- *Corresponding Author:
- S. Ma Department of Ultrasound, the Second Hospital of Yinzhou, Ningbo 315100, China
E-mail: 1466525019@qq.com
| This article was originally published in a special issue: Special issue on “Drug Development and Human Health in China” | |
| Indian J Pharm Sci 2020:82(1)spl issue2;66-70 | |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present study was to observe clinical effect of the combination of Biejiaruangan compound tablet and entecavir under the assistance of real-time shear wave elastography for treating chronic hepatitis B liver fibrosis. About 180 patients who had been treated for chronic hepatitis B liver fibrosis at the Second Hospital of Yinzhou were enrolled. The real-time shear wave elastography was applied. The patients were randomized into the research group and the control group, each containing 90 patients. The control group was treated with entecavir, while the research group was treated with a combination of Biejiaruangan compound tablet and entecavir. The therapeutic efficacy was compared between both groups. Patients’ liver stiffness was obviously decreased after treatment (p<0.05). The research group had better hepatic function index than the control group (p<0.05). Moreover, a greater decrease in the hepatic fibrosis index and abdominal ultrasonography was observed in both groups after treatment, compared to those before therapy. The therapeutic efficacy of the research group was significantly better as well (p<0.05). For treating chronic hepatitis B liver fibrosis, a combination of Biejiaruangan compound tablet and entecavir with the aid of real-time shear wave elastography could achieve good clinical efficacy as well as significantly improve the therapeutic efficiency.
Keywords
Chronic hepatitis B liver fibrosis, RTSWE, Biejiaruangan compound tablet, entecavir
The continuous replication of hepatitis B virus in patients can cause liver cell damage and inflammatory reaction, resulting in cellular degeneration and necrosis. Moreover, the reactive proliferation of hepatic sinus collagen tissue is observed, which could further result in liver fibrosis[1-3]. Without in-time treatment, it will gradually develop into liver cirrhosis and even liver cancer. According to relevant surveys, the proportion of liver cirrhosis induced by hepatitis B virus infection is 30 % and proportion of liver cancer by hepatitis B virus infection is 45 %, respectively. The corresponding incidence rates in China are 60 and 80 %, respectively, which have caused great concern to the society[4,5].
Liver fibrosis is more common in chronic hepatitis B patients. The treatment for chronic hepatitis B-induced hepatic fibrosis involves etiological and antiinflammatory treatments. Entecavir is a guanine nucleoside analogue, which can effectively inhibit polymerase and the revertase of the hepatitis B virus. Entecavir is recognized as a major drug to treat hepatitis B virus in the Asia-Pacific region. Biejiaruangan compound tablet is a traditional Chinese medicine (TCM) preparation to treat liver fibrosis. It could activate blood circulation and dissipate stasis, producing ideal therapeutic effect on liver fibrosis. The real-time shear wave elastography (RTSWE) and pathological examination can lead to more accurate assessment of liver fibrosis. In this work, the effect of a combination of Biejiaruangan compound tablet and entecavir in treating chronic hepatitis B liver fibrosis under realtime shear wave elastography was investigated.
About 180 chronic hepatitis B liver fibrosis patients who were treated at the Second Hospital of Yinzhou were enrolled. Figure 1 shows the image of a chronic hepatitis B liver fibrosis patient. Patients were randomized into the research group and the control group, each containing 90 patients. The research group contained 50 male and 40 female patients, with an average age of 45.68±3.25 y. The control group contained 46 male and 44 female patients with an average age of 43.29± 4.02 y. Demographical data (sex, ages, degree of education, family situation) obtained from both groups were comparable.
