- *Corresponding Author:
- Xiaofeng Rongn
Department of Integrated Traditional Chinese and Western Medicine,
The First Affiliated Hospital of Chongqing Medical University,
Chongqing 400016
E-mail: 202194@hospital.cqmu.edu.cn
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “237-245” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
One of the leading causes of death in women around the word is breast cancer. For the treatment of most cancers, chemotherapy is a good option. The most treatment approaches for breast cancer are radiotherapy, surgery and chemotherapy. There are some restrictions and limitations in diagnostic approaches and treatment methods. They result in the progress of new diagnosis and treatment approaches to improve the life quality of patients. Application of nanotechnology in the treatment of cancers currently has received much attention. In cancer treatments, nanotechnology plays a significant role in the drug delivery. Tumors can be targeted by nanoparticles. They have important role in controlling the drug release to accurate sites. Therefore, nanoparticles improve the drug efficiency and reduce the normal organ’s toxicity. Another ability of nanoparticles is activation of immune cells against tumors. As a result, nanoparticles can be used as a proper and efficient tool in the treatment and diagnosis of cancer. In this review we discuss about using nanotechnology and application of different nanoparticles in diagnosis and treatment of breast cancer.
Keywords
Breast cancer, nanotechnology, nanoparticles, magnetic resonance imaging
The incidence and mortality of cancer are rising around the world. Early detection is important to the successful treatment of cancer and greatly decreased cancer mortality [1]. Currently, some methods such as imaging techniques, cytology and histology help in early detection of cancer. Imaging techniques that are used widely in early detection, only diagnose cancers when the tissue is obviously changed [1]. Until then, many cancer cells had grown. On the other hand, differentiation between benign and malignant lesions is not possible by imaging techniques [2]. In addition, at an early stage, histopathology and cytology cannot be effectively used for diagnosis of cancer [2]. Therefore, detecting cancer at early stages is a challenge and new technologies should be developed to cope with this challenge.
Breast cancer is a malignant tumor that begins in the breast cells. Screening for and diagnosis of breast cancer should perform as early as possible, because of high speed of metastasis of breast cancer to the lymph nodes and bones.
In the management of breast cancer, targeted therapy has played an essential role. Compared to chemotherapy, targeted therapy highly improved clinical results [3]. New systems of drug delivery apply novel methods and technologies to transport drugs towards target sites. Nanotechnology is one of new medical systems and technologies that have been developed for treatment and diagnosis of diseases. It is a novel method for monitoring and manipulating molecules and atoms that have been developed and designed in the 1990s [4]. At the molecular level, nanomaterials permit unparalleled interaction with biological systems [5]. The wide application of nanotechnology is due to the progress of nanomaterial such as nanoparticles [6,7]. This fastgrowing method develops novel approaches in the treatment and diagnosis of cancers.
Nanoparticles can be applied in nanomedicine for treatment of different disease. The total aim of nanomedicine is for accurate detection and effective treatment without adverse effects. There are various targeted nanoparticles for breast cancer detection and treatment. Some of nanoparticle properties for the drug targeting systems include specificity, stability, self-assembly, drug encapsulation and biocompatibility [6]. There are different methods and approaches for using nanoparticles in nanomedicine. Both inorganic and organic nanomaterials have been investigated in clinical experiments for treatment of cancer [6,8].
In this review, application of nanotechnology and different nanoparticles in diagnosis and treatment of breast cancer is investigated and discussed.
Epidemiology of Breast Cancer
Breast cancer is the most frequent cancer in women and the second most reason for female death caused by cancer worldwide. About 2.3 million females were detected with breast cancer and the number of deaths caused by this disease was 685 000 women in 2020. Strategies for the prevention and control of breast cancer must be a high priority in different populations. In addition, it is essential to enhance awareness of early detection and risk factors in developing countries [9].
Using Nanotechnology in Cancer
An American physicist introduced nanotechnology in 1959. A single atom was produced at the atomic level in 1989. But, the official birth of nanotechnology was marked in 1990. Nanotechnology was developed for manipulating and monitoring molecules and atoms with the size of 0.1-100 nm [4].
Nanomaterials have various specific chemical and physical features compared with macroscopic material. They have a higher catalytic capacity, stronger adsorption capacity, higher surface reaction activity and higher surface area.
