- *Corresponding Author:
- M. A. Alzahrani
Department of Urology, College of Medicine, Majmaah University, Al-Majmaah 15341, Saudi Arabia
E-mail: ma.alzahrani@mu.edu.sa
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “67-78” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Our major aim was to systematically study the effectiveness of phosphodiesterase type 5 inhibitors in treating chronic prostatitis stage III and performed meta-analysis to evaluate the changes occurred in posttreatment scores of National Institutes of Health chronic prostatitis symptom index, international index of erectile function and international prostate symptom score. This meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analyses guidelines. Comprehensive research was performed by using online resources like PubMed and the Wiley online library database to gather the relevant literature produced from the y 2010 to 2022. Two authors were assigned to independently collect the relevant information including author name, region of study, study design, treatment, publication year, sample size, voiding parameters, study inclusion and exclusion criteria, urological conditions, drug type, international prostate symptom score, international index of erectile function score, etc. In final screening 17 relevant studies were found. Out of these 17, 5 contained surgical procedure while 4 were narrative analysis. One study was incomplete regarding information. Finally, 7 studies were included for analysis. Total of 584 patients were involved in the selected studies. Three out of seven are from Italy region while two are from Japan and one was from Korea and Egypt respectively. Most of these studies used tadalafil monotherapy while one placebo-controlled trial. A significant difference in pain score, international prostate symptom score domain and National Institutes of Health chronic prostatitis symptom index were observed after consuming 5 mg tadalafil monotherapy. Everyday consumption of oral phosphodiesterase type 5 gave positive outcomes in terms of voiding. A significant difference was observed after using tadalafil monotherapy in the majority of the studies. Hence, tadalafil alone can be used to treat the type III chronic prostatitis.
Keywords
Phosphodiesterase type 5 inhibitors, chronic prostatitis, tadalafil, ciprofloxacin
The prostate is the male sexual gland that has ability to secrete prostatic fluid, which forms the main part of the ejaculated sperm volume. The prostate grows under the testosterone control where the hormone made by the testicles and adrenal glands[1,2]. Chronic prostatitis as a usual disease, is categorized as Category III prostatitis by the United States National health institution. It has a lifetime prevalence rate about 1.8 %-8.2 %[3]. Chronic prostatitis is categorized as category III prostatitis by the United States National health institution. This condition is characterized by severe abdominal, pelvic and perineal pain associated with irritating low urinary tract symptoms. This condition is also marked by the absence of Urinary Tract Infection (UTI) and is associated with sexual dysfunction or painful voiding. Patients of chronic prostatitis also reported Lower Urinary Tract Symptoms (LUTS) including urgency, frequency, hesitancy and poor interrupted flow[4-6]. A patient with chronic prostatitis presents with pelvic pain or discomfort for more than 3 mo and this forms the basis of clinical diagnosis. Previous literature has reported chronic prostatitis prevalence ranging between 2.2 %-13.8 %. There has been a number of studies which reports association between bacterial infection or pelvic floor dysfunction and chronic prostatitis, however the etiology still remains unknown[7,8]. Although it is more commonly seen in younger age groups, but it can affect individuals of any age groups[9]. The quality of life of an individual is also compromised as it causes severe pain and sexual disorder. The sexual disorder can present with a myriad of symptoms, most common being erectile dysfunction, which is reported in 30 %-50 % of cases. Evidences suggest that Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) is an independent risk factor for erectile dysfunction with an odds ratio of 3.62. Severe pain and sexual problem affect the patient’s quality of life. The nature of sexual diseases is highly heterogeneous however 30 % to 50 % of cases of chronic prostatitis reported erectile dysfunction. This indicates that CP/CPPS is the independent factor causing erectile dysfunction with 3.62 odd ratios[10-12]. After observing symptoms like chronic pain, mental stress and sexual dysfunction, patients generally feel depression and worry, which makes patients incorrect cognition of the illness and therapy[3].
The common pathophysiological pathways of prostatitis pain and sexual dysfunction are not completely understood however previous literatures claim that elevated Rho-kinase activation and impaired nitric oxidase synthase in pelvic muscles may cause high intraprostatic pressure and thus interferes with smooth muscle relaxation of penile tissues this in turn results in prostatitis or erectile dysfunction[12-14]. Atherosclerosis and metabolic syndrome also plays a major role in prostate-associated sexual dysfunction[13]. Growing evidence suggests that Phosphodiesterase Type 5 (PDE5) inhibitors may have a major role in the treatment of CP/CPPS[15,16].
Tadalafil is a successful oral medication to inhibit PDE5 and doctors use it as a treatment option for men who have erectile dysfunction[17]. Studies prove that tadalafil can decrease Rho-kinase activity. Studies conducted on male rats reported that tadalafil treatment significantly suppresses pelvic pain and prostatic inflammation. This treatment also regulates the Nitric Oxide (NO)/cyclic Guanosine Monophosphate (cGMP) which helps in reducing prostatic smooth muscle contractions. Additionally, tadalafil also decreases the inflammatory markers and minimize atherosclerosis and inflammation[18-20]. A study by Grimsley et al.[21] reported that PDE5 inhibitors treatment can significantly decrease the prostatitis symptoms in CP/CPPS patients associated with erectile dysfunction.
However, only a few studies are conducted to measure the effectiveness of PDE5 inhibitors for treating chronic prostatitis patients[22-25]. In view of this, our study aims to systematically review all the relevant literature conducted in the years between 2010 and 2022 and perform meta-analysis to conclude the results.
Materials and Methods
This meta-analysis study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Comprehensive literature search was done using online database like PubMed and the Wiley online library to gather the relevant literature between the y 2010 to 2022. Following keywords were used with various combinations to search the relevant database: Chronic prostatitis, type III prostatitis, CPPS, chronic prostatitis treatment, oral phosphodiesterase inhibitors, tadalafil, Viagra, tamsulosin, ciprofloxacin. Manual cross-referencing of the reference list of the selected articles was done to ensure that no relevant article is missed in the study.
