- *Corresponding Author:
- H. B. Long
Department of Spine Surgery,
Zibo Central Hospital,
Zibo, Shandong 255036,
China
E-mail: ti94017@126.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “113-117” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To compare and discuss the early efficacy of infliximab and adalimumab for the treatment of ankylosing spondylitis and their impacts on inflammatory markers is the objective of the study. A total of 180 patients with ankylosing spondylitis admitted to our hospital from June 2017 to May 2020 were selected and divided into an infliximab group and an adalimumab group by block randomization, with 90 cases in each group. The infliximab group was treated with infliximab and the adalimumab group was treated with adalimumab. Morning stiffness duration, the visual analog scale score for lower back pain and the bath ankylosing spondylitis disease activity index before and after treatment were evaluated, and the levels of serum C-reactive protein, interleukin-6, tumor necrosis factor alpha and erythrocyte sedimentation rate were measured. After treatment, the morning stiffness duration, visual analog scale score for low back pain, bath ankylosing spondylitis disease activity index and serum C-reactive protein, interleukin-6, tumor necrosis factor alpha and erythrocyte sedimentation rate levels in both groups were decreased compared with the conditions before treatment (p<0.05). The morning stiffness time, visual analog scale score for lower back pain and bath ankylosing spondylitis disease activity index in the adalimumab group were lower than the infliximab group (p<0.05), while serum C-reactive protein, interleukin-6, tumor necrosis factor alpha and erythrocyte sedimentation rate were higher than the infliximab group (p<0.05). For ankylosing spondylitis patients, adalimumab had a greater impact than infliximab on morning stiffness duration, visual analog scale score and bath ankylosing spondylitis disease activity index but a less impact than infliximab on serum inflammatory cytokines.
Keywords
Infliximab, adalimumab, ankylosing spondylitis, inflammatory markers, serum C-reactive protein, interleukin-6, tumor necrosis factor alpha, erythrocyte sedimentation rate
Ankylosing Spondylitis (AS) is a chronic progressive inflammatory disease primarily affecting the axial and sacroiliac joint of the spine. AS has long duration and a relatively high disability rate, greatly affecting the health of patients[1]. The treatment objectives of AS are to alleviate clinical symptoms and signs, restore physical functions, prevent joint injury and spinal complications, and improve quality of life[2]. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) can quickly improve the morning stiffness and lower back pain of AS patients, reduce joint swelling, increase the range of motion of joints and have become the first choice for the treatment of AS. However, there are a wide range of medications under the NSAID category and the efficacy of these medications is also basically the same for AS. Patients who are symptomatic or in the active stage have to receive continuous administration[3]. Slow-Acting Anti-Rheumatic Drugs (SAARDs) can improve peripheral arthritis in AS patients, but evidence showing the effect of such drugs on axial joint lesions is lacking[4]. Because the cause of AS is not completely clear yet, it’s difficult to have a breakthrough on treatment. Traditional anti-rheumatic drugs cannot control the disease quickly and effectively, and have serious adverse reactions, which limits their clinical application[5]. The appearance of biological agent Tumor Necrosis Factor alpha (TNF-α) antagonist greatly improves the prognosis of AS. TNF-α is a cytokine present in the immune response and inflammatory response. It has an elevated level in the synovial fluid of AS patients and plays an important role in joint destruction and pathological inflammation[6]. With the deepening of research, people found that TNF-α plays an important role in the cellular and molecular levels of AS and tried treating AS with its antagonists and the treatment results were relatively satisfactory[7]. TNF-α antagonists currently used include monoclonal antibodies (infliximab and adalimumab) and TNF receptor-antibody fusion proteins (Yisaipu®, a brand name of Etanercept). Studies found that TNF-α monoclonal antibody had a significant effect on the treatment of AS and could inhibit the level of inflammatory cytokines in AS[8,9]. The purpose of this study was to study the efficacy of infliximab and adalimumab for the treatment of AS and their impacts on serum inflammatory cytokines, to explore the differences in the treatment effect of different TNF-α antagonists on AS and to provide some reference for clinical promotion and