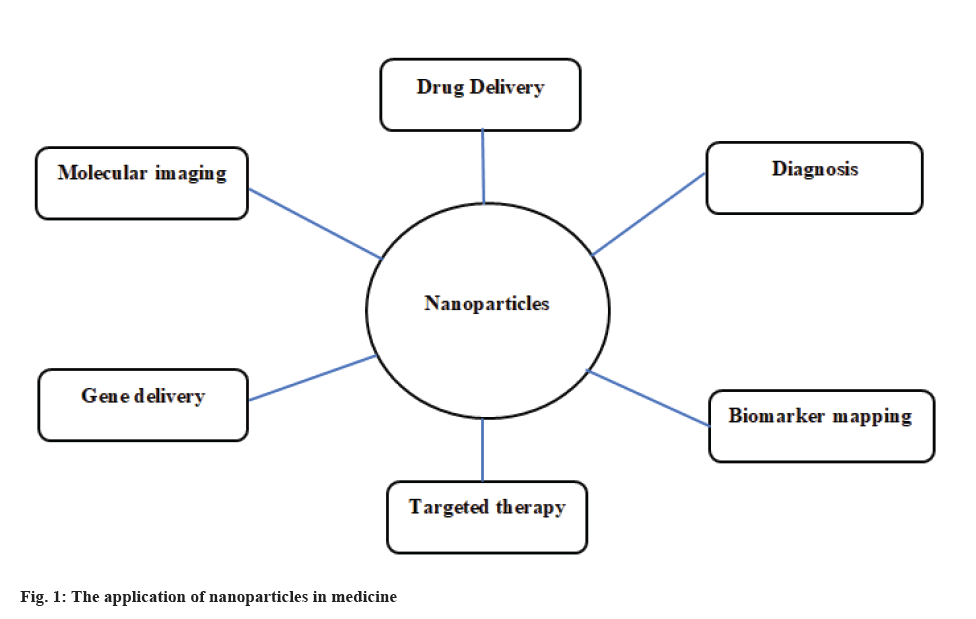
In addition, there are various other properties like higher diffusivity, higher reaction activity and lower melting point [10]. Nanomedicine was formed by merging nanotechnology and medicine. Therefore, by applying nanotechnology, life information is achieved at a single-molecule level [11] (fig. 1).
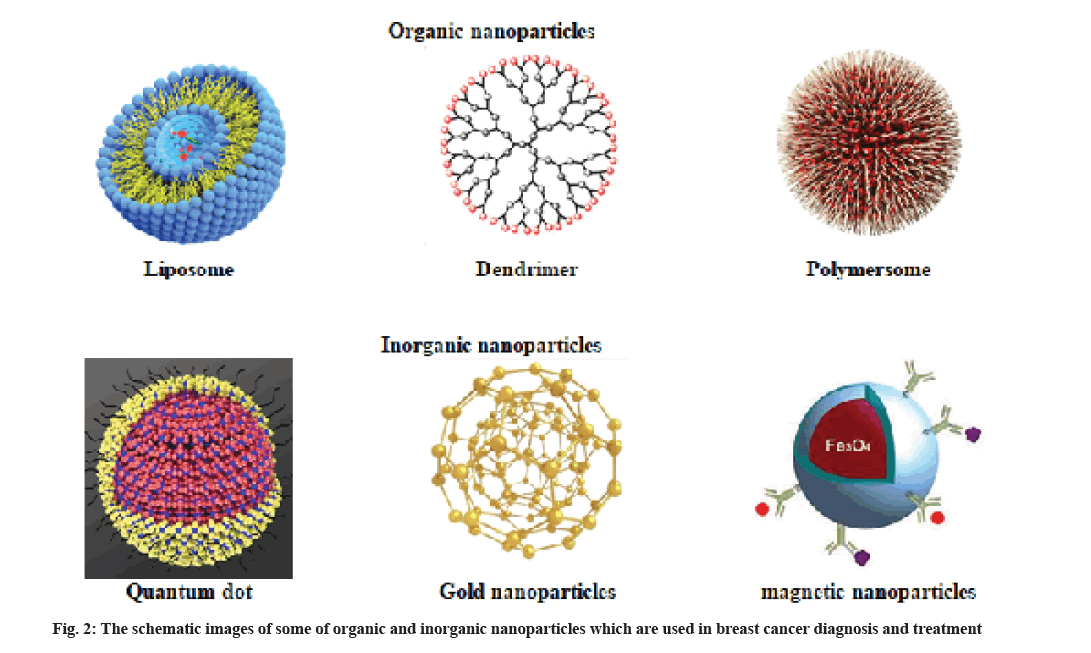
Nanoparticles are categorized into two major categories. Particles that contain organic molecules as the major building material and the second group that commonly have minerals and metals as the core (fig. 2).