Study selection:
The selection of the articles was done based on the following inclusion and exclusion criteria.
Inclusion criteria: Patients sexually active, age between ≥18 to ≤45 y old with persistent prostatitis like symptoms for more than 3 mo that are documented by history, clinical examination and Meares-Stamey 4-glass test and classified as category IIIA or IIIB; patients with type III CP/CPPS associated with erectile dysfunction for at least 6 mo; National Institutes of Health-Chronic Prostatitis Symptom Index (NIH‐CPSI) pain sub‐score≥4; total International Prostate Symptom Score (IPSS)≥8.
Exclusion criteria: The studies were not conducted on participants having active UTI, symptoms for less than 3 mo; those studies in which patients were diagnosed with UTI, acute and chronic bacterial prostatitis, benign prostatic hyperplasia, having sexually transmitted disease or documented infection, invasive prostate-related procedures (transurethral resection of the prostate, transurethral incision of the prostate or transurethral needle ablation), LUTS without significant pain, significant signs and symptoms of obstructive voiding, or prostate volume of >50 cm were excluded; studies that performed urethral catheterization on urethral stricture or reported peptic ulcer, neurogenic bladder dysfunction or bladder calculi.
Information sources:
Comprehensive research was performed using online resources like PubMed and Wiley online library database to gather the relevant literature from 2010 to 2022.
Search strategy:
Following keywords with various combinations were used to access the data: chronic prostatitis, Type III prostatitis, CPPS, chronic prostatitis treatment, oral phosphodiesterase, tadalafil, Viagra, tamsulosin, ciprofloxacin. To identify the additional studies, we also performed manual research on the reference list of selected articles.
Study selection process:
Two independent authors, Sheetal Kalra and Puneeta, having 15 y of experience in handling meta-analysis, were assigned to review the patient’s age, diagnostic method, duration of erectile dysfunction, NIH‐CPSI pain sub‐score and IPSS scores. Baseline parameters were critically reviewed and those studies with less or zero baseline and post-treatment outcomes were omitted. These two authors also rejected all the case studies and duplicate articles. Those studies that met the following inclusion criteria were finalized for the study and meta-analysis.
Data extraction:
Two authors were assigned to independently collect the relevant information, including author sir name, region of study, study design, treatment name, publication year, sample size, voiding parameters, study inclusion and exclusion criteria, urological conditions, drug type, IPSS, International Index of Erectile Function (IIEF), etc. In the case of disagreement, a third reviewer was asked to resolve the matter with consensus. After the data collection process, two independent authors processed the quality assessment of included articles.
Outcomes or endpoints of research:
The primary outcomes were phosphodiesterase inhibitor drug characteristics, case and control group mean age, drug type, duration, and pre and post-treatment scores, including mean NIH-CPSI score, mean IPSS score, and mean IIEF scores. Adverse events and discontinuation of the drugs were considered as study endpoints. However, the secondary outcomes involve quality of life, urinary domain, voiding parameters and LUTS.
Quality assessment:
Cochrane risk of bias was used for quality assessment with scores ranges from 0-7 scales. The main items of this scoring include title, abstract, sample size calculations, allocation of patients and allocation concealment.
Statistical analysis:
The relevant information was conceived on the excel sheet. Statistical Package for the Social Sciences (SPSS) version 23.0 was used for data analysis. The quantitative parameters are summarized as mean and Standard Deviation (SD). Pooled prevalence was estimated by using the DerSimonian-Laird technique with a fixed-effects model. It was used to generate proportions with 95 % Confidence Intervals (CI) and model-fitted weights. Cochrane Q test and I square (I2) statistics were used to determine the heterogeneity across the studies. I2>50 % and p<0.10 was considered significant. Publication bias was estimated by using the Egger’s regression analysis and its significance was set at p value<0.05.
Results and Discussion
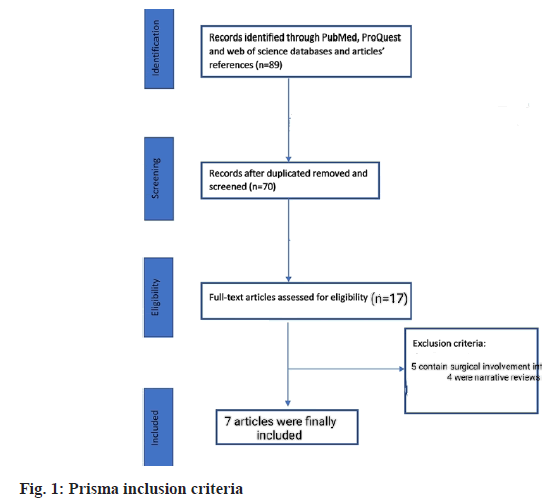
In final screening 17 studies were found relevant. Out of these 17, 5 contained surgical involvement while 4 were narrative analysis. One study was incomplete regarding information. Finally, 7 studies were included for analysis. Total of 584 patients were involved in selected studies. 3 out of 7 were of Italy region while 2 were of Japan and 1 was of Korea and Egypt respectively. Most of these studies used tadalafil monotherapy while one placebo-controlled trial (fig. 1).