Liver real-time shear wave elastography was applied to all patients. A color Doppler ultrasound instrument was adopted in this study. Supersonic aixplorer type, carrying convex array probe SC6-1, with the frequency of 1-6 MHz. The elastography sampling frame size was 4×3 cm. The patient was kept in the supine position and the probe was placed in the right intercostal space with RTSWE mode. Then, the probe was kept away from the thick vascular structure in the liver and the upper edge of the elastography sampling frame was placed about 1 cm below the liver capsule[6]. At the same time, the corresponding mean value was recorded in the imaging area using a 3 cm diameter circular quantitative detection zone. The liver stiffness (kPa) was measured 10 times and then averaged. The interquartile range should be less than 1/3 of the median, and a success rate of more than 60 % should be obtained, making the data a reliable result. The range of measurement was from 2.4 to 75.4 kPa.
The control group was treated with entecavir, while the research group was given a combination of Biejiaruangan compound tablet and entecavir. Each patient was given entecavir orally (Boluding, Sino American Shanghai Squib Pharmaceutical Ltd. H20052237), 0.5 mg each time, once a day, combined with antiviral therapy. Moreover, Biejiaruangan compound tablet (Inner Mongolia Furui medical Polytron Technologies Inc, Z19991011) was also taken orally, 2.0 mg each time, 3 times a day. Treatments were given for 48 consecutive weeks for both groups.
Liver function was detected by an automatic biochemical analyzer. Enzyme-linked immunosorbent assay was carried out to examine serum markers of hepatitis B virus. Polymerase chain reaction was performed to detect hepatitis B virus DNA. Color Doppler ultrasound was used to examine the diameter of the portal vein, portal vein blood flow, mean blood flow velocity, the long diameter and thickness of spleen.
SPSS21.0 Software was used for statistical analysis. All quantitative data were expressed as mean±standard deviation, with t test used for intergroup comparison. Enumeration data were expressed in the form of natural number (n)+percent (%), with chi-square test for intergroup comparison. The intergroup difference was considered statistically significant when p<0.05.
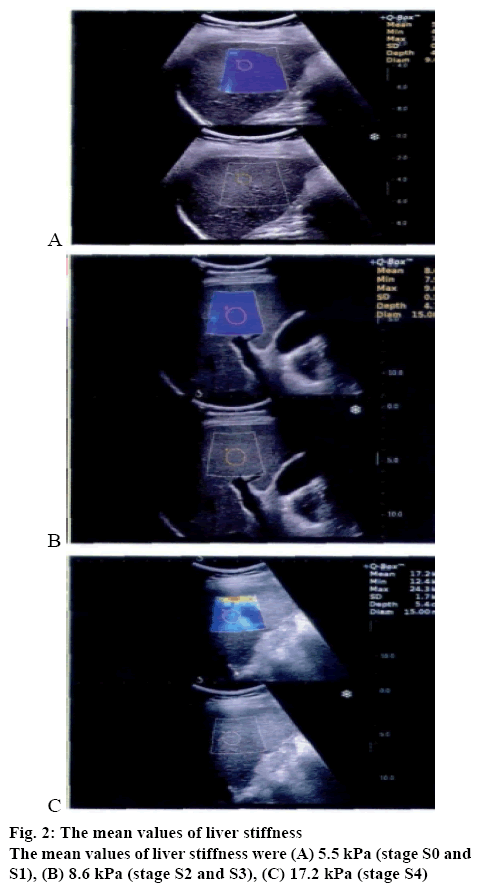
As shown in figures 2A, B and C, ultrasonography and RTSWE were applied to both groups. Before treatment, the mean liver stiffness of stage S0 and S1 was 5.9± 1.2 kPa, the mean liver stiffness of stage S2 and S3 was 10.8±2.1 kPa, and the mean liver stiffness of stage S4 was 20.6±3.5 kPa. Results showed that the liver stiffness was significantly decreased after treatment. The research group had significantly improved liver function indices. After treatment, the liver fibrosis indices were significantly improved in the research group than that in the control group.
Chronic hepatitis B is the main cause of liver fibrosis in China[7]. Liver fibrosis is a transition stage from chronic hepatitis B to liver cancer. The effective counter measures is to block or reverse the liver fibrosis[8]. Clinically, effective antifibrosis therapy includes etiological treatment, antiinflammatory treatment, and degradation of collagen, of which the etiological treatment is still a primary intervention. The important principle for treating chronic hepatitis B with liver fibrosis is to inhibit the replication of hepatitis B virus.
