- *Corresponding Author:
- W. L. Zhu
Department of Pharmacy,
The Second Affiliated Hospital,
Zhejiang University School of Medicine,
Hangzhou 310027,
China
E-mail: 0015317@zju.edu.cn
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “1-12” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Liver fibrosis is an outcome of many chronic liver diseases and often results in cirrhosis, liver failure and even hepatocarcinoma. Zhuyu zhenggan decoction as a classical traditional Chinese medicine formula is used to liver fibrosis in clinical practice while its mechanism is unclear. The aim of this study was to investigate the anti-fibrosis effect of Zhuyu zhenggan decoction and to explore the molecular mechanisms by combining network pharmacology and animal experiment. The carbon tetrachloride-induced liver fibrosis rats were treated with three doses of Zhuyu zhenggan decoction. The liver fibrosis and function were evaluated by histopathological examination and biochemical detection. The fibrosis related protein phosphoinositide 3-kinase, phosphorylated phosphoinositide 3-kinase, protein kinase B and phosphorylated protein kinase B were assessed by Western blot. The herb-component-target network was constructed combined the network pharmacology. Network pharmacology found that 119 potential active compounds were obtained through the analysis of bioavailability and drug-like properties, involving 142 anti-liver fibrosis targets. Gene ontology function enrichment analysis yielded 625 gene ontology entries, most of which were related to biological processes and molecular functions. Kyoto encyclopedia of genes and genomes pathway enrichment screening yielded 117 signal pathways, including phosphoinositide 3 kinase-protein kinase B signal pathway, mitogen-activated protein kinase signal pathway, tumor necrosis factor pathway, etc. Compared with the model group, Zhuyu zhenggan decoction can ameliorate the pathological damage and fibrosis of rat liver tissues, significantly reduce rat serum aminotransferase, aspartate aminotransferase, malondialdehyde levels and significantly increase serum superoxide dismutase levels and can significantly inhibit tumor necrosis factor alpha, interleukin-1 beta and interleukin-6 level. Western blot analysis showed that Zhuyu zhenggan decoction can down-regulate the expression of phosphorylated-phosphoinositide 3-kinase and phosphorylated protein kinase B protein. These results suggested that Zhuyu zhenggan decoction markedly alleviated carbon tetrachloride-induced liver fibrosis by regulating anti-oxidation, inhibiting the expression of pro-inflammatory factors and phosphoinositide 3-kinase-protein kinase B signaling pathway.
Keywords
Zhuyu zhenggan decoction, liver fibrosis, network pharmacology, phosphoinositide 3-kinaseprotein kinase B signaling pathway
Liver Fibrosis (LF) is the common pathological basis of many chronic liver diseases and the necessary pathological process for the development of various chronic liver diseases to cirrhosis. At present, there are still no ideal anti-LF drugs at home and abroad for clinical use[1]. LF is characterized by a large amount of Extracellular Matrix (ECM) deposition in the liver[2]. The abnormal production of inflammatory factors and cytokines during inflammation leads to the activation of Hepatic Stellate Cells (HSC) and increased ECM production[3]. Excessive fibrosis can reduce the total number of liver cells and eventually lead to liver cirrhosis. Due to the many factors and complex pathogenesis of LF, although modern pharmacological research on anti-LF has been conducted for many years, some progress has been made in some animal models and the research ideas and drug targets are also very clear. However, in terms of therapeutic application, the research of anti-LF drugs has not made a major breakthrough. In recent years, herbal medicines as alternative methods have attracted great interest, especially because of their advantages in the treatment of LF and few side effects[4].
