- *Corresponding Author:
- N. B. Amor
Department of Biotechnology, Higher Institute of Biotechnology of Beja, University of Jendouba, Beja, Jendouba 8189, Tunisia
E-mail: nmmor@kfu.edu.sa
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “22-29” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Platelets are small circulating anucleated cells, resulting from the fragmentation of megakaryocytes. They are directly involved in primary hemostasis, where they are among the first elements to intervene in stopping bleeding. Platelet count or functional alteration was reported to be correlated to different types of diseases. Since antiquity, plants have been used to cure panoply of diseases. Nigella sativa, black cumin, is an annual Mediterranean plant. Nigella sativa's main essential oil constituent, thymoquinone (30 %-48 %) is responsible for a number of its biological activity. Various drugs and plant substances can induce thrombocytopenia. Some studies describe a dose and time-dependent decrease of platelet count induced by consumption of these substances. Thymoquinone is a quinine-like compound; quinines are involved in drug-induced immune thrombocytopenia. Indeed, human consumption and rat administration of black seed oil for 4 and 12 w, respectively, induce thrombocytopenia. To date, the thymoquinone effect on platelets is not fully understood. Here we bring new elements on the effects of thymoquinone on platelet aggregation. Our results show that thymoquinone by itself is able to induce platelet aggregation, but needs a relatively long exposure (28 min). We also show that thymoquinone-induced platelet aggregation is probably mediated through cytochrome metabolites but mainly via the mitochondrial pathway. Results demonstrate that platelets pre-incubation with thymoquinone abrogate thrombin-induced platelet aggregation in a concentration-dependent manner. In conclusion, black seeds and thymoquinone consumption as nutritional complement or as alternative medicine should be in low concentration and under phyto-specialists or medical supervision.
Keywords
Thymoquinone, platelet aggregation, cytochrome P450, mitochondria
Blood platelets are small circulating anucleated cells, resulting from the fragmentation of their hematopoietic precursor, resident in the marrowbone, the megakaryocyte. Platelets are directly involved in primary hemostasis, where they will be among the first elements to intervene in stopping bleeding. They will locally undergo various changes related to their hemostatic activity. Platelet aggregation might be either reversible or irreversible. The reversible aggregation will take place as a mission that the repair of the small damages that take place in the sub- endothelium and vascular tissue, while the phenomenon of irreversible aggregation will take place when the damage is very serious and the formation of a platelet thrombus is required[1]. Platelet count or functional alteration is associated to a number of diseases[2-4]. Thrombocytopenia is the decrease of platelet count to less than 150 000/ml and caused by platelet production decrease or high consumption and sequestration.
Various drugs and plant substances can induce thrombocytopenia. Some studies report 25 non-quinine substances from foods, beverage, herbal and nutritional supplements rather than medicines are associated to thrombocytopenia[5]. They also describe a dose and time-dependent decrease of platelet count induced by these substances[6].
Since ancient times, plants have been used to cure a plethora of disorders. Nigella sativa (N. sativa), black cumin is an annual Mediterranean plant. Its oil was used in Arabic medicine to heal arthritis, lung disease and hypercholesterolemia[7]. Studies have shown that the biological activity of N. sativa seeds is mainly due to its essential oil, mainly composed of Thymoquinone (TQ) (30 %-48 %)[8].
The therapeutic potential of TQ has attracted the attention of a number of research groups to characterize its molecular mechanism. Indeed many scientists underline TQ high scavenging proprieties of superoxide and free radicals[9]. This ability gives TQ the ability to modulate cellular antioxidant defense enzymes[10]. Moreover, several studies attribute to TQ the capability to inhibit tumor cell proliferation via several signaling pathways[11-13].
TQ does not only have beneficial virtues. TQ is quinine like compound and quinines are involved in drug-induced immune thrombocytopenia[14]. Thus, TQ might act through the same mechanism that induces thrombocytopenia[15]. Indeed, oral administration of N. sativa fixed oil in mice and rats during 12 w causes 15 % to 35 % decrease in platelet count[16]. In humans, consumption of black seed oil for 1 mo has been reported to induce thrombocytopenia[17]. Also, TQ poses an apoptotic effect on platelets mediated by G protein-coupled receptors that may explain platelet count decrease[18]. Until now, the effect of TQ on platelets has not been fully understood. Here we shed new light on the effect of TQ on platelet aggregation. We demonstrate for the first time that TQ by itself is capable to stimulate platelet aggregation and abrogates the effect of thrombin.