Entecavir is a guanine nucleoside analogue inhibiting the reverse transcription of the pregenome mRNA in hepatitis B virus as well as prevents the polymerase initiation and formation of HBV-DNA positive strand. Meanwhile, it could block the HBV-DNA replication, and exert favorable antiviral efficacy. Resistance to this drug is low, which makes it an important antiviral drug suitable for treating large-scale replication of chronic hepatitis B.
Traditional Chinese medicine has the quality of multicomponent, multi-link and multi-target, endowing it the advantages in the treatment of complex diseases. The main components of Biejiaruangan compound tablet are, Biejia, Curcuma zedoary, red peony, Ziheche, Cordyceps sinensis, Ban-lan-gen, Forsythia, Angelica, Sanqi, Dangshen, and Astragalus. Studies have shown that Biejiaruangan compound tablet can enhance the biological activity of matrix metalloproteinases and effectively degrade the over deposited extracellular matrix (ECM). It can not only inhibit the activation and proliferation of hepatic stellar cells (HSC), but also promote apoptosis, reducing the number of activated HSCs. In addition, it can inhibit the production of transforming growth factor-β (TGF-β) by ECM, and inhibit the effective scavenging of free radicals by hepatocyte lipid peroxidation, thus affecting the formation of liver fibrosis by multiple targets and exerting the effect of antiliver fibrosis.
RTSWE is a reliable indicator of liver fibrosis, which is safe, easy to operate, highly reproducible, and quantitative. Application of RTSWE can prevent 50- 60 % of patients from undergoing liver puncture. This study proved that the research group had better liver stiffness, liver function indexes and liver fibrosis indexes than the control group, which is consistent with the relevant findings[9-15] (Tables 1 and 2).
Table 1: Changes in the Liver Function Indexes of Two Groups
| Groups | Time | ALT (U/l) | AST (U/l) | TBIL (μmol/l) | ALB (g/l) |
|---|---|---|---|---|---|
| Research group (n = 90) | Before treatment | 115.90±60.96 | 98.03±60.12 | 22.30±10.19 | 38.56±3.20 |
| 48 weeks after treatment | 31.28±10.25 | 33.90±22.36 | 14.28±3.21 | 46.90±3.12 | |
| Control group (n = 90) | Before treatment | 116.88±71.28 | 97.80±56.31 | 22.91±9.84 | 38.77±5.62 |
| 48 weeks after treatment | 66.04±12.39 | 53.18±30.28 | 19.03±3.52 | 40.25±6.08 |
Mean±standard deviation
Table 2: Comparison of the Liver Fibrosis Indexes Between Two Groups
| Groups | Time | Diameter of the portal vein (cm) | Blood flow velocity (cm/s) | Long diameter of spleen (cm) | Thickness of spleen (cm) | Portal vein blood flow (ml/min) |
|---|---|---|---|---|---|---|
| Research group (n = 90) | Before treatment | 1.69±0.84 | 13.03±2.12 | 12.09±0.20 | 4.96±0.21 | 1346.96±180.84 |
| 48 weeks after treatment | 1.22±0.25 | 16.90±1.34 | 10.28±0.21 | 4.90±0.67 | 1225.79±146.03 | |
| Control group (n = 90) | Before treatment | 1.88±1.20 | 13.08±1.31 | 12.91±0.88 | 5.10±0.62 | 1339.05±167.46 |
| 48 weeks after treatment | 1.39±0.41 | 13.18±1.90 | 11.78±0.52 | 4.79±0.08 | 1289.00±190.52 |
Mean±standard deviation
RTSWE could accurately determine the degree of liver fibrosis. Moreover, it could minimize the chances of invasive examination, reduce patients’ pain and alleviate the economic burden. The drug combination of Biejiaruangan compound tablet and entecavir could achieve good clinical efficacy for chronic hepatitis B liver fibrosis. Entecavir could inhibit the polymerase and the revertase of the hepatitis B virus. Biejiaruangan compound tablet is a TCM preparation that could activate blood circulation and dissipate stasis, producing ideal therapeutic effect on liver fibrosis. In conclusion, the combination of Biejiaruangan compound tablet and entecavir under the assistance of real-time shear wave elastography can achieve good results for treating chronic hepatitis B liver fibrosis.