application. The report is as follows. Patients who were admitted to our hospital and initially diagnosed with AS from June 2017 to May 2020 were selected. These patients met the diagnostic criteria for AS specified in the guidelines for the diagnosis and treatment of AS issued by Chinese Rheumatology Association of Chinese Medical Association, were in the active stage of the disease and voluntarily participated in this study and signed the informed consent form. Patients who had other osteoarticular disorders, rheumatic immune diseases, acute or chronic infectious diseases, malignant tumors or had received biological agent treatment and antirheumatic drug treatment, or had used glucocorticoids or antibiotics within 2 mo were excluded. Finally, a total of 180 cases were included and divided into an infliximab group and an adalimumab group by block randomization, with 90 cases in each group. The two groups of AS patients had no intestinal diseases or peripheral joint inflammation. X-ray examination showed that the joint space became wider, the edges blurred, the articular cartilage had an increased bone density and a beaded decreased-density zone inside. There was no significant difference in age, sex distribution, disease duration, Human Leukocyte Antigen B27 (HLA-B27) positive rate and family history between the two groups (p>0.05), as shown in fig. 1. This experimental scheme has been approved by the medical ethics committee of our hospital. Treatment methods in this study are shown below. Infliximab group includes intravenous infliximab (Shanxi Sun Biotechnology Co., Ltd., lot No. 151021, 160942; specification-10 ml:100 mg) 20 mg on 1 w, 2 w, 6 w and after that, every 8 w, 5 courses in total. Adalimumab group includes subcutaneous adalimumab (Shanghai Tongji Pharmaceutical Co., Ltd., lot no. 151106B, 170112A; specification-0.8 ml:40 mg) 40 mg, every 2 w. The clinical and serological markers before treatment and after 6 w of treatment (after treatment) were observed. Observation measures were recorded. Morning stiffness duration and Visual Analog Scale (VAS) score for lower back pain before and after treatment were recorded. Among them, morning stiffness refers to a long period of stiffness in the joint after immobility which gradually reduces after appropriate activity. Morning stiffness usually lasts no more than half an hour. Bouchard’s nodules and Heberden’s nodules can be seen in physical examination if hand joints are involved; a sense of friction can be touched if knee joints are involved, with no extraarticular manifestations such as vasculitis and subcutaneous nodules. The VAS ranges from 0-10 points according to the severity of pain. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is composed of 5 items including fatigue, spinal pain, arthralgia, enthesitis and spinal inflammation, in which spinal inflammation=(morning stiffness severity+morning stiffness duration)×0.5, and the scores of the other 4 items range from 0~10 points. The BASDAI=The sum of scores of the 5 items×0.2. Serum C-Reactive Protein (CRP), Interleukin-6 (IL-6), TNF-α and Erythrocyte Sedimentation Rate (ESR) levels were measured. Peripheral venous blood was extracted before and after treatment and serum was separated. Serum CRP was determined by immunoturbidimetry (reagents were purchased from Sigma, USA). Serum IL-6 and TNF-α were determined by the Enzyme-Linked Immunosorbent Assay (ELISA), (ELISA kits were purchased from Sigma, USA). ESR was determined by the Westergren method. Statistical Package for the Social Sciences (SPSS) 20.0 software was used for analysis. Measurement data were expressed as (x?±s); the independent samples t-test was used for comparison between the two groups and the paired samples t-test was used for comparison before and after treatment. Statistical significance could be attained when the p value was less than 0.05. Clinical measures before and after treatment between the groups was compared. Before treatment, there were no statistically significant differences in morning stiffness duration, VAS and BASDAI between the two groups (p>0.05). After treatment, the above measures in both groups were decreased than the conditions before treatment (p<0.05) and the adalimumab group was lower than the infliximab group (p<0.05) (fig. 2). Serum inflammatory markers between the groups were compared. Before treatment, there was no significant difference in serum CRP, IL-6, TNF-α and ESR levels between the two groups (p>0.05). After treatment, the above measures in both groups were decreased than the conditions before treatment (p<0.05). The adalimumab group was higher than the infliximab group (p<0.05) and the degree of decrease was also lower than the infliximab group (p<0.05) (Table 1). Adverse reactions between the groups were