Materials and Methods
Materials:
Apyrase (grade VII), Ethylene Glycol Tetraacetic Acid (EGTA) AQ1, aspirin, Bovine Serum Albumin (BSA), thrombin, TQ, rotenone and oligomycin A were from Sigma (Madrid, Spain). Calcein was from Molecular Probes (Leiden, The Netherlands). All other reagents were purchased from Panreac (Barcelona, Spain). The thrombin preparation (specific activity P2000 National Institute of Health (NIH) units/mg protein) was predominantly Alpha (α) thrombin, containing minimum autolytic digestion products, according to the manufacturer’s instructions. Therefore, most of the effects shown in the present study should be attributed to α-thrombin.
Platelet preparation:
Blood was obtained from healthy volunteers, according to the rules of the Declaration of Helsinki and mixed with one-sixth volume of acid/citrate dextrose anticoagulant containing (in mM): 85 sodium citrate, 78 citric acid and 111 D-glucose. Platelet-rich plasma was then prepared by centrifugation for 5 min at 700 g and apyrase (40 µg/ml) added. Cells were then collected by centrifugation at 350 g for 20 min and resuspended in HEPES-Buffered Saline (HBS) containing (in mM): 145 Sodium chloride (NaCl), 10 HEPES, 10 D-glucose, 5 Potassium chloride (KCl), 1 Magnesium sulfate (MgSO4), pH 7.45 and supplemented with 0.1 % w/v BSA and 40 µg/ml apyrase.
Cell viability:
Cell viability was assessed using calcein and trypan blue. For calcein loading, cells were incubated for 30 min with 5 µM Calcein-Acetoxymethyl Ester (calcein-AM) at 37°, centrifuged and the pellet was re-suspended in fresh HBS. Cells were treated with the different inhibitors, centrifuged and re-suspended in HBS. Fluorescence was recorded from 2 ml aliquots using a spectrophotometer (Varian Ltd., Madrid, Spain). Samples were excited at 494 nm and the resulting fluorescence was measured at 535 nm. The results obtained with calcein were confirmed using the trypan blue exclusion technique. 95 % of cells were viable in our platelet suspensions and no effect was observed after treatment with inhibitors.
Platelet aggregation:
The percentage, rate and lag-time of aggregation in washed platelets were monitored using a Chronolog (Havertown, PA, USA) aggregometer at 37° under stirring at 1200 rpm[19]. The percentage of aggregation or amplitude is estimated as the percentage of the difference in light transmission between the platelet suspension in HBS and HBS alone, and indicates the percentage of platelets that aggregate in response to an agonist. Resting platelets in suspension are arbitrarily considered by the aggregometer as 0 % aggregation and HBS is considered to be 100 % aggregation. The rate or slope of the aggregation is the percentage change of aggregation per minute.
Statistical analysis:
Analysis of statistical significance was performed using Student’s t test where p<0.05 was considered to be significant for a difference.
Results and Discussion
N. sativa is widely consumed in Mediterranean, Middle East and Asian countries, for its beneficial effects[19]. TQ, the main biological active compound of N. sativa, has the capacity to reduce oxidative stress and induce apoptosis in many types of cancer[12,13]. The effect of TQ in platelet physiology is controversial and a number of studies associate TQ to thrombocytopenia, induction of platelets apoptosis or no effect on platelet aggregation[17,18,20].