As we all know, Traditional Chinese Medicine (TCM) is characterized by complex chemical components and multiple targets in different pathways, which is challenging to understand its mechanism of action[5]. According to Chinese medical theory, Zhuyu Zhenggan Decoction (ZYZGD) was a classic formulation for activating blood and resolving stasis and created by Qingren Wang in Qing Dynasty. ZYZGD was composed of Bupleurum chinense DC, Platycodon grandiflorus (Jacq.) A. DC., Ligusticum chuanxiong Hort., Citrus aurantium L., Paeonia lactiflora Pall, Glycyrrhiza uralensis Fisch, Angelica sinensis (Oliv.) Diels, Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. and CA Mey., Achyranthes bidentata Bl., Safflower (Carthamus tinctorius L.) and Prunus persica (L.) Batsch, to be used for treating cardiovascular[6,7] and liver diseases[8]. In clinical studies of medicinal recipes for treating liver disease, ZYZGD has been demonstrated to improve clinical symptoms and inhibit LF caused by the hepatitis B virus[9,10]. Moreover, ZYZGD’s effect in inhibiting lung and LF has been studied in some studies[11-13]. Previous studies have reported the hepatoprotective effect of ZYZGD under different liver injury models and had a certain doseeffect relationship, which is closely related to antilipid peroxidation damage[14-16]. The induction of oxidative stress is one of the main mechanisms of liver toxicity. Therefore, antioxidants may contribute to the treatment of its hepatotoxic effects[17,18]. However, the exact mechanism of ZYZGD in liver protection is still awaiting further elucidation.
Carbon Tetrachloride (CCl4) is a typical hepatotoxic drug, which can cause lipid peroxidation and directly destroy liver cell membranes, leading to central necrosis of liver cell lobules, massive deposition of ECM and degeneration, necrosis and fibrosis of liver cells[19]. The induction of oxidative stress is one of the main mechanisms of CCl4 hepatotoxicity and has been widely used as a model for acute and chronic liver injury[20-22]. At the same time, the rat LF model induced by CCl4 is a classic model in many studies[23-25]. Therefore, the study of this overall therapeutic effect will give a deeper understanding ZYZGD in the treatment of LF. This study is based on the CCl4 induced rat LF animal model, using a combination of network pharmacology and experimental verification to explore the mechanism of action of ZYZGD active ingredients in the treatment of LF and provide a reference for its further development and clinical application.
Materials and Methods
Screening active ingredients:
All data on the chemical ingredients were derived from the Traditional Chinese Medicine Systems Pharmacology Analysis Platform (TCMSP) database (http://lsp.nwu.edu.cn/tcmsp.php)[26] and Integrative Pharmacology-based Research Platform of Traditional Chinese Medicine (TCMIP) (http://www.tcmip. cn/TCMIP/index.php/Home/Index/All)[27]. Search the prescription drugs of ZYZGD in the TCMSP and TCMIP databases and collect related chemical components in combination with the literature. Oral Bioavailability (OB) ≥30 %, Drug-Like properties (DL) ≥0.18 and intestinal epithelial permeability of human colorectal adenocarcinoma cells (Caco-2) ≥0.4 is the limiting condition, combined with the TCMIP v2.0, according to the drug-likeness grading, remove the ingredients rated as weak and obtain the active ingredients of ZYZGD with better pharmaceutical properties.
Screening disease targets:
Use the TCMSP database to search for ZYZGD-related target genes and obtain the ZYZGD active ingredientrelated target genes and the gene name through the UniProt database (https://www.uniprot.org/). Searching “LF” in the gene cards database (https://www.genecards.org/) and collect target genes related to LF (remove genes with a relevance score <1). The mined LF-related genes and ZYZGD target genes were mapped to screen out common targets and ZYZGD anti-LF targets were obtained.
Construction and analysis of active ingredient-LF target network model:
Import the relevant information of ZYZGD active ingredients and LF intersection targets into cytoscape 3.6.0 software to construct a ZYZGD active ingredient- LF target interaction network diagram.
Construction of Protein-Protein Interaction (PPI) network and acquisition of core genes:
The intersection of the obtained ZYZGD and LF protein targets was imported into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org/cgi/input.pl), the species was limited to human, the protein interaction relationship was obtained, the free nodes were removed and the histogram was drawn according to the number of node connections.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis:
Use the Database for Annotation, Visualization and Integrated Discovery (DAVID) online website to select people by species and obtain the Identity Document (ID) corresponding to the target gene. Perform GO function enrichment analysis and KEGG pathway annotation analysis in the R 3.6.3 software to visually generate histograms and bubble charts.