compared. In Infliximab group, 3 patients presented mild abnormal liver function (Alanine Transaminase (ALT) 40~50 U/l) on d 2 after medication and returned to normal in the re-examination after 1 w; 1 patient developed a rash at the injection site. In adalimumab group, 1 patient showed mild abnormal liver function (ATL 43~54 U/l) on d 2 after medication and returned to normal in the re-examination after 1 w, with no rash at the injection site. There was no significant difference between the two groups in comparison (p>0.05) (Table 2). The purpose of AS treatment is to relieve symptoms to the greatest extent, restore body functions, prevent joint injury, improve the quality of life of patients and prevent complications of spinal diseases. At present, there is no radical treatment, but timely diagnosis and rational use of drugs can control patients symptoms and improve their prognosis[10]. TNF-α antagonist is a kind of cytokines present naturally in inflammation and immune response. In the synovium with rheumatoid arthritis, TNFs are elevated and play an important role in pathologic inflammation and joint destruction. TNF-α antagonist can mediate inflammation and regulate the immunity in immune response. It mainly works to activate lymphocytes, release other cytokines, prostaglandins and metalloproteinases, etc., and promote angiogenesis and regulate adhesion molecules[11]. Infliximab, a chimeric human/mouse anti-TNF-α monoclonal antibody, was marketed in China in 2007[12]. It can be highly bound to soluble and transmembrane TNF-α, inhibiting the binding of TNF-α to receptors and thus leading to the inactivation of TNF-α[13]. A study found that infliximab could significantly improve the symptoms and functions of AS patients and could maintain remission for 5 y[14]. Adalimumab, a humanized anti-TNF-α monoclonal antibody and human TNFspecific recombinant Immunoglobulin G (IgG) antibody, was marketed in China in 2010[15]. Its specific binding to TNF-α can block the interaction of TNF-α with TNF receptors on the cell surface, thus antagonizing the activity of TNF-α[16]. Adalimumab is a completely humanized recombinant TNF-α antibody, which has lower immunogenicity than infliximab and less frequently causes autoimmune syndromes[17,18]. Moreover, it has a regulatory effect on TNF-induced biological responses and can change the adhesion molecule level in the leucocyte displacement. A study[19] found that adalimumab could effectively control the disease activity of AS and improve the physical function and quality of life of patients. Because infliximab and adalimumab have different biological structures, they show different clinical effects. The results of this study showed that both infliximab and adalimumab treatments reduced morning stiffness duration, VAS for lower back pain, BASDAI and the levels of serum CRP, IL-6, TNF-α and ESR of AS patients. The morning stiffness duration, VAS and BASDAI in the adalimumab group were lower than the infliximab group, while the serum CRP, IL-6, TNF-α and ESR levels in the infliximab group were decreased more significantly than the adalimumab group. The findings showed that both infliximab and adalimumab could effectively improve the clinical symptoms and reduce the level of serum inflammatory cytokines in patients with AS. Adalimumab was better than infliximab in improving clinical symptoms while infliximab was superior to adalimumab in reducing serum inflammatory cytokines. The mechanisms may be related to the different biological structures of the two drugs. The effect of adalimumab was due to its lower immunogenicity than infliximab and its nature as a completely humanized monoclonal antibody against TNF, but the specific reasons still need to be further studied.
| Group | CRP (mg/l) | IL-6 (ng/l) | TNF-α (ng/l) | ESR (mm/h) | |
|---|---|---|---|---|---|
| Infliximab group | Before treatment | 37.41±13.24 | 38.63±5.46 | 268.42±31.14 | 63.24±6.47 |
| After treatment | 3.11±1.04a | 23.56±3.41a | 22.40±8.64a | 21.03±3.24a | |
| Adalimumab group | Before treatment | 36.57±14.20 | 39.12±5.61 | 273.13±29.84 | 62.85±6.63 |
| After treatment | 9.73±1.31ab | 31.16±3.83ab | 53.15±12.53ab | 24.15±3.51ab |
Note: Compared with the conditions before treatment, ap<0.05; compared with the infliximab group, bp<0.05
Table 1: Comparison of Serum Inflammatory Markers between The Two Groups (x?±s, n=90)
| Group | Adverse reactions | Total number of adverse reactions |
|---|---|---|
| Infliximab group | Mild abnormal liver function (ALT 40~50 U/l) in 3 cases and rash in 1 case | 4 (4.4 %) |
| Adalimumab group | Mild abnormal liver function (ATL 43~54 U/l) in 1 case | 1 (1.1 %) |
Table 2: Comparison of Adverse Reactions between the Groups (x?±s, n=90)
Conflict of interests:
The authors declared no conflict of interest.
