández TA, Chehue-Romero A, Olvera-Hernández EG, et al. Punica protopunica Balf., the forgotten sister of the common pomegranate (Punica granatum L.): Features and medicinal properties-A review. Plants 2020;9(9):1214.
[Crossref] [Google Scholar] [PubMed]
- Lavoro A, Falzone L, Gattuso G, Salemi R, Cultrera G, Leone GM, et al. Pomegranate: A promising avenue against the most common chronic diseases and their associated risk factors. Int J Funct Nutr 2021;2(2):1-2.
- Faria A, Calhau C. The bioactivity of pomegranate: Impact on health and disease. Crit Rev Food Sci Nutr 2011;51(7):626-34.
[Crossref] [Google Scholar] [PubMed]
- Hedrick E, Cheng Y, Jin UH, Kim K, Safe S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget 2016;7(16):22245-56.
[Crossref] [Google Scholar] [PubMed]
- Banerjee N, Talcott S, Safe S, Mertens-Talcott SU. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and in vivo: Potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res Treat 2012;136(1):21-34.
[Crossref] [Google Scholar] [PubMed]
- Sharma P, McClees SF, Afaq F. Pomegranate for prevention and treatment of cancer: An Update. Molecules 2017;22(1):177.
[Crossref] [Google Scholar] [PubMed]
- Ahangari B, Sargolzaei J. Extraction of pomegranate seed oil using subcritical propane and supercritical carbon dioxide. Theor Found Chem Eng 2012;46(3):258-65.
- Howell AB, D'Souza DH. The pomegranate: Effects on bacteria and viruses that influence human health. Evid Based Complement Alternat Med 2013;2013:1-11.
[Crossref] [Google Scholar] [PubMed]
- Gautam P, Singh A, Misao N, Sharma S, Singh A. Pharmacological and photochemical study of Punica granatum: A review. Ann Romanian Soc Cell Biol 2021;2(6):19863-79.
- Giménez-Bastida JA, Ávila-Gálvez MA, Espín JC, Gonzalez-Sarrias A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci Technol 2021;114:410-23.
- Mollaei H, Mousavi-Kouhi SM, Moudi M, Hosseinzadeh MS, Ghorbany M. Evaluation of anticancer effects and molecular mechanisms of pomegranate peel extract in the mouse cellular model (4T1) of breast cancer. Gene Cell Tissue 2022;9(3):e117831.
- Hmid I, Elothmani D, Hanine H, Oukabli A, Mehinagic E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab J Chem 2017;10(2):2675-84.
- Chaudhari SM, Patel KY, Badole SL. Punica granatum (pomegranate fruit): In cancer treatment. Polyphenols Human Health Dis 2014;2:1393-1400.
- Khorsandi K, Kianmehr Z, Hosseinzadeh R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int 2020;20(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Wang N, Wang Q, Tang H, Zhang F, Zheng Y, Wang S, et al. Direct inhibition of ACTN4 by ellagic acid limits breast cancer metastasis via regulation of β-catenin stabilization in cancer stem cells. J Exp Clin Cancer Res 2017;36(1):172.
[Crossref] [Google Scholar] [PubMed]
- Moga MA, Dimienescu OG, Bălan A, Dima L, Toma SI, Bîgiu NF, et al. Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules 2021;26(4):1054.
[Crossref] [Google Scholar] [PubMed]
- Ismail T, Calcabrini C, Diaz AR, Fimognari C, Turrini E, Catanzaro E, et al. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016;8(5):151.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Luo E, Liu X, Han B, Yu X, Peng X. Delphinidin-3-glucoside suppresses breast carcinogenesis by inactivating the Akt/HOTAIR signaling pathway. BMC Cancer 2016;16(1):423.
[Crossref] [Google Scholar] [PubMed]
- Ma X, Ning S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phytotherapy Res 2019;33(1):81-9.
[Crossref] [Google Scholar] [PubMed]
- Amini AM, Spencer JP, Yaqoob P. Effects of pelargonidin-3-O-glucoside and its metabolites on lipopolysaccharide-stimulated cytokine production by THP-1 monocytes and macrophages. Cytokine 2018;103:29-33.