The sample size ranges from 14 to 80 patients. All of these studies were case-control except one in which no control group was involved. The inclusion criteria of these studies apply to sexually active patients of age group 18 or above having chronic prostatitis type III. The survey of Cantoro et al.[22] includes patients consuming phosphodiesterase inhibitors for the first time. They induce the drug when prostatitis volume reaches 15 to 25 ml. However, the analysis of Matsukawa et al.[23] has patients with 20 ml prostatitis volume. Except for these two mentioned studies, clarification of prostatitis volume was missing in others. UTI was the common exclusion criterion of the selected studies. Detailed characteristics are mentioned in Table 1[22-30].
| Study (Year) | Location (country) | Sample size | Inclusion criteria | Exclusion criteria | |
|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||
| Cantoro et al.[22] (2013) | Italy | 20 | 24 | Chronic prostatitis type III associated with erectile dysfunction since 6 mo Sexually active patients Not using any PDE-5 inhibitors before and prostate volume range 15 to 25 ml |
Urinary system infections, neoplasia, congenital disorders, previous surgeries, urolithiasis and hyperactive bladder |
| Kong et al.[26] (2014) | Korea | 48 | 40 | Fulfilled the requirements for NIH category III CP/CPPS | Symptoms for less than 3 mo, UTI, invasive prostate-related procedures, LUTS without significant pain, significant signs and symptoms of obstructive voiding or prostate volume of >50 cm3 |
| Matsukawa et al.[23] (2020) | Japan | Case: 45 | Control: 42 | Age ≥45 y, pain sub score≥4, IPSS≥8 and prostate volume≥20 ml | Bacterial prostatitis had an active UTI, neurogenic bladder dysfunction or bladder calculi |
| Benelli et al.[27] (2018) | Italy | Case: 14 | Not reported | Presence of CP/CPPS symptoms for at least 3 mo and negative Meares-Stamey test | Urinary infections or other urological disease and treated with antimicrobial drugs during the previous 3 mo |
| Yamanishi et al.[28] (2020) | Japan | 80 | 81 | Male patients with LUTS, Overactive Bladder (OAB) symptoms despite at least 8 w of treatment | Anticholinergics, cholinergics, beta (β)‐agonists or antagonists, α‐blockers, UTI etc. |
| Tawfik et al.[29] (2022) | Egypt | 59 | 56 | Recurrent/persistent symptoms after previous treatment | Mild symptoms (total CPSI score≤14), IIEF-5≥22, abnormal Digital Rectal Examination (DRE) or Prostate-Specific Antigen (PSA) values, bladder stones, lower UTI |
| Sebastianelli et al.[30] (2019) | Italy | 25 | 50 | Age>40 to 80 y, mild to severe erectile dysfunction, IPSS>7 | Prostatic cancer, bladder lithiasis, UTI, bladder neck obstruction |
Table 1: Characteristics of The Selected Studies
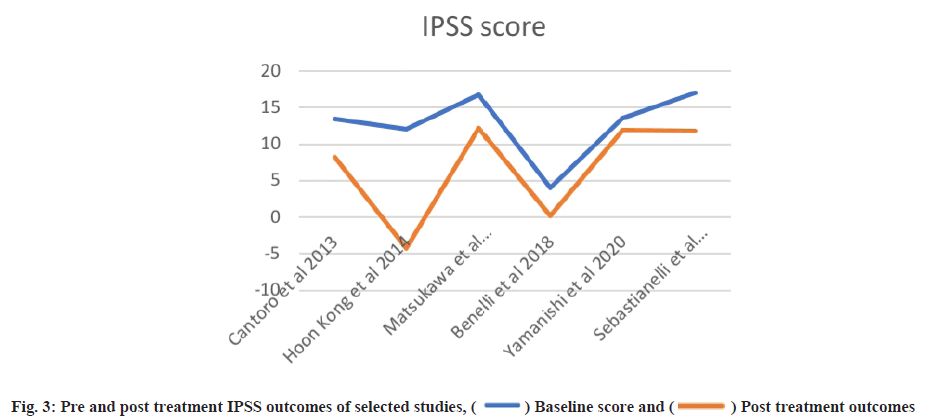
Pre-treatment outcomes were explained here. Patients with a mean age of 32.04 y were observed in a study by Cantoro et al.[22] with mean pre-treatment IPSS, IIEF and NIH-CPSI scores of 13.52±1.49, 12.41±0.66 and 17.51±1.92, respectively. They examined the overall pre-treatment scores before classifying patients into case-control groups. However, the study of Kong et al.[26] used both monotherapy and combined therapy using levofloxacin. The mean NIH-CPSI, IPSS and IIEF scores in the monotherapy group were 22.1±1.5, 12.0±1.2 and 18.8±6.2. The combined therapy group reported 14.1±1.0 baseline score of IPSS, 18.2±6.0 for IIEF and a comparatively lower NIH-CPSI score of 19.5±1.6. A study by Benelli et al.[27] only reported case groups. NIH-CPSI score was not mentioned in the study of Sebastianelli et al.[30] while the study of Yamanishi et al.[28], Benelli et al.[27] and Matsukawa et al.[23] missed the IIEF scores. Two studies also missed the IPSS score information. Detailed data presentation of baseline outcomes is shown in Table 2.