CCl4 induced LF rat animal experiment:
Materials and reagents: Olive oil was obtained from Aladdin Industrial Corporation (Shanghai, China). Colchicine was obtained from KPC Pharmaceuticals, Inc. (Kunming, China); CCl4, xylene, absolute ethanol and methanol were purchase from Sino pharm chemical reagent Co., Ltd. (Shanghai, China); 4 % paraformaldehyde, Sirius Red (SR) staining solution (Wuhan Google Biotechnology Co., Ltd., Wuhan, China); Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), total Superoxide Dismutase (SOD), Malondialdehyde (MDA), Tumor Necrosis Factor-alpha (TNF-α), leukocyte-mediated detection kit for Interleukin-1 beta (IL-1β) and Interleukin-6 (IL-6) (NanJing JianCheng Bioengineering Institute, Nanjing, China). 4 % paraformaldehyde solution, Phosphate Buffered Saline (PBS) solution, heparin sodium, Phosphoinositide 3-Kinases (PI3K) p85α antibody (Affinity), pan-Protein Kinase B (Akt) 1/2/3 antibody (Affinity), anti-Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) antibody (Abcam), antibody diluent, Bicinchoninic Acid (BCA) protein quantitative reagent Box (Solarbio) and Polyvinylidene Difluoride (PVDF) membrane (GE Healthcare Life).
Preparation of ZYZGD extract: All medicinal materials were purchased from Huadong Medicine Co., Ltd. (Hangzhou, China). Take proper mass of herb to a ceramic pot (5 l), add 8-fold volume of distilled water (v/w), soak for 30 min, then boiling for 1 h, 45 min and 30 min, respectively. Combination of the three extracts then filtration with filter paper and condense to 1 g/ml.
Animals: 60 adult male Sprague-Dawley rats (Specific-Pathogen Free (SPF) grade, 180-200 g) were provided by the experimental animal center of Zhejiang University and the experimental animal qualification license number No. SCXK2008-0033. The rats are reared in separate cages and adaptive feed for 1 w. The indoor temperature of the breeding environment is set at 22°-26° and the relative humidity is 40 %-70 %.
Experimental design: After 1 w of adaptive feeding, the rats in the all groups were intraperitoneally injected with 50 %CCl4 in an olive oil solution at 1 ml/kg bodyweight twice weekly for 6 w. At the end of the 4 w, the rats in theCCl4-treated group were randomly divided into six groups as control group, model group, colchicine treatment group (0.1 mg/kg/d, Orally (PO)), Low-dose ZYZGD (ZYZGD-L) group (5 g/ kg, PO), Middle-dose ZYZGD (ZYZGD-M) group (10 g/kg/d, PO) and High-dose ZYZGD (ZYZGD-H) group (20 g/kg/d, PO). The rats in the model group were administered the corresponding amount of water. The rats were sacrificed by cervical dislocation after the last administration, blood samples were collected through the abdominal aorta. The serum was separated by centrifugation at 13 000 rpm at 4° for 10 min and stored at -80° for biochemical analysis. The liver was quickly dissected, weighed and cut into small pieces and immediately perfused with pre-chilled saline, then a part of the liver was collected in 10 % formalin buffer and the remaining tissues were quickly frozen in liquid nitrogen and preserved at -80° until analysis.
Detection of liver function biomarkers: Enzyme- Linked Immunosorbent Assay (ELISA) method was used to detect the contents of ALT, AST in liver and TNF-α, IL-1β, IL-6, MDA and SOD in serum.
Histopathological examination: The left lobe of liver tissue was cut out, fixed with 4 % paraformaldehyde, embedded in paraffin, serial sections, 5 μm thick, stained with Hematoxylin and Eosin (HE) and SR, respectively. And observed the pathological changes of liver tissue under an optical microscope.
Protein expression of phosphorylated PI3K (p-PI3K) and phosphorylated Akt (p-Akt) in liver tissue assay using Western blot: Take liver tissue about 1 cm3 in size and place it in a 1.5 ml Eppendorf (EP) tube on ice, add Radioimmunoprecipitation Assay (RIPA) lysis solution and Phenylmethylsulfonyl Fluoride (PMSF) (PMSF:RIPA=1:100) and homogenize. Put the EP tube with the lysate into the ice box for storage and ultrasound. Centrifuge at low temperature at 4°, 12 000 rpm for 10 min. After centrifugation, use a 20 μl pipette tip to carefully aspirate the transparent part of the supernatant into a new EP tube and insert it into ice to wait for the next step of detection. Use the BCA kit to detect the protein concentration of the labeled sample. Protein denaturation was done by calculating the amount of buffer added according to the protein detection results, adjust to the required protein concentration, mix well and put the protein sample in a metal bath at 98° for 8 min; prepare 8 % separation gel. Electrophoresis was done first at 80 v, 30-40 min; then 120 v, 1 h. Then transfer membrane at 300 mA for 1 h. Seal at room temperature for 1 h. Primary antibody incubation was done, take the membrane out of the blocking solution, add 6 ml of Tris Buffered Saline with Tween (TBST) to the labeled incubator and wash 3 times on a shaker, 10 min each time. Remove the membrane in the order of labeling and add the corresponding diluted primary antibody. After labeling, incubate at 4° overnight. Secondary antibody incubation was done, take out the membrane, add 6 ml of TBST to the labeled incubator, wash 3 times on a shaker, 10 min each time, add the secondary antibody diluted in the corresponding proportion and incubate for 1 h at room temperature on a shaker development.