Organic nanoparticles:
Liposomes are applied as drug carriers in the chemotherapy of different human cancers, such as breast cancer. Liposomes are vesicles with a cave sphere structure that have both hydrophilic and hydrophobic groups. Liposomes can be considered as a great drug carrier because hydrophobic molecules can be encapsulated within the lamellae and hydrophilic molecules can be encapsulated within the aqueous core by liposomes [3].
Dendrimers are applied in Magnetic Resonance Imaging (MRI) as contrast agents and help in detecting different pathological processes. Dendrimers are large spherical molecules in which all bond radially out of a central focal point. Dendrimers are commonly branched molecules made from natural or synthetic elements like nucleotides, sugars and amino acids [3,12]. Dendrimers contain an external surface with functional groups, an internal dendritic structure and a central core. The diverse combination of these components produces various products with different sizes and shapes that shield internal cores. Some of the applications of dendrimers include gene transfection, drug delivery, catalysis and energy harvesting.
Aptamers are peptide and oligonucleotide molecules that bind with high specificity and affinity to their target molecule. They are tools for diagnosing and treating diseases such as cancer. Ribonucleic Acid (RNA) aptamers are more flexible, but Deoxyribonucleic Acid (DNA) aptamers are more stable comparing with RNA aptamers. Peptide aptamers are built of a variable peptide loop that is attached to a protein scaffold at the end. Aptamers can also be applied in drug delivery systems. They bind to the cells surface receptors and transfer into the cell [13].
Given that the use of conventional methods of tumor diagnosis and imaging have drawbacks and limitations, the use of nanotechnologies such as aptamers can be a new way to detect cancer early, especially breast cancer. In addition to diagnosing cancer, aptamers are also used in the treatment. Aptamer technology has been developed as a valid and effective technology and many studies have been done on the users of aptamers and today aptamers are used in various aspects as a diagnostic and therapeutic tool in the development of new drugs and drug delivery systems. However, aptamers have not yet found their place in medical treatment and diagnosis. Limited information is available on the toxicity of aptamers, so it is essential to know the toxicity of aptamer therapy [14].
Small interfering RNAs (siRNAs) are now used as a new technique in therapy. Their role is to induce informational RNA (iRNA), which regulates the expression of a particular gene. Safe, specific and efficient delivery of siRNAs into specific cells is of great importance in the field of therapy [15].
Camel and shark species have antibodies that do not have a light chain and only contain a heavy chain, and they are called Heavy Chain-only Antibodies (HCAbs). These antibodies lack the constant domains, CH1 domain but have CH2 and CH3 domains that are very similar to common antibodies. Therefore, the constant fragment (Fb) portion that binds to the antigen in common antibodies is limited to one alternative domain in HCAbs and as mentioned, contains only a heavy chain called Human Herpesvirus (HHV). Due to its small size, which is in the nanometer range, they are known as nanobodies and have many applications in nanotechnology.
Today, bacteria can produce monoclonal nanobodies and because of their small size, they are more stable compared with conventional antibodies, have high solubility and have a high specificity and affinity for their antigen, so they can be used for cancer diagnosis and treatment.
Polymeric nanoparticles (Polymersomes) are selfassembled micelles and composed of synthetic or natural polymer. Polymersomes are very specific with high stability.
The use of nanobodies in mice has reduced the rate of metastasis of mammary gland cancer cells [16]. In 20 % to 30 % of breast cancer cases, it has been observed that the expression and activity of 2 surface antigens, Human Epidermal Growth Factor Receptor 1 (HER1) and Human Epidermal Growth Factor Receptor 2 (HER2), increase [17]. By designing and producing nanobodies specific to these two types of surface receptors, cancer can be diagnosed and treated.
Inorganic nanoparticles:
The structure of most inorganic nanoparticles has a central core which shows electrical, magnetic and fluorescence features. Their surface is coated by an organic protective coating. Therefore, environmental degrading agents cannot achieve the core. On the other hand, these inorganic nanoparticles can bind to positively charged molecules through electrostatic or/and covalent bonding [5].