[Crossref] [Google Scholar] [PubMed]
- Qi Q, Chu M, Yu X, Xie Y, Li Y, Du Y, et al. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev Int 2022;1-29.
- Gonçalves AC, Nunes AR, Falcão A, Alves G, Silva LR. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021;14(7):690.
[Crossref] [Google Scholar] [PubMed]
- Wang N, Wang ZY, Mo SL, Loo TY, Wang DM, Luo HB, et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat 2012;134(3):943-55.
[Crossref] [Google Scholar] [PubMed]
- Rahmani AH, Alsahli MA, Almatroodi SA. Potential antitumor effects of pomegranates and its ingredients. Pharmacogn Rev 2017;11(22):136-40.
[Crossref] [Google Scholar] [PubMed]
- BenSaad LA, Kim KH, Quah CC, Kim WR, Shahimi M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A and B isolated from Punica granatum. BMC Complement Altern Med 2017;17(1):47.
[Crossref] [Google Scholar] [PubMed]
- Akpinar-Bayizit A, Ozcan T, Yilmaz-Ersan L. The therapeutic potential of pomegranate and its products for prevention of cancer. In: Georgakilas AG editors. Cancer prevention: From mechanisms to translational benefits. London: IntechOpen Limited; 2012. p. 331-73.
- Ceci C, Lacal PM, Tentori L, de Martino MG, Miano R, Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients 2018;10(11):1756.
[Crossref] [Google Scholar] [PubMed]
- Zare M, Shaverdi H, Kalaee SE. Anti-cancer effects of pomegranate seed oil on esophageal cancer cell line (KYSE-30). Gene Cell Tissue 2021;8(1):1-10.
- Shirode AB, Bharali DJ, Nallanthighal S, Coon JK, Mousa SA, Reliene R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int J Nanomedicine 2015;10(1):475-84.
[Crossref] [Google Scholar] [PubMed]
- Jeune ML, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food 2005;8(4):469-75.
[Crossref] [Google Scholar] [PubMed]
- Sudha T, Mousa DS, El-Far AH, Mousa SA. Pomegranate (Punica granatum) fruit extract suppresses cancer progression and tumor angiogenesis of pancreatic and colon cancer in chick chorioallantoic membrane model. Nutr Cancer 2021;73(8):1350-6.
[Crossref] [Google Scholar] [PubMed]
- Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat 2002;71(3):203-17.
[Crossref] [Google Scholar] [PubMed]
- Hussein AM, El-Beih NM, Swellam M, El-Hussieny EA. Pomegranate juice and punicalagin-mediated chemoprevention of hepatocellular carcinogenesis via regulating miR-21 and NF-κB-p65 in a rat model. Cancer Cell Int 2022;22(1):333.
[Crossref] [Google Scholar] [PubMed]
- Chan HJ, Petrossian K, Chen S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and-resistant breast cancer cells. J Steroid Biochem Mol Biol 2016;161:73-83.
[Crossref] [Google Scholar] [PubMed]
- Hilborn E, Stal O, Jansson A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017;8(18):30552-62.
[Crossref] [Google Scholar] [PubMed]
- Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res 2010;3(1):108-13.
[Crossref] [Google Scholar] [PubMed]
- Sreeja S, Kumar TR, Lakshmi BS, Sreeja S. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. J Nutr Biochem 2012;23(7):725-32.
[Crossref] [Google Scholar] [PubMed]
- Sreekumar S, Sithul H, Muraleedharan P, Azeez JM, Sreeharshan S. Pomegranate fruit as a rich source of biologically active compounds. Biomed Res Int 2014;2014:686921.
[Crossref] [Google Scholar] [PubMed]
- Rocha A, Wang L, Penichet M, Martins-Green M. Pomegranate juice and specific components inhibit cell and molecular processes critical for metastasis of breast cancer. Breast Cancer Res Treat 2012;136(3):647-58.