Statistical analysis:
The Statistical Package for the Social Sciences (SPSS) 19.0 software was used for statistical analysis and the statistical indicators were expressed as mean±Standard Deviation (SD) (x?±s) and the t-test was used for comparison between groups. p<0.05 and p<0.01 indicated that the difference is statistically significant.
Results and Discussion
A total of 1688 compounds were collected through TCMSP database search and combined with literature search. With OB≥30 %, DL≥0.18, Caco-2≥0.4 as the screening conditions, excluding active ingredients without corresponding target genes and drug-likeness grading as weak, a total of 119 potential active chemical ingredients in ZYZGD were obtained.
The TCMSP database was used to search ZYZGD corresponding target genes, among which 158 potential active compounds were screened corresponding to genes. The GeneCards database was used to search for genes related to LF and genes with a score lower than 1 was removed. A total of 4454 genes were collected. The target gene corresponding to the potential active compound and the target gene corresponding to LF was crossed and 142 genes related to both the potential active compound of ZYZGD and LF was obtained.
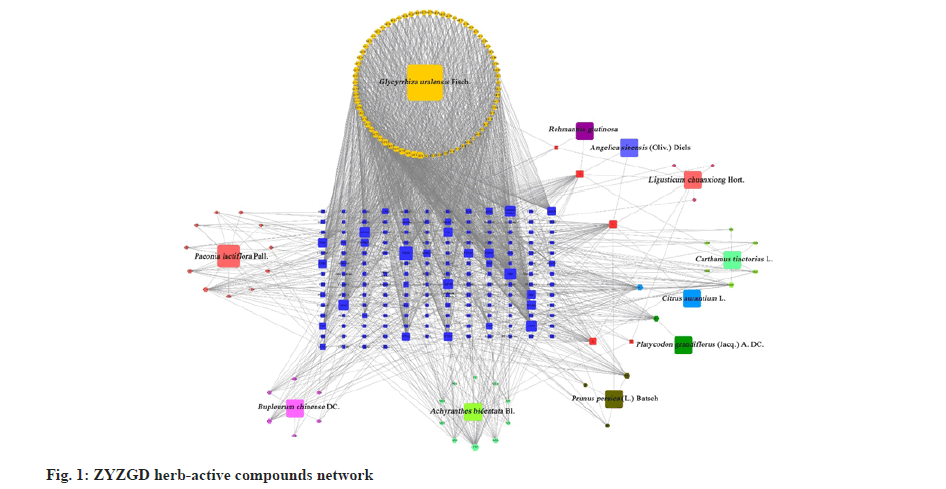
With the obtained intersection target gene as the anti-LF target, the cytoscape was used to construct the ZYZGD active ingredient-target-LF interaction network as shown in fig. 1, including inflammation, immune function regulation, promoting cell apoptosis and other biological processes, such as cell apoptosis, PI3K/ Akt, TNF and other signaling pathways. These signal pathways are closely related to the occurrence and development of LF. The above suggests that the PI3K/Akt signal pathway may be one of the mechanisms for the active ingredients of ZYZGD to treat LF.