Quantum dots fluorescent nanoparticles (10-2 nm) consisting of a central core up to thousands of atoms of group V (such as Indium), group III (such as tantalum), group VI and II elements (including selenide, zinc, technetium and cadmium). Quantum dots consist of a central core of cadmium selenide and a zinc sulfide coating surrounded by a coating of amphiphilic polymer and ligand. Because of the toxic influences of heavy metal cores, using quantum dots in therapeutic and imaging applications in vivo is limited [5,18].
Another sensitive technique is Surface Enhanced Raman Scattering (SERS) that applied to determine the spectroscopic identification of multiple molecules and targets [5,19]. Some of modern types of this technique contain a silica surface layer which is used to conjugate the protein, a reporter molecule which is used to determine the spectroscopic spectrum and silver or gold in the core which is used to increase light sensitivity. The use of these nanoparticles is to detect very specific and low concentrations of drugs [5,20].
Gold Nanoparticles (AuNPs) are applied in diagnosis, imaging and treatment of breast cancer. These particles are applied as contrast agents and drug carrier. Conjugating various peptides to AuNP can enhance biocompatibility, stability and specific targeting.
The mechanism of AuNPs action in the body has several steps. First, AuNPs attach to their receptors. Then, they undergo oscillation upon exposure to radiofrequency pulses or light. As a result, the light is absorbed and AuNPs release heat. Therefore, the surrounding area of the cell is heated. Therefore, irreversible thermal cellular destruction is occurred [6].
Noninvasive methods like MRI can detect magnetic nanoparticles. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) and Monocrystalline Iron Oxide Nanoparticles (MION) are used in specific delivery of drugs [6].
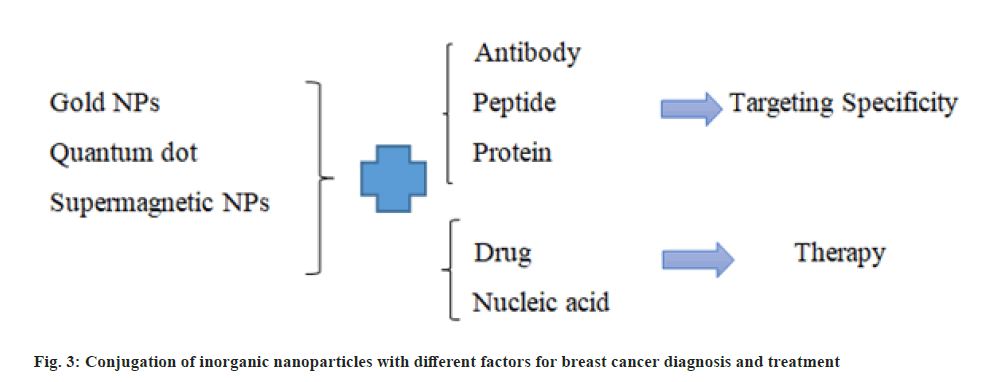
SPIONs are proper for increasing contrast and are used in MRI. Magnetic nanoparticles with an outer coating of an organic substance are suitable for conjugating to biomolecules and can be used to carry drugs in the cancer treatment. Iron oxide magnetic nanoparticles are applied as a magnetic resonance agent for cell trafficking researches, angiogenesis and gene expression researches [21]. Different conjugation of inorganic nanoparticles with different factors and their applications for breast cancer diagnosis and treatment are presented in fig. 3.
Nanoparticles in Diagnosis of Breast Cancer
Common diagnosis methods for breast cancer are clinical examination accompanied with imaging. Pathological assessment then is used for confirming the diagnosis results. The clinical examinations include palpation of breasts and lymph nodes, metastases assessment and medical history of individuals and their family. Imaging includes ultrasound and mammography of lymph nodes and breast. The pathological assessment should report HER2, grade and histological type and Estrogen Receptor (ER).
The expression level of ER in breast cancers is associated with the profit of endocrine treatments. The overexpression of HER2 protein shows profit from trastuzumab, a monoclonal antibody [22].
Recently, different studies have been investigated for novel contrast agents that result in non-invasive and reliable detection of breast cancer [3]. The progress of nanostructured contrast agents that are target-specific improved sensitivity for different imaging methods such as Positron Emission Tomography (PET), Computed Tomography (CT) and MRI. In MRI, magnetic iron oxide nanoparticles can increase the T2-weighted negative contrast of target tissues. Surface engineering of magnetic iron oxide nanoparticles with specific markers of tumor can create a flexible platforms for site-specific tumor localization and imaging of early breast cancer through MRI [3,23]. Tissue immunochemistry is one of the standard methods for determining the expression of hormone receptor or HER2, which has limitations such as signal degradation and background noise [22].