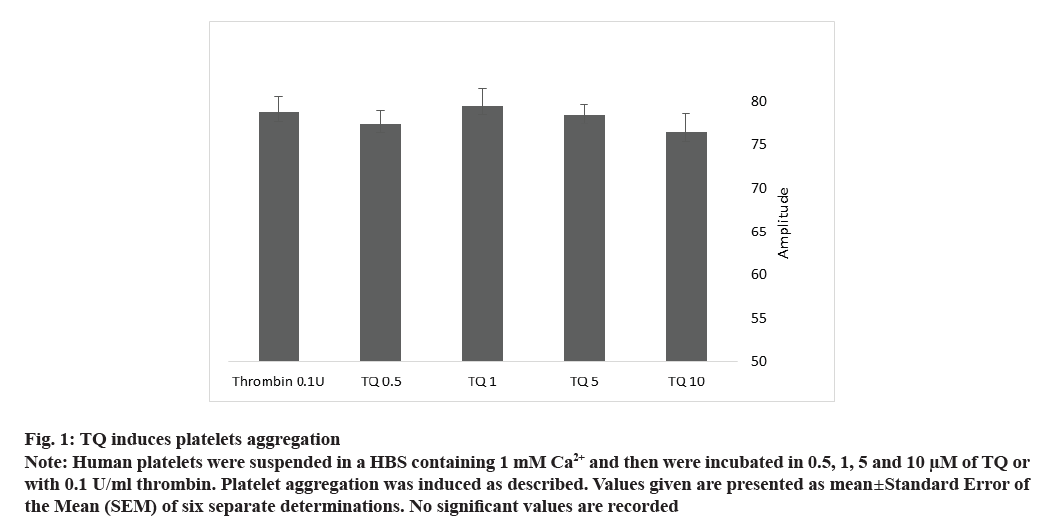
To explore the effect of TQ on human platelet aggregation, we incubated cells with 0.5, 1, 5 and 10 µM of TQ at 37° and 1200 rpm stirring. We noticed that, all the concentrations of TQ used, caused platelet aggregation with the same amplitude as 0.1 U/ml of thrombin (fig. 1 and Table 1, no significant values comparing to 0.1 U/ml of thrombin; n=10). Interestingly, the lag-time for TQ-induced platelet aggregation was very long, about 28 min for 5 and 10 µM without affecting the slope compared to 0.1 U/ml thrombin (Table 1). TQ, 1 and 0.5 µM are also able to induce slow platelet aggregation, (lag-time was 2130±276 and 1229±607 s, respectively) with a smaller slope (40.5±4.33 and 47.66±2.84, respectively) (Table 1).
| Stimulus | Amplitude | Slope | Lag-time |
|---|---|---|---|
| Thrombin 0.1 U | 78.7±1.8 | 65.2±2.9 | 35.8±4.9 |
| TQ 10 µM | 76.4±2.2 | 65.0±2.8 | 1707.0±35.6** |
| TQ 5 µM | 78.5±1.1 | 64.4±4.6 | 1596.0±84.5** |
| TQ 1 µM | 79.5±1.9 | 40.5±4.3* | 2130.0±276.1** |
| TQ 0.5 µM | 77.3±1.6 | 47.6±2.8* | 1229.0±607.0** |
Note: Values given are presented as mean±SEM of six separate determinations; *p<0.05, **p<0.001 compared to thrombin-induced response in the absence of agents
Table 1: TQ Effect on Platelets Aggregation
Fig. 1: TQ induces platelets aggregation
Note: Human platelets were suspended in a HBS containing 1 mM Ca2+ and then were incubated in 0.5, 1, 5 and 10 μM of TQ or
with 0.1 U/ml thrombin. Platelet aggregation was induced as described. Values given are presented as mean±Standard Error of
the Mean (SEM) of six separate determinations. No significant values are recorded
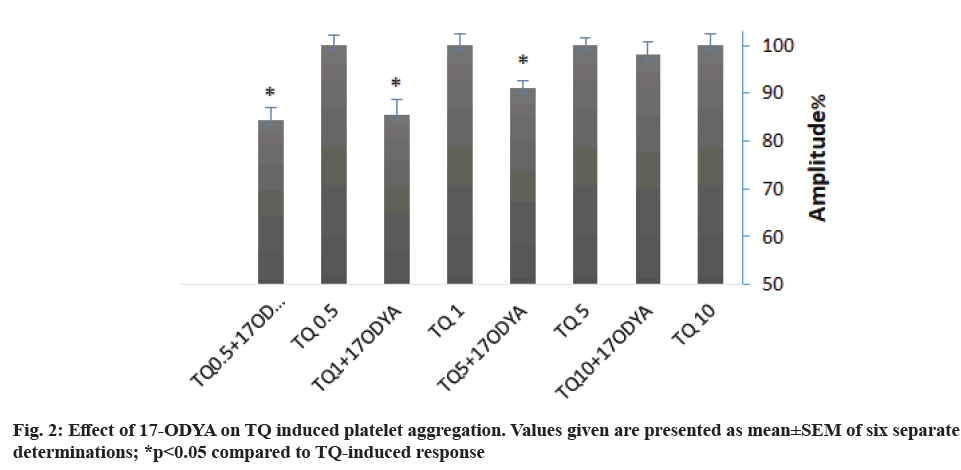
Arachidonic acid metabolites are one of the main platelet aggregation pathways. To investigate the role of Cytochrome P450 (CYP-450) in TQ-induced platelet aggregation, we preincubated platelets with 10 µM of 17-Octadecynoic acid (17-ODYA), a CYP- 450 inhibitor for 10 min. Treatment with 17-ODYA attenuated TQ-induced aggregation by 15 % for 0.5 and 1 µM TQ and 10 % for 5 µM TQ. 17-ODYA seems no effect on aggregation amplitude when 10 µM of TQ was used (fig. 2 and Table 2). We noticed that 17- ODYA also reduces the lag-time of TQ-induced platelet aggregation (Table 2).