| Study (Year) | Age Mean/Median/SD | Chronic prostatitis type* | Mean IPSS | Mean IIEF | Mean NIH-CPSI |
|---|---|---|---|---|---|
| Cantoro et al.[22] (2013) | 32.04±3.15 y (All cases) | Type III | 13.52±1.49 | 12.41±0.66 | 17.51±1.92 |
| Kong et al.[26] (2014) | 44.2±6.9 45.3±7.0 y |
Type III | Group L: 12.0±1.2 Group ML: 14.1±1.0 |
Group L: 18.8±6.2 Group ML: 18.2±6.0 |
Group L: 22.1±1.5 Group ML: 19.5±1.6 |
| Matsukawa et al.[23] (2020) | Case group: 67.9±6.9 Control group: 65.9±9.9 |
Type III | Case: 16.8±4.5 Control: 16.5±6.2 |
Not reported | 19.7±4.6 |
| Benelli et al.[27] (2018) | 40.14±8.63 | Type III | 4±2.85 | Not reported | 27.57±4.18 |
| Yamanishi et al.[28] (2020) | Group 1: 72.3±8.0 Group 2: 72.4±7.4 |
Type III | Group 1: 13.637±6.751 Group 2: 13.506±5.872 |
Not reported | Group 1: 12.363±5.733 Group 2: 12.333±5.438 |
| Tawfik et al.[29] (2022) | Case: 39.9±3.9, Control: 39.7±4.7 |
Type III | Not reported | 17.6±2.2 | 24.21±5.05 |
| Sebastianelli et al.[30] (2019) | Case: 65.5±6.3 Control: 65.7±9.1 |
Type III | Case: 17±6.1 Control: 18.8±5.9 |
Case: 13.8±5.2 Control: 12±3.5 |
- |
Note: *NIH classification; Group L: Only levofloxacin and Group ML: Monotherapy and combined therapy using levofloxacin
Table 2: Pre-Treatment Findings of Patients Mentioned In Selected Studies
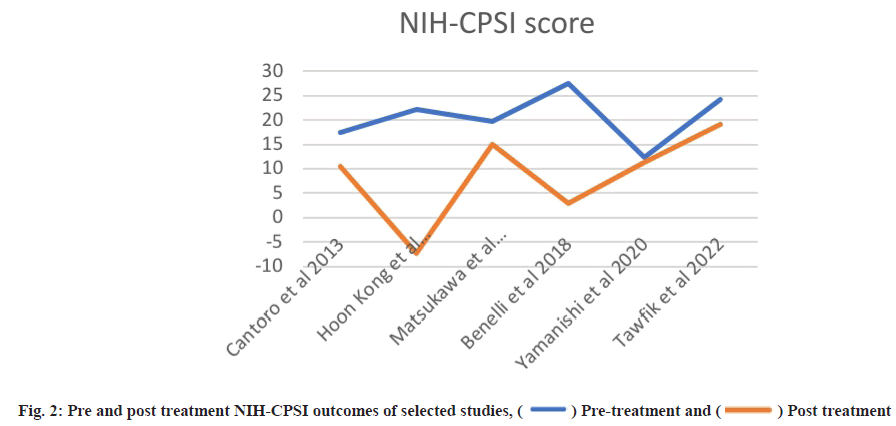
Post-treatment outcomes were shown here. Posttreatment IPSS, IIEF and NIH-CPSI scores are detailed in Table 3 and Table 4, while subdomain analysis of NIH-CPSI scores constitutes in Table 5. One study missed the NIH-CPSI score, whereas IIEF posttreatment outcomes were missing in three studies. IPSS scores were also not observed in two studies. Subdomain analysis of NIH-CPSI score was conducted in all studies except two. The subdomains include pain, urinary symptoms and quality of life. However, the overall score was mentioned in all studies. Analysis of voiding parameters also noted many missing outcomes, including Qmax range, volume and Post-Void Residual (PVR) volume. Detailed analysis is shown in Table 5. IPSS subdomain analysis is shown in Table 6. In fig. 2 and fig. 3, significant changes were observed in pre and post-treatment NIH-CPSI and IPSS scores.
| Study (Year) | Mean IPSS | Mean IIEF | Mean NIH-CPSI | Follow up duration | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study group | Control group or combined therapy group | Group 1 | Group 2 | Group 1 | Group 2 | ||||||
| Cantoro et al.[22] (2013) | 8.23±0.72 | 8.07±0.91 | 17.83±1.46 | 18.75±1.24 | 10.54±1.35 | 9.74±1.98 | More than 60 d | Tamsulosin therapy has the same effectiveness of the most expensive combination therapy (tamsulosin and sildenafil) | |||
| Kong et al.[26] (2014) | −4.3±0.2 | −1.1±0.2 | 7.8±1.8 | 0.2±2.4 | −7.2±0.1 | −3.2±0.2 | 6 w | No significant outcomes reported | |||
| Matsukawa et al.[23] (2020) | 12.2±5.2 | 14.0±6.4 | - | - | 15.1±4.7 | 12.8±5.2 | 12 w | Significant outcomes of tadalafil observed | |||
| Benelli et al.[27] (2018) | 0.214286±0.42 | - | 2.928571±2.43 | 12 w | Reduction in pain and significant improvement in quality of life | ||||||
| Yamanishi et al.[28] (2020) | 11.934±7.204 | 9.961±6.478 | Not reported | Not reported | 11.303±6.769 | 8.584±5.334 | 12 w | Tadalafil/mirabegron combination therapy is better than tadalafil monotherapy | |||
| Tawfik et al.[29] (2022) | - | - | 21±1.8 | 17.46±3.56 | 19.1±5.26 | 23.79±5.2 | 6 w | Significant improvement of all CPSI domains (pain, micturition, Quality of Life (QOL) and total scores) compared to baseline | |||
| Sebastianelli et al.[30] (2019) | 11.8±6.3 | 11.5±5.4 | 19.9±5.1 | 17.7±3.3 | - | - | 12 w | Tadalafil 5 mg daily monotherapy is able to improve erectile dysfunction and overall LUTS | |||
Table 3: Post Treatment Outcome of PDE5 Inhibitors
| Study (Year) | NIH-CPSI | Study group (Monotherapy) | Control group (Combined therapy or α-blockers) |
|---|---|---|---|
| Cantoro et al.[22] (2013) | NIH-CPSI/Total post treatment % Reduction |