Diagnostic approaches which are based on nanotechnology are evolving and developing as a suitable tool for cancer detection and diagnosis. Nanoparticles have expanded rapidly to image tumors, show cancer biomolecules or biomarkers and targeted drug delivery. Nanotechnology has been used in various medical screens and tests like using AuNPs in pregnancy tests [24]. For cancer detection, nanoparticles are used to capture cancer biomarkers [25]. A cancer biomarker is a biological molecule that can be measured in tissues, fluids or blood. It shows the existence of cancer in the body. Cancer biomarkers can be nucleic acids, carbohydrates or proteins [26,27]. Early diagnosis of cancer can be performed by the measurement of the level of a specific cancer biomarker levels, therefore, it assist monitoring and screening of therapy efficacy. Many barriers limit biomarkers usage such as heterogeneity in the abundance and low concentrations of biomarker in body fluids.
Nanotechnology which is based on biosensor can enhance the clinical diagnosis sensitivity compared with traditional approaches. Therefore, early detection can be occurred more accurately. Nanotechnology proposes high sensitivity and selectivity and makes it possible to measure various targets simultaneously. On the other hands, using nanoparticles lead to enhanced surfaceto- volume ratio and therefore biosensors become more sensitive to special bimolecular diagnostics [28]. Three common nanoparticle probes are Polymer Dots (PDs), AuNPs and quantum dots which can be applied and used in cancer diagnosis [29].
Quantum dots which are fluorescent nanoparticle can bind to various antibodies and targeted to specific proteins. The resulting spectrum of quantum dots that are conjugated to various proteins is determined simultaneously by spectroscopy. Fluorescent emission of these conjugated nanoparticles is related to protein expression [30].
The luminous fluorescent of quantum dots identifies targets at low concentrations in cancer cells and increases sensitivity. Application of multiple quantum dots results in detection of target molecules with highsensitivity [ 31].
The best way to conjugate quantum dots by peptides and antibodies is to apply biotin and streptavidin as adapter molecules [5,32]. Direct conjugates of quantum particles maintain high affinity and minimize nonspecific connections. A quantum dot-based assay method for the detection and measurement of Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2), progesterone and ERs in cultured breast cancer cells was developed. These quantum dots conjugate directly to the antibodies of these three proteins [30].
Quantum particles are available in different emission spectra and different sizes and can identify multiple proteins in a small tumor sample simultaneously. In a study, six proteins in breast cancer were identified simultaneously using direct conjugates of quantum particles to antibodies on paraffin-embedded tumor specimens [33].
Fluorescent In Situ Hybridization (FISH) is a standard approach used for detecting gene amplification or distribution of matrix RNA using fluorescent-labeled DNA or fluorescent-labeled RNA probes, but with some limitations. Nanotechnology overcomes these problems in FISH. Quantum dots that are conjugated applied as fluorescent tags with oligonucleotide probes and create stable and clear fluorescent signals [34]. As a result, using quantum dot probes and conjugates, it is possible to determine the amount of multiple proteins simultaneously on a small cancer specimen or a single tumor piece and the final treatment strategy is based on these outcomes.
The use of aptamers conjugated with quantum dot, fluorophore or other materials such as gadolinium is useful for MRI. The advantage of using aptamers for imaging is that they are non-toxic because oligonucleotides are naturally present in the human body. Aptamers have a high specificity for their purposes and are rapidly released into the bloodstream. The use of these molecules can increase the validity of the results of clinical or research analyzes.
Nanoparticles in Breast Cancer Treatment
Targeted therapy for special receptors become an important approach for management of breast cancer and clinical results are improved compared to old treatment approach. The way that nanoparticles can diffuse and affect the targeted site is the most important concern in using such nanoparticles for cancer detection and treatment. One of the major difficulties in chemotherapy is Multidrug Resistance (MDR). Some types of nanoparticles have demonstrated the ability to dominate MDR.
For improving treatment of breast cancer, nanotechnology was applied to design efficient drug delivery systems. The carrier should be produced on the scale of industry and must be non-toxic [35]. In addition, maximum specificity into tumors is an important feature of nano drug which should be noticed to enhance the active drug delivery toward cancer cells and avoid adverse effects [36]. To balance the drug dosage, the amount of drug molecules loaded in the carrier should be optimized [37]. The suitable size of nanoparticles is crucial because nano carrier greater than 100 nm are simply detected and cleared.