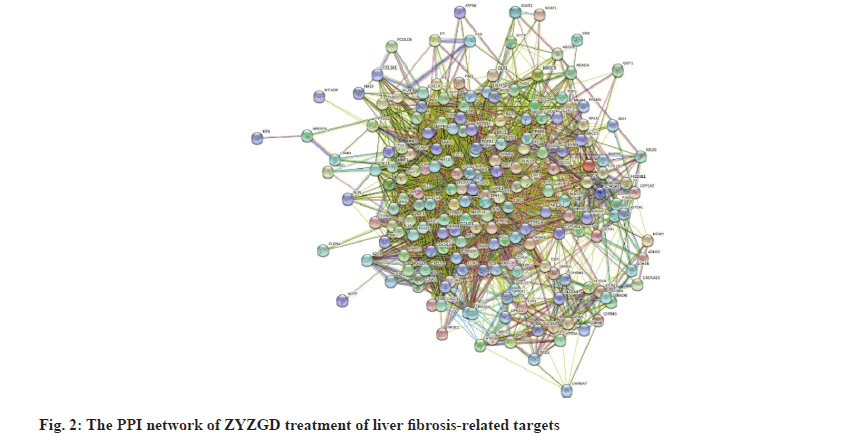
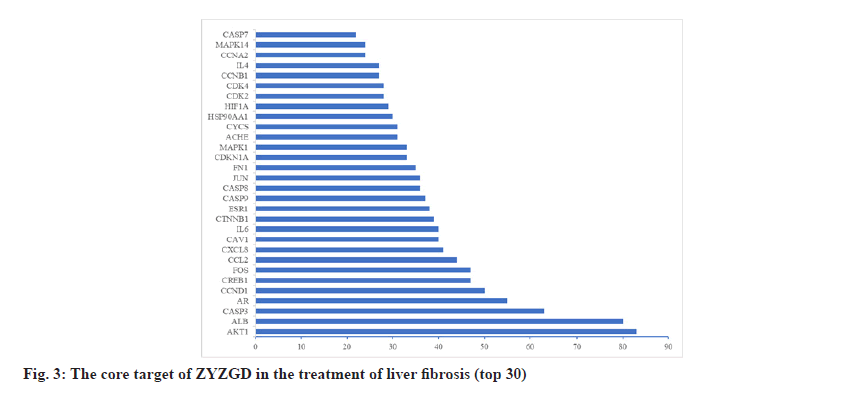
In order to further understand the interaction between the ZYZGD target and the LF target at the protein level. This study imported 142 cross target genes screened into the STRING database, the free target protein was removed and the protein interaction was obtained. The relationship network involves a total of 142 nodes and 2116 edges. In the PPI network, the more connections of the target point, the more important the target point is to play its role as shown in fig. 2. Count the number of connections between each target point and other target points and draw the graph according to the 30 points before the target point connection as shown in fig. 3. There are 22 kinds of genes with ≥30 links, of which Akt1 and ALB have the most links were 83 and 80, respectively.
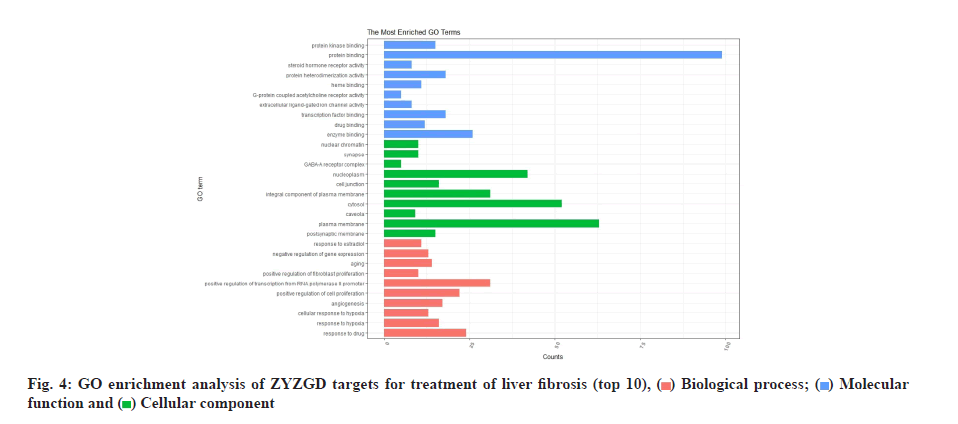
The 142 target genes intersected by ZYZGD and LF were analyzed by GO enrichment analysis and 625 GO entries were obtained, including 471 Biological Process (BP), 44 Cellular Component (CC) and 110 Molecular Function (MF). According to queue, select the top 10 with high enrichment of BP, CC and MF for visualization as shown in fig. 4. The main factors related to BPs are response to drug, response to hypoxia, cellular response to hypoxia, etc. The main ones related to cell composition are response to drug, response to hypoxia, cellular response to hypoxia, etc., postsynaptic membrane, plasma membrane and caveola. The molecular functions are mainly enzyme binding, drug binding, transcription factor binding, etc.