References
- Zão A, Cantista P. The role of land and aquatic exercise in ankylosing spondylitis: A systematic review. Rheumatol Int 2017;37(12):1979-90.
[Crossref] [Google Scholar] [PubMed]
- Hanson A, Brown MA. Genetics and the causes of ankylosing spondylitis. Rheum Dis Clin North Am 2017;43(3):401-14.
[Crossref] [Google Scholar] [PubMed]
- Bidad K, Gracey E, Hemington KS, Mapplebeck JC, Davis KD, Inman RD. Pain in ankylosing spondylitis: A neuro-immune collaboration. Nat Rev Rheumatol 2017;13(7):410-20.
[Crossref] [Google Scholar] [PubMed]
- Allouch H, Shousha M, Böhm H. Surgical management of ankylosing spondylitis (Bechterew's disease). Z Rheumatol 2017;76(10):848-59.
[Crossref] [Google Scholar] [PubMed]
- Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis-recent advances and future directions. Nat Rev Rheumatol 2017;13(6):359-67.
[Crossref] [Google Scholar] [PubMed]
- Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm 2017;23(8):859-67.
[Crossref] [Google Scholar] [PubMed]
- Kobayashi S, Yoshinari T. A multicenter, open-label, long-term study of three-year infliximab administration in Japanese patients with ankylosing spondylitis. Mod Rheumatol 2017;27(1):142-9.
[Crossref] [Google Scholar] [PubMed]
- Kwon SR, Jung KH, Lim MJ, Son MJ, Choi BH, Park SG, et al. The effect of anti-TNF treatment on osteoblastogenesis in ankylosing spondylitis: The number of circulating osteoblast-lineage cells in peripheral blood decreased after infliximab therapy in patients with ankylosing spondylitis. Clin Exp Rheumatol 2017;35(5):837-43.
[Google Scholar] [PubMed]
- Kaushik VV. Review of biosimilars of adalimumab. J Assoc Physicians India 2017;65(5):15-21.
- Chen J, LV LJ, Bao C. Research progress on prediction indexes of therapeutic effect of tumor necrosis factor alpha antagonists on ankylosing spondylitis. Chin J Rheumatol 2011;15(10):713-6.
- Li WS, Li QX, Cao SY. Mechanism of TGP in the treatment of inactive ankylosing spondylitis. China New Med 2014;6(9):601-7.
- Park W, Yoo DH, Miranda P, Brzosko M, Wiland P, Gutierrez-Ureña S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis 2017;76(2):346-54.
[Crossref] [Google Scholar] [PubMed]
- Kim BY, Kim HS. Phlegmonous gastritis in an ankylosing spondylitis patient treated with infliximab. Korean J Intern Med 2017;32(5):945.
[Crossref] [Google Scholar] [PubMed]
- Osman MS, Maksymowych WP. An update on the use of tumor necrosis factor alpha inhibitors in the treatment of ankylosing spondylitis. Expert Rev Clin Immunol 2017;13(2):125-31.
[Crossref] [Google Scholar] [PubMed]
- Rosas J, Llinares-Tello F, Senabre-Gallego JM, Barber-Vallés X, Santos-Soler G, Salas-Heredia E, et al. Obesity decreases clinical efficacy and levels of adalimumab in patients with ankylosing spondylitis. Clin Exp Rheumatol 2017;35(1):145-8.
[Google Scholar] [PubMed]
- Zhang Y, Mu XP, Lan MD, Luo YM, Ou YF, Li ZH, et al. Systematic evaluation of efficacy and safety of Adamu monoclonal antibody in the treatment of ankylosing spondylitis. Shandong Med J 2018;58(44):61-4.