10.54±1.35 | 9.74±1.98 |
| Kong et al.[26] (2014) | NIH-CPSI/Total post treatment % Reduction |
15.1±4.7 | 12.8±5.2 |
| Benelli et al.[27] (2018) | NIH-CPSI/Total post treatment % Reduction |
2.928571±2.43 | - |
| Yamanishi et al.[28] (2020) | NIH-CPSI/Total post treatment % Reduction |
11.303±6.769 | 8.584±5.334 |
| Tawfik et al.[29] (2022) | NIH-CPSI/Total post treatment % Reduction |
19.1±5.26 | 23.79±5.2 |
| Sebastianelli et al.[30] (2019) | NIH-CPSI/Total post treatment % Reduction |
- | - |
Table 4: Comparison of NIH-CPSI After Treatment
| Study (Year) | Voiding parameters | Study group (Monotherapy) | Control group (Combined therapy or α-blockers) | p-value |
|---|---|---|---|---|
| Cantoro et al.[22] (2013) | Qmax, ml/s | - | - | - |
| Voided volume, ml | - | - | - | |
| PVR, ml | - | - | - | |
| Kong et al.[26] (2014) | Qmax, ml/s | 1.1±2.1 | 1.1±2.1 | |
| Voided volume, ml | −1.7±0.3 | 1.2±0.8 | ||
| PVR, ml | ||||
| Matsukawa et al.[23] (2020) | Qmax, ml/s | 11.2±3.9 | 10.8±3.8 | |
| Voided volume, ml | 171±100 | 164±73 | ||
| PVR, ml | 20 (10‐40) | 30 (15‐60) | ||
| Benelli et al.[27] (2018) | Qmax, ml/s | 20.14±3.50 | - | <0.698 |
| Voided volume, ml | 15.71±21.38 | - | <0.822 | |
| PVR, ml | - | - | - | |
| Yamanishi et al.[28] (2020) | Qmax, ml/s | 11.985±6.283 | 12.440±5.485 | 0.09 |
| Voided volume, ml | 161.142±94.051 | 179.303±100.098 | 0.048 | |
| PVR, ml | 20.908±24.742 | 31.643±28.523 | 0.25 | |
| Tawfik et al.[29] (2022) | Qmax, ml/s | - | - | - |
| Voided volume, ml | - | - | - | |
| PVR, ml | - | - | - | |
| Sebastianelli et al.[30] (2019) | Qmax, ml/s | 11.8±4 | 14.5±3.7 | 0.027 |
| Voided volume, ml | - | - | - | |
| PVR, ml | - | - | - |
Table 5: Voiding Parameters Outcomes between The Two Groups
| Study (Year) | Voiding parameters | Study group (Monotherapy) | Control group (Combined therapy or α-blockers) | p-value |
|---|---|---|---|---|
| Cantoro et al.[22] (2013) | IPSS total score | - | - | - |
| IPSS‐voiding | - | - | - | |
| IPSS‐storage | - | - | - | |
| IPSS‐QOL | 2.02±0.56 | 1.82±0.25 | 0.574 | |
| Kong et al.[26] (2014) | IPSS total score | −4.3±0.2 | −1.1±0.2 | p<0.05 |
| IPSS‐voiding | −3.0±0.2 | −0.7±0.1 | p<0.05 | |
| IPSS‐storage | −1.3±0.1 | −0.4±0.1 | p<0.05 | |
| IPSS‐QOL | −0.2±0.1 | −0.1±0.1 | p<0.05 | |
| Matsukawa et al.[23] (2020) | IPSS total score | 12.2±5.2 | 14.0±6.4 | - |
| IPSS‐voiding | 7.2±3.8 | 8.0±4.1 | - | |
| IPSS‐storage | 5.0±2.4 | 6.0±2.9 | - | |
| IPSS‐QOL | 3.1±1.3 | 3.4±1.1 | - | |
| Benelli et al.[27] (2018) | IPSS total score | 0.214286±0.42 | - | - |
| IPSS‐voiding | - | - | - | |
| IPSS‐storage | - | - | - | |
| IPSS‐QOL | - | - | - | |
| Yamanishi et al.[28] (2020) | IPSS total score | 11.934±7.204 | 9.961±6.478 | - |
| IPSS‐voiding | 3.868±4.199 | 3.675±4.266 | - | |
| IPSS‐storage | 6.882±3.216 | 5.403±2.725 | - | |
| IPSS‐QOL | 3.974±1.286 | 3.455±1.401 | - | |
| Tawfik et al.[29] (2022) | IPSS total score | - | - | - |
| IPSS‐voiding | - | - | - | |
| IPSS‐storage | - | - | - | |
| IPSS‐QOL | - | - | - | |
| Sebastianelli et al.[30] (2019) | IPSS total score | 11.8±6.3 | 11.5±5.4 | 0.084 |
| IPSS‐voiding | 8±4.7 | 5.1±2.7 | 0.006 | |
| IPSS‐storage | 3.8±3.4 | 5.3±2.7 | 0.08 | |
| IPSS‐QOL | 2.1±1.7 | 2.1±1 | 0.321 |
Table 6: Post-Treatment LUTS Outcomes between the Two Groups
The main items of Cochrane risk of bias were concerned with research title, abstract, sample size calculations, allocation of patients and allocation concealment. The quality assessment revealed that a single study had a sufficient amount of information while many of them neglect the element of blinding in their research. Sample size calculation was also not adequately addressed in the studies. So, overall two studies scored 5 points, two scored 4, one study scored 3 points while one scored at least 2 points. The highest score was observed as 7 in Tawfik’s study and focused on set of all the points to minimize the risk of bias[29].