Acknowledgements:
Zhejiang Provincial Medical Science and Technology Planning Project (No. 2016KYB273); the first two authors (Ye Fang and Long Sun) contributed equally to this work.
References
- Liu BX, Liang JY, Xie XY. Preliminary comparative study of shear wave elastography versus quasi-static elastography on evaluation of thyroid nodules. Chin J Med Ultrasound 2014;11(11):925-31.
- Ding Y, An ZY, Wang SJ. Efficacy evaluation of entecavir anti-fibrosis treatment in chronic hepatitis B with real-time shear wave elastography and ultrasound quantitative score. J China Clin Med Imag 2016;27(06):407-10.
- Duan XL, Li GY Li SM. Meta-analysis of influences of FufangBiejiaRuanganPian combined with entecavir on serum liver fibrosis markers of chronic hepatitis B. Chin J Hosp Pharm 2015;35(19):1762-5.
- Wroblewska K, Kucinska M, Murias M, Lulek J. Characterization of new eye drops with choline salicylate and assessment of their irritancy by in vitro short time exposure tests. Saudi Pharm J 2017;23(4):407-12.
- Moty SGA, Hussein MA, Aziz SAA, Abou-Salim MA. Design and synthesis of some substituted thiazolo [3, 2-a] pyrimidine derivatives of potential biological activities. Saudi Pharm J 2016;24(2):119-32.
- Hazra M, Mandal DD, Mandal T, Bhunia S, Ghosh M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharm J 2018;23(4):429-36.
- Mo JR, Huang SY. Clinical Efficacy of FufangBiejiaRuangan Tablet Combined with Entecavir in Treating Chronic Hepatitis B Patients with Liver Fibrosis. Chin Foreign Med Res 2018;16(17):11-13.
- Xu Z. Clinical study of compound Biejiaruangan tablets combined with entecavir in treatment of chronic hepatitis with hepatic fibrosis. Chin Commun Doctors 2018;34(19):113-14.
- Ofori-Kwakye K, Mfoafo KA, Kipo SL, Kuntworbe N, ElBoakye-Gyasi M. Development and evaluation of natural gum-based extended release matrix tablets of two model drugs of different water solubilities by direct compression. Saudi Pharm J 2016;24(1):82-91.
- Attari Z, Bhandari A, Jagadish PC, Lewis S. Enhanced ex vivo intestinal absorption of olmesartan medoxomil nanosuspension: Preparation by combinative technology. Saudi Pharm J 2016;24(1): 57-63.
- Mehta BK, Banerjee S. Characterization of Cognitive Impairment in Type 2 Diabetic Rats. Indian J Pharm Sci 2017;79(5):785-793.
- Lungu M, Romila A, Nechita A. Neurological Manifestations in Thyroiditis. Acta Med Med 2017;33(3):369-76.
- Nawaz A, Batool Z, Ahmed S, Tabassum S, Khalig S, Mehdi BJ, et al. Enriched Environment Palliates Nicotine-Induced Addiction and Associated Neurobehavioral Deficits in Rats. Pak J Pharm Sci 2017;30S(6):2375-2381.
- Xu K, He G, Qin J. High-Efficient Extraction of Principal Medicinal Components from Fresh Phellodendron Bark (Cortex phellodendri). Saudi J Biol Sci 2018;25(4):811-5.
- Ozgun A, Sargin A, Karaman S. The Relationship between the Trendelenburg Position and Cerebral Hypoxia in Patients Who Have Undergone Robot-Assisted Hysterectomy and Prostatectomy. Turk J Med Sci 2017;47(6):1797-803.