- Lie E, Lindström U, Zverkova-Sandström T, Olsen IC, Forsblad-d'Elia H, Askling J, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: Results from the Swedish biologics register. Ann Rheum Dis 2017;76(9):1515-21.
[Crossref] [Google Scholar] [PubMed]
- Luchetti MM, Benfaremo D, Ciccia F, Bolognini L, Ciferri M, Farinelli A, et al. Adalimumab efficacy in enteropathic spondyloarthritis: A 12-mo observational multidisciplinary study. World J Gastroenterol 2017;23(39):7139.
[Crossref] [Google Scholar] [PubMed]
- Mease PJ, Van den Bosch F, Sieper J, Xia Y, Pangan AL, Song IH. Performance of 3 enthesitis indices in patients with peripheral spondyloarthritis during treatment with adalimumab. J Rheumatol 2017;44(5):599-608.
[Crossref] [Google Scholar] [PubMed]
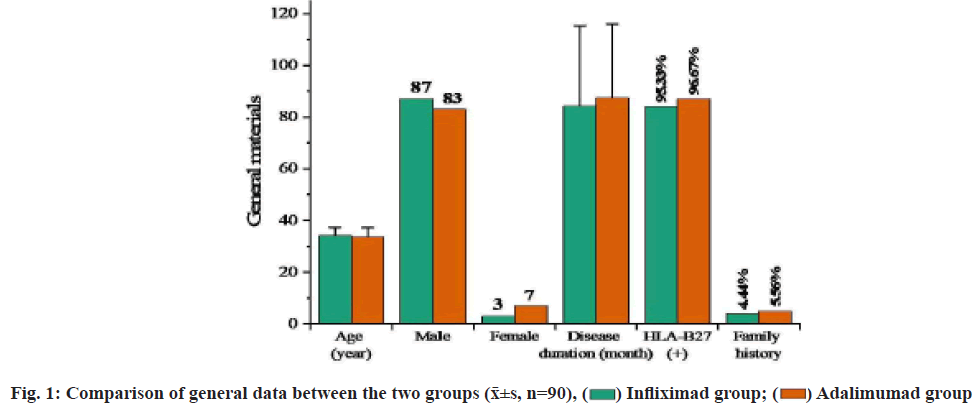
 Infliximad group;
Infliximad group;  Adalimumad group
Adalimumad group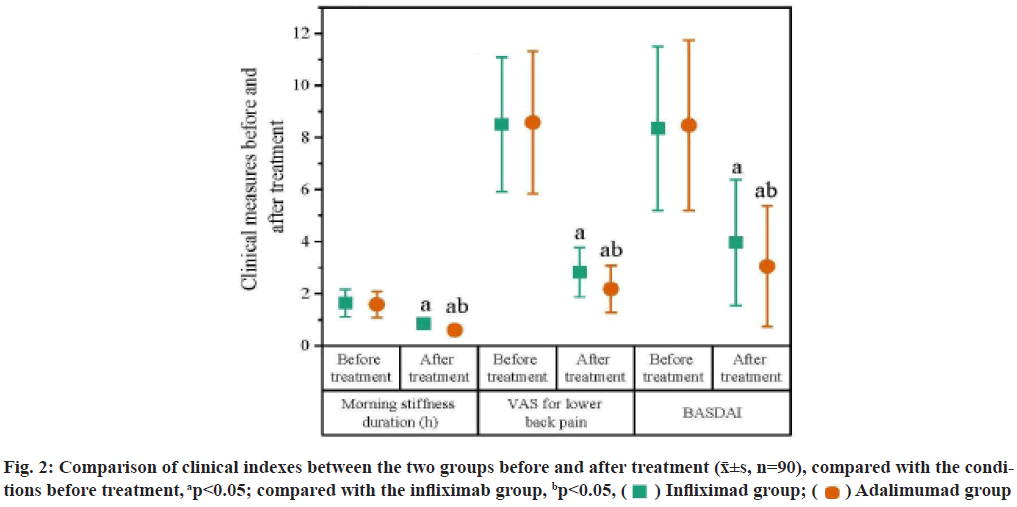
 Infliximad group;
Infliximad group;  Adalimumad group
Adalimumad group