| Agent | Stimulus | Amplitude | Slope | Lag-time (s) |
|---|---|---|---|---|
| Thrombin | 78.7±1.8 | 65.2±2.9 | 35.8±4.9 | |
| 17-ODYA | TQ 10 µM | 74.8±2.0 | 51.5±4.3* | 1439.0±73.9**† |
| 17-ODYA | TQ 5 µM | 71.4±1.2*† | 49.2±4.2* | 1362.0±54.5**† |
| 17-ODYA | TQ 1 µM | 68.0±2.6*† | 43.6±2.5** | 1004.6±184.1**† |
| 17-ODYA | TQ 0.5 µM | 65.2±2.1*† | 51.0±3.7* | 625.1±60.3**† |
| Rot+Oligo | TQ 10 µM | 62.7±0.4*† | 77.2±5.4*† | 1592.2±32.2**† |
| Rot+Oligo | TQ 5 µM | 67.0±1.5*† | 72.5±10.1 | 1266.0±33.0**† |
Note: Values given are presented as mean±SEM of four to six separate determinations; *p<0.05, **p<0.01 compared to thrombin-induced response in the absence of agents; †p<0.05 compared to TQ-induced response
Table 2: TQ Induces Platelet Aggregation via CYP and Mitochondrial Pathway
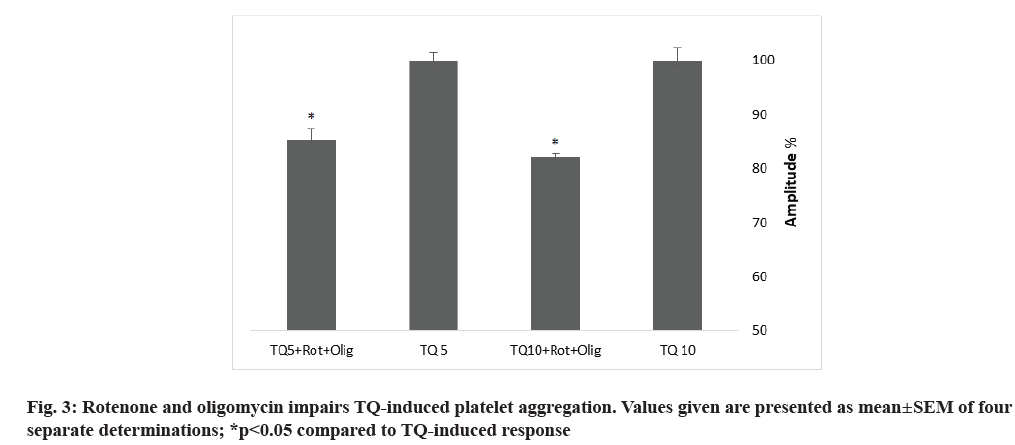
Towhid and coworkers reported a mitochondrial membrane depolarization in TQ-induced platelet apoptosis[18]. To assess the contribution of mitochondria in TQ-induced platelet aggregation, we incubated platelets for 10 min with 10 µM rotenone, an electron transport chain Complex I inhibitor and 10 µM oligomycin A, an inhibitor of Adenosine Triphosphate (ATP) synthase (used to avoid ATP depletion), prior to platelet stimulation with 5 and 10 µM of TQ. Treatment with oligomycin A and rotenone resulted in attenuation of the amplitude of platelet aggregation and increase in the lag-time (fig. 3 and Table 2), thus suggesting that mitochondria plays a relevant role in platelet aggregation induced by TQ.