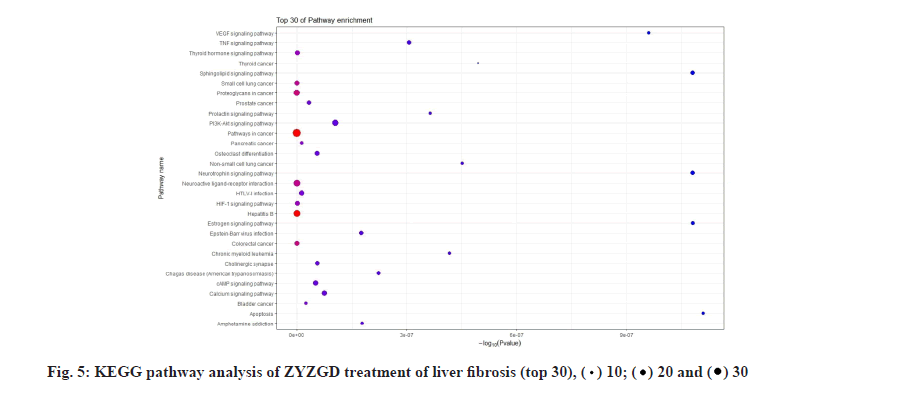
KEGG analysis of the target genes of ZYZGD and LF intersection showed that the key target genes are enriched in 117 pathways, involving inflammation, immune and other pathways, including inflammation-related pathways: Hepatitis B, TNF signaling pathway; signal transduction related pathways: PI3K-Akt signaling pathway, Hypoxia-Inducible Factor 1 (HIF-1) signaling pathway, cyclic Adenosine Monophosphate (cAMP) signaling pathway and other signaling pathways. The protective effect of some of the signal pathways on LF has been reported in related literature [28-30], which also provides a basis for further biological experiments to verify the mechanism of ZYZGD’s anti-LF. According to the top 30 results, a KEGG functional enrichment bar chart is formed as shown in fig. 5.
After 6 w of modeling, the rats showed symptoms such as getting together, hunched back and lying prone, unresponsive, irritable, dirty anus, loose stools, yellow and loose hair, slower weight gain and oral secretions. The rats in the control group were in good mental state and had a strong appetite. In Table 1, the model group rats have poor hair loss, slower body mass growth and most rats have poor appetite. The rats in each dose of ZYZGD and the colchicine group had better mental status and the overall status was better than that of the model group. Compared with the control group, the increase in body mass of the model group was significantly reduced (p<0.01) and the liver index increased (p<0.01). Compared with the model group, only the ZYZGD-H group had a significant increase in body mass (p<0.01) and each administration group could significantly reduce the liver index (p<0.05).
| Group | Dose | Body weight (g) | Liver index (%) | |
|---|---|---|---|---|
| 1 w | 6 w | |||
| Control | - | 196.38±8.64 | 297.72±16.50 | 3.05±0.09 |
| Model | - | 197.78±9.04 | 236.73±22.84** | 4.32±0.44** |
| Colchicine | 0.2 mg/kg | 194.61±7.62 | 246.23±23.21 | 3.80±0.26# |
| ZYZGD-L | 5 g/kg | 195.51±7.13 | 240.85±26.04 | 3.69±0.39# |
| ZYZGD-M | 10 g/kg | 198.02±8.54 | 247.19±17.98 | 3.97±0.73 |
| ZYZGD-H | 20 g/kg | 197.84±7.63 | 265.20±30.02## | 3.56±0.46## |
Note: *p<0.05 vs. control; **p<0.01 vs. control; #p<0.05 vs. model and ##p<0.01 vs. model
Table 1: The Effect of ZYZGD on the Body Weight and Liver Index (x?±s, n=10).