Extensive research has been performed on using of nanoparticles that can protect healthy tissues and have lethal effects on cancer cells. For instance, liposomal anthracycline was applied to treat all degrees of breast cancer [38], but its use is limited because of its toxic influence on the heart. This combination with trastuzumab, which is a monoclonal antibody against ERBB2, has a better effect [39].
Doxorubicin-containing liposomes and PEGylated liposome (PEGLip) have been reported to be used in the treatment of metastatic breast cancer. Preclinical studies have shown that paclitaxel in albuminencapsulated nanoparticles has better penetrating power than conventional paclitaxel. Targeted delivery of tamoxifen occurs in all degrees of breast cancer [40]. Tamoxifen is a non-steroidal anti-estrogen drug that is highly hydrophobic. The use of nanoparticles with this drug increases its permeability to tumor tissue. Its toxic effects on healthy non-tumor tissue cells are also less [41].
Nanoparticles conjugated to antibodies can be applied to simultaneously detect multiple molecular targets in small tumor fragments. Also, considering the side effects of anti-cancer drugs, it seems necessary to use safe drug delivery systems with biocompatibility, including solid lipid nanoparticles and liposomes that deliver the drug to the target tissue with high specificity. The basis of this method is to get sufficient amounts of the drug to the tumor site for a specified period of time and to reduce the harmful effects of the drug on other organs. Solid lipid nanoparticles are a complex system with unique advantages and disadvantages that distinguish it from other colloidal systems. Further studies using nuclear MRI are needed to elucidate the drug delivery mechanism using this system. A type of drug delivery system that is widely used is Nanoparticle albumin-bound (Nab) technology that use albumin to transport and bind hydrophobic molecules. In addition, albumin can mediate Nab endothelial transcytosis and identify the albondin (gp60) glycoprotein receptor [42].
Some drug delivery systems which were mostly studied include metal and polymeric nanoparticles proteinbased nanocages and dendrimers. Using the cavities of their structure, they can be conjugated and loaded with drugs [3].
The liposome can be applied as a carrier for administration of drugs and nutrients. Some disruption methods of biological membrane such as sonication can be used for preparing liposomes. Because of their structure, liposomes are compatible with lipid bilayer structure. In the design of liposomes, surface ligands may be used to bind to unhealthy tissue [43].
Nanoparticles Toxicity
The research in nanotechnology and nanoparticles field is fast growing. In addition these researches investigate the toxicity features of nanoparticles. The hydrophobicity and small size of these nanoparticles facilitate the permeability of nanoparticles through the membranes of different organisms. There are different processes in nanoparticles production such as vapor attrition, gas phase, deposition and dispersion. After exposure through dermal, ingestion or inhalational administration, these particles can be transferred to several body organs. Nanoparticles have large surface areas and small particle size, therefore they can accumulate in various organs such as brain, heart, liver and lungs. As a results nanoparticle accumulation caused organ toxicity [6]. Through endocytosis, the nanoparticles accumulate inside the cells, despite the fact that the body has defense mechanisms. Numerous toxic effects have been reported such as nephrotoxicity, neurotoxicity, fibrosis and dermatitis [6,44]. Understanding the nontoxicity mechanism is necessary to prevent the potential toxic influences of nanoparticles. It requires instruction and research in designing, development and using of nanoparticles [45]. Single doses or short periods of use of cancer drugs can pose serious risks to human health, but the use of biodegradable nanoparticles for a long time or even a period of treatment can cause adverse side effects.
Nanotechnology Future
Emerging advanced treatments and novel methods in drug delivery open a new season in targeted chemotherapy. Recently, targeted nanoparticles have decreased the prevalence of breast cancer and the death resulted from this cancer. There are still limitations and challenges for using nanoparticles in medicine. It is hoped that in the near future, synthesis costs will decrease and pharmacokinetic properties will increase to enhance the efficiency of nanoparticles and overcome the limitations of their use. Totally, different researches demonstrated that the therapeutic index of various anticancer drugs when encapsulated in nanoparticles increased in solid breast tumors. Nanotechnology provides promising standards especially in diagnosis and treatment of cancers.
Conclusion
In this study, nanoparticles, their benefits and applications in the diagnosis and treatment of breast cancer were discussed. Nanoparticles can be applied in medicine in the fields of disease diagnosis and treatment, drug delivery and bioimaging. Advances in nanotechnology in oncology will provide special facilities for identifying multiple molecular targets simultaneously in small tumor specimens to adopt a treatment strategy. Using nanoparticles in the in vivo tumor imaging is rapidly advancing and the identification and targeting of related antigens of cancer are made possible at the same time. In the future, nanotechnology will revolutionize not only oncology but all stages of medical science.