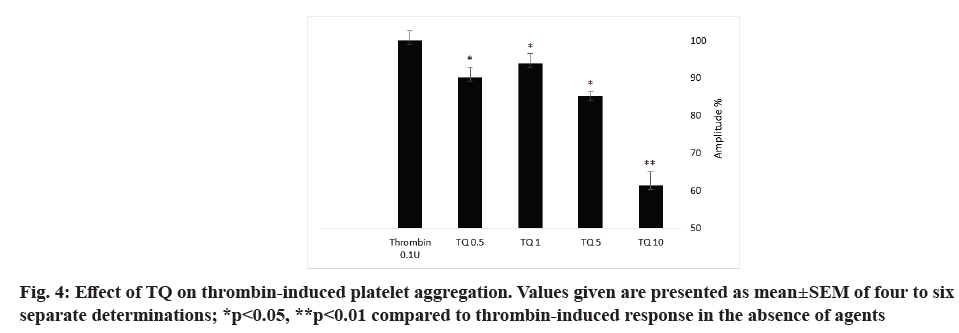
Thrombin is a strong platelet physiological agonist that induces cytosolic calcium mobilization, cytoskeletal reorganization, granule secretion, changes in cell shape and aggregation. In order to investigate the role of TQ on thrombin-evoked platelet aggregation, platelets were pretreated with different concentration (0.5, 1, 5 and 10 µM) of TQ at 37° for 15 min under stirring conditions (1200 rpm) and then stimulated with 0.1 U/ml thrombin. As shown in fig. 4 and Table 3, TQ significantly decreased the aggregation amplitude and the slope of thrombin-induced platelet aggregation but without effect on the lag-time. The maximum effect was recorded with 10 µM TQ with a 38±3 % inhibition in the amplitude and 38±1 % in the slope (p<0.05; N=6).
| Agent | Stimulus | Amplitude | Slope | Lag-time (s) |
|---|---|---|---|---|
| Thrombin | 78.7±1.8 | 65.2±2.9 | 35.8±4.9 | |
| TQ 10 µM | Thrombin | 48.3±2.8** | 38.0±1.0** | 39.5±0.3 |
| TQ 5 µM | Thrombin | 67.0±1.0* | 53.3±6.5* | 33.0±5.0 |
| TQ 1 µM | Thrombin | 74.0±2.0* | 48.6±6.9* | 42.5±1.5 |
| TQ 0.5 µM | Thrombin | 71.0±2.0* | 59.0±2.0* | 45.0±13.0 |
Note: Values given are presented as mean±SEM of four to six separate determinations; *p<0.05, **p<0.01 compared to thrombin-induced response in the absence of agents
Table 3: TQ Effect on Thrombin Induced Platelets Aggregation
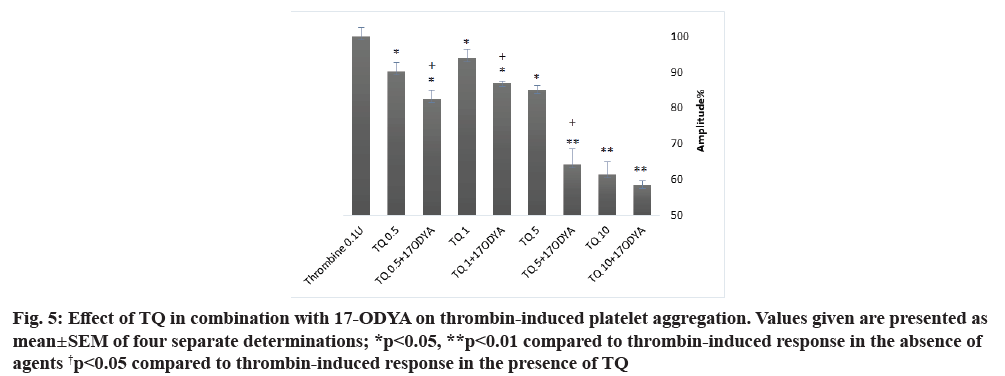
As shown in fig. 5 and Table 4, platelet treatment with 0.5, 1, 5 and 10 µM of TQ at 37° for 15 min in combination with 10 µM of 17-ODYA for 10 min significantly reduced thrombin-induced aggregation as compared to their respective controls. The aggregation amplitude and the slope were decreased and the lag- time was enhanced. When platelets were treated with TQ in combination with 17-ODYA, the inhibitory effect on thrombin-induced aggregation was greater than that observed with TQ alone (fig. 5 and Table 4; p<0.05 n=4).