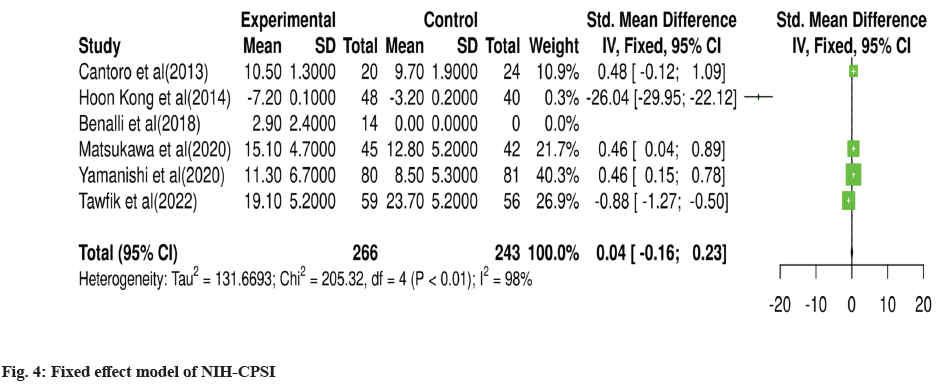
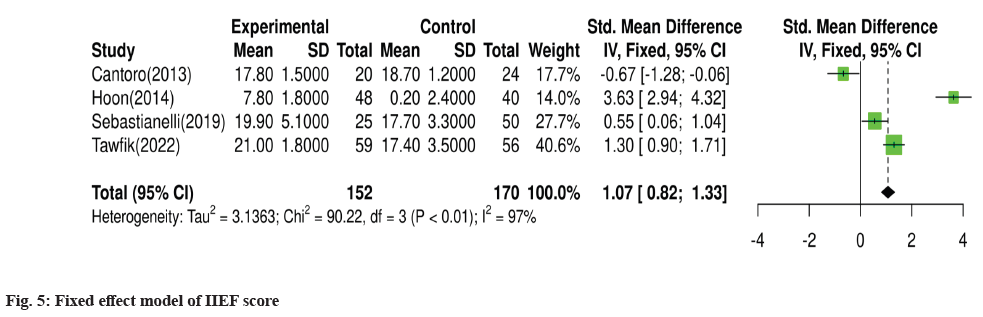
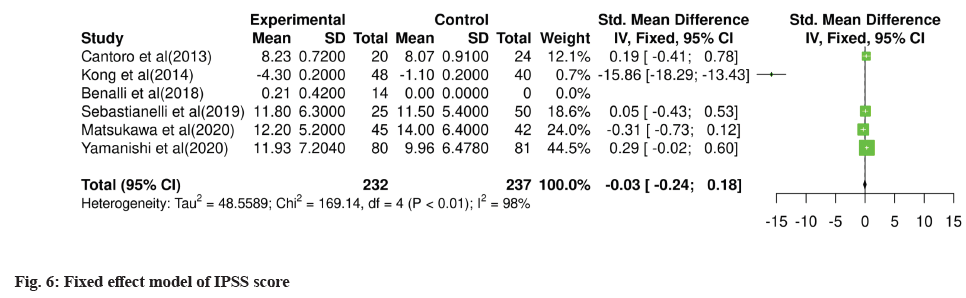
For meta-analysis, Wald test was performed to evaluate the effective size of mentioned studies. Cochrane diagnostic test was performed along with Roswin and Egger model to evaluate the risk of bias. The pooled prevalence of NIH-CPSI score was reported as 40 % with 98 % heterogeneity. The overall NIH-CPSI score ranges from -0.16 to 0.23 at 95 % CI. The study conducted by Yamanishi et al.[28], which weighted higher than the other studies, reported all the NIHCPSI parameters including pain, voiding, urinary symptoms and quality of life. The Chi-square score of these studies was reported as 205.32 with a Degrees of Freedom (DF) value of 4. Egger regression test value was observed as 0.0951 which indicates a significant publication bias between studies (fig. 4). Meanwhile, the IPSS score had 98 % heterogeneity with an overall 30 % prevalence (95 % CI of -0.24 to 0.18). The Chisquare value was reported as 169.14 with a significant publication bias of 0.0446 (fig. 5). Only 10 % of the studies reported IIEF scores with 97 % significant heterogeneity results. Odds ratio of IIEF was reported as 1.07 (0.82 to 1.33). The study of Cantoro et al.[22] and Kong et al.[26] reported all the variables including total NIH-CPSI score, total IPSS score and total IIEF score with the weighted prevalence of 10.9 % vsl. 0.3 %, 12.1 % vsl. 0.7 % and 17.7 % vsl. 14.0 % (fig. 6).
Traditionally antibiotics, anti-inflammatory and Alpha (α)-blockers were used to treat chronic prostatitis however these drugs failed to give successful outcomes[31]. Tadalafil at the dose of 5 mg daily shows improvement in LUTS and erectile dysfunction[32]. Experimental studies have shown positive outcomes of PDE5 inhibitors in the management of prostatitis[15,16,18]. Unfortunately, most of the literature was formed on Benign Prostatic Hyperplasia (BPH)-associated CP/ CPPS or combination with other medications. Thus, we could only find two case-control studies[22,23]. In a study by Benelli et al.[27], a significant improvement was observed in all NIH-CPSI domains. The treatment duration was of 3 mo, while improvement in symptoms was noted within a month of treatment. Pain score was reduced from 13.7±3.7 to 5.4±2.2. Further reduction was observed by the 2nd and 3rd mo of the trial. A reduction of more than 25 % in CPSI score is assumed to show clinically significant improvement after treatment[33-35]. In the case-control study conducted by Tawfik et al.[29], 50.8 % of respondents have been observed to have signs of clinical improvement. Likewise, a study by Hiramatsu et al.[25] observed a reduction in pain score after a 3 mo treatment of tadalafil in chronic prostatitis patients associated with LUTS. They reported that the pre-treatment mean pain score as -10.0±7.8 which was improved to be −4.4±4.5 post-treatment.
Quality of life was also reportedly improved during the treatment. Tadalafil monotherapy was used in the study of Matsukawa et al.[23]. The study reports that only 4 of their patients showed ≥50 % improvement in CPSI score. Similarly, a study by Pineault et al.[24] conducted on 25 patients observed a significant reduction in CPSI total pain, urinary symptoms and quality of life scores after everyday consumption of 5 mg tadalafil for 1.5 y. In contrast, a placebo-controlled clinical trial observed no significant improvement in pain scores in both placebo and control groups. The short period of medication could be the reason for the results. However, PDE5 inhibitors show significant results in treating erectile dysfunction in mild and moderate cases. Improved IIEF-5 can be observed in the tadalafil group[29].