Author’s contributions:
Teng Wang and Chunhao Cao contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J Hematol Oncol 2019;12(1):1-3.
- Choi YE, Kwak JW, Park JW. Nanotechnology for early cancer detection. Sensors 2010;10(1):428-55.
[Crossref] [Google Scholar] [PubMed]
- Marta T, Luca S, Serena M, Luisa F, Fabio C. What is the role of nanotechnology in diagnosis and treatment of metastatic breast cancer? Promising scenarios for the near future. J Nanomater 2016;2016.
- Li J, Yao M, Shao Y, Yao D. The application of bio-nanotechnology in tumor diagnosis and treatment: A view. Nanotechnol Rev 2018;7(3):257-66.
- Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O'Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol 2006;7(8):657-67.
[Crossref] [Google Scholar] [PubMed]
- Hussain Z, Khan JA, Murtaza S. Nanotechnology: An emerging therapeutic option for breast cancer. Crit Rev Eukaryot Gene Expr 2018;28(2):163-75.
[Crossref] [Google Scholar] [PubMed]
- Semiglazov VF, Paltuev RM, Remizov AS, Semiglazov VV, Dashian GA, Bessonov AA, et al. The role of nanotechnology in creating novel antitumor agents. Vopr Onkol 2011;57(5):636-40.
[Google Scholar] [PubMed]
- Vinod P, Siddik U, Viswanathan Mariammal Berlin G, Chandrasekharan G. Nanoparticles in drug delivery and cancer therapy: The giant rats tail. J Cancer Ther 2011;2011.
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev 2016;17(sup3):43-6.
[Crossref] [Google Scholar] [PubMed]
- Qiu H, Min Y, Rodgers Z, Zhang L, Wang AZ. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2017;9(5):e1456.
[Crossref] [Google Scholar] [PubMed]
- Akhtar MJ, Ahamed M, Alhadlaq HA, Alrokayan SA, Kumar S. Targeted anticancer therapy: Overexpressed receptors and nanotechnology. Clin Chim Acta 2014;436:78-92.
[Crossref] [Google Scholar] [PubMed]
- Kaminskas LM, McLeod VM, Ryan GM, Kelly BD, Haynes JM, Williamson M, et al. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J Control Release 2014;183:18-26.
[Crossref] [Google Scholar] [PubMed]
- Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes 2020;2020.
[Crossref] [Google Scholar] [PubMed]
- Liu M, Yu X, Chen Z, Yang T, Yang D, Liu Q, et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J Nanobiotechnology 2017;15(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Hosseini-Zijoud SM, Zarei S, Ghasemi H, Mahmoodi M, Abbasalipourkabir R. Aptamers and their biological-therapeutical applications: A review article. Pajouhan Sci J 2013;12(1):11-26.
- van Impe K, Bethuyne J, Cool S, Impens F, Ruano-Gallego D, de Wever O, et al. A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res 2013;15(6):1-5.
[Crossref] [Google Scholar] [PubMed]
- Moghimi SM, Rahbarizadeh F, Ahmadvand D, Parhamifar L. Heavy chain only antibodies: A new paradigm in personalized HER2+ breast cancer therapy. Bioimpacts 2013;3(1):1.
[Crossref] [Google Scholar] [PubMed]
- Hardman R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Perspect 2006;114(2):165-72.
[Crossref] [Google Scholar] [PubMed]
- Moore BD, Stevenson L, Watt A, Flitsch S, Turner NJ, Cassidy C, et al. Rapid and ultra-sensitive determination of enzyme activities using surface-enhanced resonance Raman scattering. Nat Biotechnol 2004;22(9):1133-8.
[Crossref] [Google Scholar] [PubMed]
- Faulds K, Smith WE, Graham D, Lacey RJ. Assessment of silver and gold substrates for the detection of amphetamine sulfate by surface enhanced Raman scattering (SERS). Analyst 2002;127(2):282-6.
[Crossref] [Google Scholar] [PubMed]
- Perez OD, Nolan GP. Simultaneous measurement of multiple active kinase states using polychromatic flow cytometry. Nat Biotechnol 2002;20(2):155-62.