| Agent | Stimulus | Amplitude | Slope | Lag-time (s) |
|---|---|---|---|---|
| Thrombin | 78.7±1.8 | 65.2±2.9 | 35.8±4.9 | |
| TQ 10 µM | 76.3±2.2 | 66.3±4.7 | 1707.0±35.6** | |
| TQ 10 µM | Thrombin | 48.3±2.8** | 38.0±1.0** | 39.5±0.3 |
| TQ 10 µM+17-ODYA | Thrombin | 46.0±1.0** | 37.0±0.5** | 42.0±6.0 |
| TQ 5 µM+17-ODYA | Thrombin | 50.5±3.5** | 46.0±7.0* | 54.0±1.0* |
| TQ 1 µM+17-ODYA | Thrombin | 68.5±0.4* | 44.3±3.1** | 43.0±1.7** |
| TQ 0.5 µM+17-ODYA | Thrombin | 65.0±4.0* | 50.5±0.5* | 49.0±5.0* |
Note: Values given are presented as mean±SEM of four separate determinations; *p<0.05, **p<0.01 compared to thrombin-induced response in the absence of agents
Table 4: TQ and 17-ODYA Inhibit Thrombin Induces Platelets Aggregation
Fig. 5: Effect of TQ in combination with 17-ODYA on thrombin-induced platelet aggregation. Values given are presented as mean±SEM of four separate determinations; *p<0.05, **p<0.01 compared to thrombin-induced response in the absence of agents †p<0.05 compared to thrombin-induced response in the presence of TQ
The effect of TQ on platelet physiology remains controversial, indeed, some studies report that only high doses of TQ (20-100 µg/ml) inhibit collagen and Adenosine Diphosphate (ADP) induced platelet aggregation in rats[20] and some others describe no effect on platelet aggregation[20-22].
To investigate the effect of TQ on human platelet aggregation, we incubated cells with increasing concentrations of TQ (0.5, 1, 5 and 10 µM). Our results showed for the first time that all TQ concentrations used enhanced platelet aggregation with similar amplitude as 0.1 U/ml of thrombin. Interestingly, TQ- induced platelet aggregation shows a significantly longer lag-time than stimulation with thrombin. No previous studies report that TQ is able to induce platelet aggregation in contrast they describe that platelet incubation with TQ was without effect[22]. This can be explained by the short time of TQ incubation (3 min). Our lag-time for platelet aggregation is in agreement with the study by Towhid et al.[18] reporting that 10 µM of TQ activates Phosphoinositide 3-kinases (PI3K), caspase 3 and increase in cytosolic calcium concentration, so platelet activation, after 30 min of TQ incubation.
Mitochondria play an important role in platelet functionality via ATP synthesis, redox state modulation and caspase activation[23,24]. By uncoupling mitochondria with rotenone in the presence of oligomycin A, we have found that mitochondria play a relevant role in TQ-induced human platelet. These findings are in accordance with a previous study describing that inhibition of mitochondria electron transport chain or oxidative phosphorylation reverse the effect of hyperglycemia on Reactive Oxygen Species (ROS) generation, suggesting a mitochondrial role. In addition, mitochondria can produce superoxide, responsible of platelets dysfunction in diabetic patients[25].
It was previously reported that TQ-induced apoptosis in chondrocytes and ovarian cancer cells via ROS generation and may participate in membrane electron-transport chains between complex I and complex III leading to superoxide anion and hydroxyl radical production[26-28].
We have further found that arachidonic acid metabolism might also play a role in the mechanism underlying platelet aggregation upon stimulation with TQ, as demonstrated by the inhibitory effect of treatment with 17-ODYA, which prevents the metabolism of arachidonic acid by CYP-450, on TQ-evoked response; thus suggesting a possible role for CYP-450-dependent arachidonic acid metabolites in TQ-induced platelet aggregation, as previously suggested[29].
Next, we have explored the possible role of TQ on agonist-induced platelet aggregation. Our results indicate that treatment with TQ significantly decrease thrombin-induced platelet aggregation. This is in consistent with previous study describing that platelet pretreatment with 40 µM TQ for 60 min inhibit thrombin induced aggregation. Another study proposed that TQ possesses anticoagulant effect and inhibits cancer cell-induced coagulation[22].
TQ has a very similar structure to Tert- Butylhydroquinone (TBHQ), a synthetic reduced quinone[30]. We have previously shown that acidic store depletion by 20 µM TBHQ, a Sarco/Endoplasmic Reticulum (SERCA) Calcium (Ca2+)-ATPase (SERCA)-3 selective inhibitor impairs thrombin- induced platelet aggregation[31]. Although speculative, TQ might mimic the effect of TBHQ on thrombin- induced aggregation.
Our results show that when platelets were treated with TQ in combination with 17-ODYA, the inhibitory effect was greater than that observed with TQ alone.