In Table 2, the results showed that ZYZGD has a certain protective effect on liver injury in model rats. Compared with the control group, the liver tissue ALT and AST levels of the model group increased significantly (p<0.05). Compared with the model group, the levels of ALT and AST in the liver tissue of rats in the ZYZGD group and the colchicine group were significantly reduced (p<0.05) and ZYZGD showed a dose-dependent manner and the ZYZGD-H group had the best effect (p<0.01).
| Group | Dose | ALT (U/l) | AST (U/l) |
|---|---|---|---|
| Control | - | 48.27±12.44 | 65.03±15.66 |
| Model | - | 133.8±33.23** | 158.3±28.84** |
| Colchicine | 0.2 mg/kg | 84.53±22.11## | 89.47±16.35## |
| ZYZGD-L | 5 g/kg | 101.04±27.16# | 97.64±20.43## |
| ZYZGD-M | 10 g/kg | 82.14±25.71## | 94.08±16.98## |
| ZYZGD-H | 20 g/kg | 62.23±27.73## | 78.21±17.92## |
Note: *p<0.05 vs. control; **p<0.01 vs. control; #p<0.05 vs. model and ##p<0.01 vs. model
Table 2: The Effect of ZYZGD on the Levels of ALT and AST in Liver (x?±s, n=10).
As shown in Table 3, compared with the control group, the serum MDA level of rats in the model group was significantly increased (p<0.05, 0.01) and the SOD level was significantly reduced (p<0.01). Compared with the model group, the ZYZGD all groups and the colchicine group can significantly reduce the MDA level (p<0.05, 0.01) and significantly increase the serum SOD level in rats (p<0.05, 0.01). The results indicate that ZYZGD can protect the liver through its antioxidant activity.
| Group | Dose | SOD (U/ml) | MDA (nmol/ml) |
|---|---|---|---|
| Control | - | 352.28±27.13 | 5.99±0.43 |
| Model | - | 290.94±16.01* | 10.18±1.51* |
| Colchicine | 0.2 mg/kg | 337.19±20.73# | 7.62±1.15# |
| ZYZGD-L | 5 g/kg | 309.69±22.02 | 9.22±1.10 |
| ZYZGD-M | 10 g/kg | 323.89±19.12# | 8.44±0.96# |
| ZYZGD-H | 20 g/kg | 324.33±11.77# | 7.02±0.55# |
Note: *p<0.05 vs. control; **p<0.01 vs. control; #p<0.05 vs. model and ##p<0.01 vs. mode
Table 3: The Effect of ZYZGD on the Levels of SOD and MDA in the Serum (x?±s, n=10).
As shown in Table 4, compared with the control group, the serum IL-6 and TNF-α levels in the model group were significantly increased (p<0.01). Compared with the model group, ZYZGD all groups can significantly reduce the levels of IL-6 and TNF-α (p<0.01). The results indicate that ZYZGD can play an anti-hepatic fibrosis effect by regulating immunity and inhibiting the expression of pro-inflammatory cytokines and it is dose-dependent.
| Group | Dose | TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|---|
| Control | - | 656.03±28.51 | 134.31±13.31 | 107.52±12.51 |
| Model | - | 1268.21±35.09* | 271.91±13.80* | 245.61±10.48* |
| Colchicine | 0.2 mg/kg | 810.17±27.35## | 148.91±7.76## | 141.87±11.06## |
| ZYZGD-L | 5 g/kg | 1129.61±42.12 | 203.84±10.22# | 208.51±12.42# |
| ZYZGD-M | 10 g/kg | 984.88±20.93# | 181.94±9.26## | 179.10±19.21## |
| ZYZGD-H | 20 g/kg | 940.34±29.86# | 180.27±7.08## | 171.17±15.43## |
Note: *p<0.05 vs. control; **p<0.01 vs. control; #p<0.05 vs. model and ##p<0.01 vs. model
Table 4: The Effect of Zyzgd on the Serum Levels Of TNF-α, IL-1β And IL-6 (x?±s, n=10).