[Crossref] [Google Scholar] [PubMed]
- Haq AI, Zabkiewicz C, Grange P, Arya M. Impact of nanotechnology in breast cancer. Expert Rev Anticancer Ther 2009;9(8):1021-4.
- Bardhan R, Chen W, Bartels M, Perez-Torres C, Botero MF, McAninch RW, et al. Tracking of multimodal therapeutic nanocomplexes targeting breast cancer in vivo. Nano Lett 2010;10(12):4920-8.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Gao X, Liu D, Chen X. Gold nanoparticles for in vitro diagnostics. Chem Rev 2015;115(19):10575-636.
[Crossref] [Google Scholar] [PubMed]
- Jia S, Zhang R, Li Z, Li J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA and exosomes as biomarkers in colorectal cancer. Oncotarget 2017;8(33):55632.
[Crossref] [Google Scholar] [PubMed]
- Borrebaeck CA. Precision diagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat Rev Cancer 2017;17(3):199-204.
[Crossref] [Google Scholar] [PubMed]
- Ma H, Liu J, Ali MM, Mahmood MA, Labanieh L, Lu M, et al. Nucleic acid aptamers in cancer research, diagnosis and therapy. Chem Soc Rev 2015;44(5):1240-56.
[Crossref] [Google Scholar] [PubMed]
- Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assunção M, et al. Noble metal nanoparticles for biosensing applications. Sensors 2012;12(2):1657-87.
[Crossref] [Google Scholar] [PubMed]
- Harun NA, Benning MJ, Horrocks BR, Fulton DA. Gold nanoparticle-enhanced luminescence of silicon quantum dots co-encapsulated in polymer nanoparticles. Nanoscale 2013;5(9):3817-27.
[Crossref] [Google Scholar] [PubMed]
- Yezhelyev M, Morris C, Gao X, Nie S, Lewis M, Cohen C. Multiple profiling of human breast cancer cell lines with quantum dots–Ab conjugates. Proc Am Assoc Cancer Res 2005;46:510.
- Giepmans BN, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nat Methods 2005;2(10):743-9.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 2003;21(1):41-6.
[Crossref] [Google Scholar] [PubMed]
- Al-Hajj AH, Yezhelyev M, Liu T, Morris C, Gao X, Nie S, O’Regan RM. Simultaneous, quantitative detection of multiple biomarkers in breast cancers using semiconductor multicolor quantum dots. Cancer Res 2006;66 (8):841-2.
- Xiao Y, Telford WG, Ball JC, Locascio LE, Barker PE. Semiconductor nanocrystal conjugates, FISH and pH. Nat Methods 2005;2(10):723.
[Crossref] [Google Scholar] [PubMed]
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 2006;6(9):688-701.
[Crossref] [Google Scholar] [PubMed]
- Fanciullino R, Ciccolini J, Milano G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit Rev Oncol Hematol 2013;88(3):504-13.
[Crossref] [Google Scholar] [PubMed]
- MaHam A, Tang Z, Wu H, Wang J, Lin Y. Protein?based nanomedicine platforms for drug delivery. Small 2009;5(15):1706-21.
[Crossref] [Google Scholar] [PubMed]
- O'Shaughnessy J. Liposomal anthracyclines for breast cancer: Overview. Oncologist 2003;8(S2):1-2.
[Crossref] [Google Scholar] [PubMed]
- Rivera E. Current status of liposomal anthracycline therapy in metastatic breast cancer. Clin Breast Cancer 2003;4:S76-83.
[Crossref] [Google Scholar] [PubMed]
- Salehzadeh R, Abdullah R. Solid lipid nanoparticles as new drug delivery system. Int J Biotechnol Mol Biol Res 2011;2(13):252-61.
- Shenoy DB, Amiji MM. Poly (ethylene oxide)-modified poly (?-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm 2005;293(1-2):261-70.
[Crossref] [Google Scholar] [PubMed]
- Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release 2013;170(3):365-72.
[Crossref] [Google Scholar] [PubMed]
- Filipczak N, Pan J, Yalamarty SS, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev 2020;156:4-22.
[Crossref] [Google Scholar] [PubMed]
- Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev 2012;64(2):129-37.
[Crossref] [Google Scholar] [PubMed]
- Sajid M, Younus M, Khan MU, Anjum AA, Haque SE, Rafique MK, et al. Effects of lead on hematological and biochemical parameters in Lohi sheep grazing around a sewerage drain. Pak Vet J 2017;37:450-4.