It was reported that TQ downregulates cyclooxygenases, 5-lypooxygenases, inhibit thromboxane B2, prostaglandin and leukotriene B4 metabolites formation[32]. In addition, TQ has the ability to inhibit CYP-450 enzymatic function and decrease the enzyme level[33]. Thus, we suggest that TQ might impair thrombin-induced platelet aggregation through: Inhibition of arachidonic acid metabolism, indeed Enamoto et al. describes such inhibition by N. sativa methanol extract[34]. In our previous work we demonstrate that the selective depletion of the dense tubular system leads to 5,6-Epoxyeicosatrienoic acid (5,6-EET) production and activation of Store-Operated Ca2+ Entry (SOCE), which was impaired by 17-ODYA, we have also noticed that 5,6 EET-induces Ca2+ entry needs a redox state maintained by a basal level of H O [35]; decreasing cytosolic Ca2+ concentration via SOCE inhibition and impairment of ROS production necessary to maintain a minimum redox state required for platelet physiological response.
Summarizing, TQ in the low micromolar range is able to induce human platelet aggregation after 25-28 min of incubation by a mechanism that requires functional mitochondria and CYP-450. In addition, TQ is able to attenuate thrombin-evoked platelet aggregation in a concentration-dependent manner.
Acknowledgements:
The author extends his appreciation to the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, for funding this research work through grant number 1794. The author acknowledges Dr. Juan Antonio Rosado and the Cell Physiology Research Group, Veterinary College, University of Extremadura, Spain.
Conflict of interests:
The authors declared no conflict of interest.
References
- Broos K, Feys HB, de Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev 2011;25(4):155-67.
[Crossref] [Google Scholar] [PubMed]
- Patti G, di Martino G, Ricci F, Renda G, Gallina S, Hamrefors V, et al. Platelet indices and risk of death and cardiovascular events: Results from a large population-based cohort study. Thromb Haemost 2019;119(11):1773-84.
[Crossref] [Google Scholar] [PubMed]
- Tirumala V, Klemt C, Xiong L, Chen W, van den Kieboom J, Kwon YM. Diagnostic utility of platelet count/lymphocyte count ratio and platelet count/mean platelet volume ratio in periprosthetic joint infection following total knee arthroplasty. J Arthroplasty 2021;36(1):291-7.
[Crossref] [Google Scholar] [PubMed]
- Yuan Y, Zhong H, Ye L, Li Q, Fang RS, Gu W, et al. Prognostic value of platelet count in lung cancer: A systematic review and meta-analysis. BMC Pulm Med 2020;20:96.
[Crossref] [Google Scholar] [PubMed]
- Royer DJ, George JN, Terrell DR. Thrombocytopenia as an adverse effect of complementary and alternative medicines, herbal remedies, nutritional supplements, foods, and beverages. Eur J Haematol 2010;84(5):421-9.
[Crossref] [Google Scholar] [PubMed]
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357(6):580-7.
[Crossref] [Google Scholar] [PubMed]
- Khader M, Bresgen N, Eckl PM. In vitro toxicological properties of thymoquinone. Food Chem Toxicol 2009;47(1):129-33.
[Crossref] [Google Scholar] [PubMed]
- Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res 2004;18(3):195-9.
[Crossref] [Google Scholar] [PubMed]
- Vaillancourt F, Silva P, Shi Q, Fahmi H, Fernandes JC, Benderdour M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem 2011;112:107-17.
[Crossref] [Google Scholar] [PubMed]
- Mansour MA, Nagi MN, El‐Khatib AS, Al‐Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT‐diaphorase in different tissues of mice: A possible mechanism of action. Cell Biochem Funct 2002;20(2):143-51.
[Crossref] [Google Scholar] [PubMed]
- Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-κB activation pathway by thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res 2008;6(6):1059-70.
[Crossref] [Google Scholar] [PubMed]
- Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther 2008;7(7):1789-96.
[Crossref] [Google Scholar] [PubMed]
- Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-γ pathway. Biochem Pharmacol 2011;82(5):464-75.
[Crossref] [Google Scholar] [PubMed]
- Royer DJ, George JN, Terrell DR. Thrombocytopenia as an adverse effect of complementary and alternative medicines, herbal remedies, nutritional supplements, foods, and beverages. Eur J Haematol 2010;84(5):421-9.
[Crossref] [Google Scholar] [PubMed]
- Srinivasan K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents and nutraceutical effects. Food Qual Saf 2018;2(1):1-6.
- Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002;9(1):69-74.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Jiang A, Batra V. Severe thrombocytopenia associated with black seed oil and evening primrose oil. Cureus 2020;12(6):e8390.
[Crossref] [Google Scholar] [PubMed]
- Towhid ST, Schmidt EM, Schmid E, Münzer P, Qadri SM, Borst O, et al. Thymoquinone‐induced platelet apoptosis. J Cell Biochem 2011;112(11):3112-21.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Foster C, Lecchi A, Quinton TM, Prosser DM, Jin J, et al. Protease-activated receptors 1 and 4 do not stimulate GI signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of GI signaling. Blood 2002;99(10):3629-36.
[Crossref] [Google Scholar] [PubMed]
- Nemmar A, Al‐Salam S, Zia S, Marzouqi F, Al‐Dhaheri A, Subramaniyan D, et al. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol 2011;164(7):1871-82.
[Crossref] [Google Scholar] [PubMed]
- Abou-Hozaifa BM. Effect of Nigella sativa L. and thymoquinone on rat platelet aggregation. Saudi Pharm J 2002;10(4):167-76.
- Rukoyatkina N, Butt E, Subramanian H, Nikolaev VO, Mindukshev I, Walter U, et al. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis 2017;8(6):e2898.
[Crossref] [Google Scholar] [PubMed]
- Barile CJ, Herrmann PC, Tyvoll DA, Collman JP, Decreau RA, Bull BS. Inhibiting platelet-stimulated blood coagulation by inhibition of mitochondrial respiration. Proc Natl Acad Sci USA 2012;109(7):2539-43.
[Crossref] [Google Scholar] [PubMed]
- Rosado JA, Lopez JJ, Gomez‐Arteta E, Redondo PC, Salido GM, Pariente JA. Early caspase‐3 activation independent of apoptosis is required for cellular function. J Cell Physiol 2006;209(1):142-52.
[Crossref] [Google Scholar] [PubMed]
- Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50(6):1491-4.
[Crossref] [Google Scholar] [PubMed]
- Yu SM, Kim SJ. Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp Biol Med 2013;238(7):811-20.
[Crossref] [Google Scholar] [PubMed]
- Taha MM, Sheikh BY, Salim LZ, Mohan S, Khan A, Kamalidehghan B, et al. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell Mol Biol 2016;62(6):97-101.
[Google Scholar] [PubMed]
- Martinovich GG, Martinovich IV, Vcherashniaya AV, Shadyro OI, Cherenkevich SN. Thymoquinone, a biologically active component of Nigella sativa, induces mitochondrial production of reactive oxygen species and programmed death of tumor cells. Biophysics 2016;61(6):963-70.
- Caccese D, Praticò D, Ghiselli A, Natoli S, Pignatelli P, Sanguigni V, et al. Superoxide anion and hydroxyl radical release by collagen-induced platelet aggregation-role of arachidonic acid metabolism. Thromb Haemost 2000;83(03):485-90.
[Crossref] [Google Scholar] [PubMed]
- Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo (a) pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev 1999:435-40.
[Google Scholar] [PubMed]
- Amor NB, Zbidi H, Bouaziz A, Isaac J, Hernández-Cruz JM, Salido GM, et al. Acidic-store depletion is required for human platelet aggregation. Blood Coagul Fibrinolysis 2009;20(7):511-6.
[Crossref] [Google Scholar] [PubMed]
- El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett 2006;106(1):72-81.
[Crossref] [Google Scholar] [PubMed]
- Ahmad A, Khan RM, Alkharfy KM, Raish M, Al-Jenoobi FI, Al-Mohizea AM. Effects of thymoquinone on the pharmacokinetics and pharmacodynamics of glibenclamide in a rat model. Nat Prod Commun 2015;10(8):1395-8.
[Crossref] [Google Scholar] [PubMed]
- Enomoto S, Asano R, Iwahori Y, Narui T, Okada Y, Singab AN, et al. Hematological studies on black cumin oil from the seeds of Nigella sativa L. Biol Pharm Bull 2001;24(3):307-10.
[Crossref] [Google Scholar] [PubMed]
- Ben-Amor N, Redondo PC, Bartegi A, Pariente JA, Salido GM, Rosado JA. A role for 5, 6‐epoxyeicosatrienoic acid in calcium entry by de novo conformational coupling in human platelets. J Physiol 2006;570(2):309-23.
[Crossref] [Google Scholar] [PubMed]