Erection dysfunction, headaches and dyspepsia were frequently reported after using tadalafil[23,26,28,30]. However, these effects were also observed in the study that tested the new PDE5 inhibitor sildenafil. Muscle aches and dizziness were the second most frequently reported subjects. In a survey by Sebastianelli et al.[30], the treatment emergence adverse effect was noted as 16 % in the tadalafil monotherapy group, while 22 % were reported in the tadalafil combined therapy group.
The negative impact of LUTS is highly observed in elderly patients, which affects the quality of life. Besides aging, many metabolic factors affect the LUTS and erectile dysfunction, including prostatitis enlargement and inflammation. PDE-5 inhibitors are proven to significantly suppress the inflammatory factor for treating both conditions. These drugs stabilize the glandular structural anatomy, reduce the tone of bladder muscles and affect the micturition reflex[36,37]. One of the meta-analyses also observed the positive role of PDE5 inhibitors in treating benign prostatic hyperplasia[38]. Meanwhile, only four out of seven studies reported the adverse effect of PDE5 inhibitors.
Our systematic study and meta-analysis was the first one that highlights the effectiveness of PDE5 inhibitors in treating type III chronic prostatitis through the relevant data is small. A monotherapy of 5 mg tadalafil has been found to significantly reduce pain score, IPSS domain and NIH-CPSI. A daily oral dose of PDE5 has been observed to have a positive impact in reducing voiding difficulties. A significant difference has been observed after using tadalafil monotherapy in the majority of the studies. Hence, tadalafil alone can be used to treat type III chronic prostatitis.
Acknowledgements:
The authors would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Aissi A. Intracapsular prostatic omentalization: A new technique for management of prostatic abscess in dogs: Case report. Int J Adv Biol Biomed Res 2018;6(1):35-8.
- Liu H, Gao Y, Vafaei S, Gu X, Zhong X. The prognostic value of plasma cell-free DNA concentration in the prostate cancer: A systematic review and meta-analysis. Front Oncol 2021;11:599602.
[Crossref] [Google scholar] [PubMed]
- Wang Z, Tan H, Liu S, Zhang G. Effect of cognitive behavior intervention on improving psychological emotion and erectile dysfunction of chronic prostatitis patients. Acta Med Mediterr 2020;36(4):2507-13.
- Schaeffer AJ. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med 2006;355(16):1690-8.
- Krieger JN, Nyberg Jr L, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA 1999;282(3):236-7.
[Google scholar] [PubMed]
- Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: Consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology 1999;54(2):229-33.
[Crossref] [Google scholar] [PubMed]
- Bartoletti R, Cai T, Mondaini N, Dinelli N, Pinzi N, Pavone C, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: Results of a multicenter case-control observational study. J Urol 2007;178(6):2411-5.
[Crossref] [Google scholar] [PubMed]
- Marszalek M, Wehrberger C, Hochreiter W, Temml C, Madersbacher S. Symptoms suggestive of chronic pelvic pain syndrome in an urban population: Prevalence and associations with lower urinary tract symptoms and erectile function. J Urol 2007;177(5):1815-9.
[Crossref] [Google scholar] [PubMed]
- Alexander RB, Trissel D. Chronic prostatitis: Results of an internet survey. Urology 1996;48(4):568-74.
[Crossref] [Google scholar] [PubMed]
- Rees J, Abrahams M, Doble A, Cooper A, Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: A consensus guideline. BJU Int 2015;116(4):509-25.
[Crossref] [Google scholar] [PubMed]
- Hao ZY, Li HJ, Wang ZP, Xing JP, Hu WL, Zhang TF, et al. The prevalence of erectile dysfunction and its relation to chronic prostatitis in Chinese men. J Androl 2011;32(5):496-501.
[Crossref] [Google scholar] [PubMed]
- Köhler TS, McVary KT. The relationship between erectile dysfunction and lower urinary tract symptoms and the role of phosphodiesterase type 5 inhibitors. Eur Urol 2009;55(1):38-48.
[Crossref] [Google scholar] [PubMed]
- Hatzimouratidis K. A review of the use of tadalafil in the treatment of benign prostatic hyperplasia in men with and without erectile dysfunction. Ther Adv Urol 2014;6(4):135-47.
[Crossref] [Google scholar] [PubMed]
- McVary KT. Unexpected insights into pelvic function following phosphodiesterase manipulation-what’s next for urology? Eur Urol 2006;50(6):1153-6.
[Crossref] [Google scholar] [PubMed]
- Kurita M, Yamaguchi H, Okamoto K, Kotera T, Oka M. Chronic pelvic pain and prostate inflammation in rat experimental autoimmune prostatitis: Effect of a single treatment with phosphodiesterase 5 inhibitors on chronic pelvic pain. Prostate 2018;78(15):1157-65.
[Crossref] [Google scholar] [PubMed]
- Sugimoto M, Zhang X, Ueda N, Tsunemori H, Taoka R, Hayashida Y, et al. A phosphodiesterase 5 inhibitor, tadalafil, suppresses stromal predominance and inflammation in a rat model of nonbacterial prostatitis. BMC Urol 2019;19(1):1-7.
[Crossref] [Google scholar] [PubMed]
- Okuyucu EE, Guven O, Ucar E, Duman T. Assessment of headache in men taking phosphodiesterase-5 inhibitor (tadalafil) for erectile dysfunction. Acta Med 2014;30:1007.
- Kedia GT, Ückert S, Kedia M, Kuczyk MA. Effects of phosphodiesterase inhibitors on contraction induced by endothelin-1 of isolated human prostatic tissue. Urology 2009;73(6):1397-401.