[Crossref] [Google Scholar] [PubMed]
- Sturgeon SR, Ronnenberg AG. Pomegranate and breast cancer: Possible mechanisms of prevention. Nutr Rev 2010;68(2):122-8.
[Crossref] [Google Scholar] [PubMed]
- Grossmann ME, Mizuno NK, Schuster T, Cleary MP. Punicic acid is an omega-5 fatty acid capable of inhibiting breast cancer proliferation. Int J Oncol 2010;36(2):421-6.
[Google Scholar] [PubMed]
- Caruso A, Barbarossa A, Tassone A, Ceramella J, Carocci A, Catalano A. Pomegranate: Nutraceutical with promising benefits on human health. Appl Sci 2020;10(19):6915.
- Costantini S, Rusolo F, de Vito V, Moccia S, Picariello G, Capone F, et al. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules 2014;19(6):8644-60.
[Crossref] [Google Scholar] [PubMed]
- Shirode AB, Kovvuru P, Chittur SV, Henning SM, Heber D, Reliene R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol Carcinog 2014;53(6):458-70.
[Crossref] [Google Scholar] [PubMed]
- Chen HS, Bai MH, Zhang T, Li GD, Liu M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int J Oncol 2015;46(4):1730-8.
[Crossref] [Google Scholar] [PubMed]
- Seoane J, Gomis RR. TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol 2017;9(12):a022277.
[Crossref] [Google Scholar] [PubMed]
- Bishayee A, Mandal A, Bhattacharyya P, Bhatia D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr Cancer 2016;68(1):120-30.
[Crossref] [Google Scholar] [PubMed]
- Mandal A, Bishayee A. Mechanism of breast cancer preventive action of pomegranate: Disruption of estrogen receptor and Wnt/β-catenin signaling pathways. Molecules 2015;20(12):22315-28.
[Crossref] [Google Scholar] [PubMed]
- Villalba KJ, Barka FV, Pasos CV, Rodríguez PE. Food ellagitannins: Structure, metabolomic fate and biological properties. In: Aires A, editor. Tannins-structural properties, biological properties and current knowledge. London: IntechOpen Limited; 2019. p. 26-46.
- Kujawska M, Jodynis-Liebert J. Potential of the ellagic acid-derived gut microbiota metabolite-Urolithin A in gastrointestinal protection. World J Gastroenterol 2020;26(23):3170.
[Crossref] [Google Scholar] [PubMed]
- Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 2006;136(10):2481-5.
[Crossref] [Google Scholar] [PubMed]
- Raimundo AF, Ferreira S, Tomás-Barberán FA, Santos CN, Menezes R. Urolithins: Diet-derived bioavailable metabolites to tackle diabetes. Nutrients 2021;13(12):4285.
[Crossref] [Google Scholar] [PubMed]
- Ceci C, Graziani G, Faraoni I, Cacciotti I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020;31(38):382001.
[Crossref] [Google Scholar] [PubMed]
- Alfei S. Nanotechnology applications to improve solubility of bioactive constituents of foods for health-promoting purposes. Nano Food Eng 2020;189-257.
- Kim B, Park JE, Im E, Cho Y, Lee J, Lee HJ, et al. Recent advances in nanotechnology with nano-phytochemicals: Molecular mechanisms and clinical implications in cancer progression. Int J Mol Sci 2021;22(7):3571.
[Crossref] [Google Scholar] [PubMed]
- Paliwal R, Paliwal SR. Advances in Nanochemoprevention: Controlled delivery of phytochemical bioactives. 1st ed. Springer: Nature; 2021.
- Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly (lactic acid)/poly (lactic-co-glycolic acid) micro and nanoparticles. J Pharm Invest 2019;49(4):347-80.
- Metin E. Targeted co-delivery of doxorubicin and TPGS to breast cancer cells by PLGA coated magnetic nanoparticles (Master's thesis). Middle East Technical University; 2017.
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release 2012;161(2):505-22.
[Crossref] [Google Scholar] [PubMed]