[Crossref] [Google scholar] [PubMed]
- Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 2007;148(3):1019-29.
[Crossref] [Google scholar] [PubMed]
- Roumeguere T, Boudjeltia KZ, Babar S, Nuyens V, Rousseau A, van Antwerpen P, et al. Effects of phosphodiesterase inhibitors on the inflammatory response of endothelial cells stimulated by myeloperoxidase-modified low-density lipoprotein or tumor necrosis factor alpha. Eur Urol 2010;57(3):522-9.
[Crossref] [Google scholar] [PubMed]
- Grimsley SJ, Khan MH, Jones GE. Mechanism of phosphodiesterase 5 inhibitor relief of prostatitis symptoms. Med Hypotheses 2007;69(1):25-6.
[Crossref] [Google scholar] [PubMed]
- Cantoro U, Catanzariti F, Lacetera V, Quaresima L, Muzzonigro G, Polito M. Comparison of tamsulosin vs. tamsulosin/sildenafil effectiveness in the treatment of erectile dysfunction in patients affected by type III chronic prostatitis. Arch Ital Urol Androl 2013;85(3):109-12.
[Crossref] [Google scholar] [PubMed]
- Matsukawa Y, Naito Y, Funahashi Y, Ishida S, Fujita T, Tochigi K, et al. Comparison of cernitin pollen extract vs. tadalafil therapy for refractory chronic prostatitis/chronic pelvic pain syndrome: A randomized, prospective study. Neurourol Urodyn 2020;39(7):1994-2002.
[Crossref] [Google scholar] [PubMed]
- Pineault K, Ray S, Gabrielson A, Herati AS. Phosphodiesterase type 5 inhibitor therapy provides sustained relief of symptoms among patients with chronic pelvic pain syndrome. Transl Androl Urol 2020;9(2):391-7.
[Crossref] [Google scholar] [PubMed]
- Hiramatsu I, Tsujimura A, Soejima M, Yoshiyama A, Nagashima Y, Ishikawa K, et al. Tadalafil is sufficiently effective for severe chronic prostatitis/chronic pelvic pain syndrome in patients with benign prostatic hyperplasia. Int J Urol 2020;27(1):53-7.
[Crossref] [Google scholar] [PubMed]
- Kong DH, Yun CJ, Park HJ, Park NC. The efficacy of mirodenafil for chronic prostatitis/chronic pelvic pain syndrome in middle-aged males. World J Mens Health 2014;32(3):145-50.
[Crossref] [Google scholar] [PubMed]
- Benelli A, Mariani S, Varca V, Gregori A, Barrese F, Cappa M. Once-daily 5 mg tadalafil oral treatment for patients with chronic prostatitis/chronic pelvic pain syndrome. Ther Adv Urol 2018;10(12):377-81.
[Crossref] [Google scholar] [PubMed]
- Yamanishi T, Kaga K, Sakata K, Yokoyama T, Kageyama S, Fuse M, et al. A randomized controlled study of the efficacy of tadalafil monotherapy versus combination of tadalafil and mirabegron for the treatment of persistent overactive bladder symptoms in men presenting with lower urinary tract symptoms (Contact study). Neurourol Urodyn 2020;39(2):804-12.
[Crossref] [Google scholar] [PubMed]
- Tawfik AM, Radwan MH, Abdulmonem M, Abo-Elenen M, Elgamal SA, Aboufarha MO. Tadalafil monotherapy in management of chronic prostatitis/chronic pelvic pain syndrome: A randomized double-blind placebo controlled clinical trial. World J Urol 2022;40(10):2505-11.
[Crossref] [Google scholar] [PubMed]
- Sebastianelli A, Spatafora P, Frizzi J, Saleh O, Sessa M, de Nunzio C, et al. Tadalafil 5 mg alone or in combination with tamsulosin 0.4 mg for the management of men with lower urinary tract symptoms and erectile dysfunction: Results of a prospective observational trial. J Clin Med 2019;8(8):1126.
[Crossref] [Google scholar] [PubMed]
- Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and network meta-analysis. JAMA 2011;305(1):78-86.
[Crossref] [Google scholar] [PubMed]
- Zeltner R, Hilgers KF, Schmieder RE, Porst M, Schulze BD, Hartner A. A promoter polymorphism of the alpha 8 integrin gene and the progression of autosomal-dominant polycystic kidney disease. Nephron Clin Pract 2008;108(3):c169-75.
[Crossref] [Google scholar] [PubMed]
- Propert KJ, Litwin MS, Wang Y, Alexander RB, Calhoun E, Nickel JC, et al. Responsiveness of the National Institutes of Health chronic prostatitis symptom index (NIH-CPSI). Qual Life Res 2006;15(2):299-305.
[Crossref] [Google scholar] [PubMed]
- Nickel JC. Treatment of chronic prostatitis/chronic pelvic pain syndrome. Int J Antimicrob Agents 2008;31(1):112-6.
- Porst H, Rajfer J, Casabé A, Feldman R, Ralph D, Vieiralves LF, et al. Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med 2008;5(9):2160-9.
[Crossref] [Google scholar] [PubMed]
- Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, de Nunzio C, et al. Metabolic syndrome and benign prostatic enlargement: A systematic review and meta‐analysis. BJU Int 2015;115(1):24-31.
[Crossref] [Google scholar] [PubMed]
- Andersson KE. Storage and voiding symptoms: Pathophysiologic aspects. Urology 2003;62(5):3-10.
[Crossref] [Google scholar] [PubMed]
- Gacci M, Sebastianelli A, Spatafora P, Corona G, Serni S, de Ridder D, et al. Best practice in the management of storage symptoms in male lower urinary tract symptoms: A review of the evidence base. Ther Adv Urol 2018;10(2):79-92.
[Crossref] [Google scholar] [PubMed]
 Pre-treatment and
Pre-treatment and  Post treatment
Post treatment