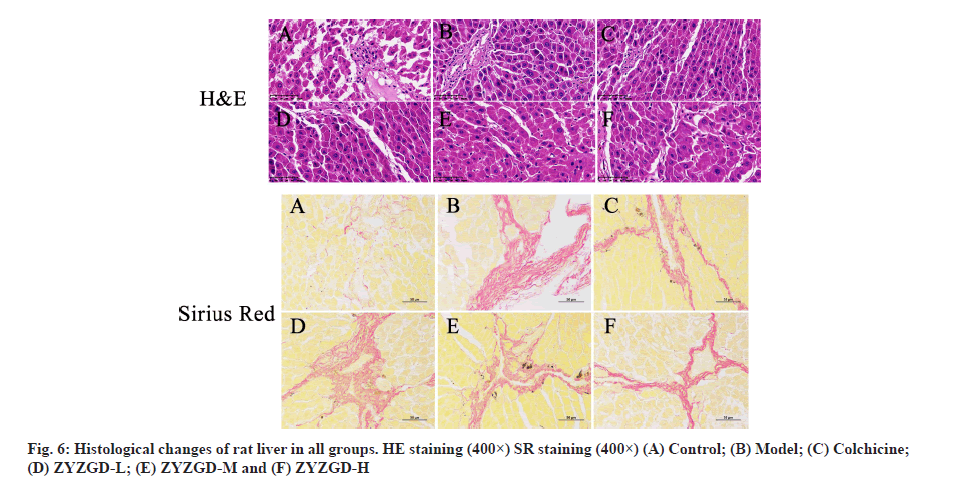
As shown in fig. 6, HE staining shows that the liver cells of the control group are arranged radially with the central vein as the center. The liver lobules are in a complete and clear structure and are arranged in an orderly manner to form hepatic cords. The liver cells have not undergone degeneration or necrosis. The liver cells are arranged neatly without collagen fibers. In the model group, most of the liver lobule structure was damaged; the liver cells were fatty degeneration, edema and inflammatory cells and necrotic cells increased. Some cells are necrotic and the liver cord structure is disordered. The ZYZGD all groups and the colchicine group all improved the degree of fibrosis to varying degrees. The ZYZGD-H group had a small amount of collagen fiber deposition and inflammatory cell infiltration, the liver cords were still neatly arranged and no false lobules were formed; the colchicine group was similar to the ZYZGD-L and ZYZGD-M groups, with varying degrees degeneration and necrosis of liver cells, infiltration of a large number of inflammatory cells, proliferation of collagen fibers, a small number of incomplete pseudo-lobule formation and disorder of liver cord structure.
SR staining showed that the lobular structure of the control group was complete and clear and there was no fibrosis as shown in fig. 6. In the model group, the lobular structure was destroyed, the portal area was enlarged and there was a large amount of fibrous tissue hyperplasia. The proliferated fibrous tissue extended from the portal area to the lobule and a small part reached the central vein of the liver lobule. The central vein showed fibrosis and formed fibers. The interval destroys the boundary plate of the liver lobules and the liver lobules are not formed completely in some rats. ZYZGD all groups can reduce collagen fibrous tissue proliferation and fibrosis to varying degrees, especially the high dose group has a significant effect.
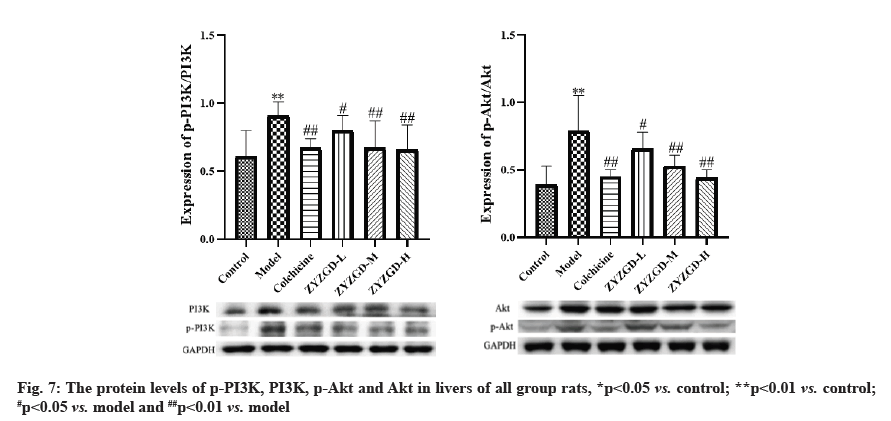
In fig. 7, compared with the control group, the expression of p-PI3K and p-Akt protein in the liver tissue of the model group increased significantly (p<0.01). Compared with the model group, the expressions of p-PI3K and p-Akt protein in the liver tissues of rats in the ZYZGD treated groups and the colchicine group were significantly reduced (p<0.05, 0.01), among which the ZYZGD-H group was the most down-regulated.
LF is a process in which the liver is damaged, causing inflammation and immune response in the liver, which in turn leads to tissue self-repair. The essence of LF is the pathological process in which a large amount of ECM with collagen as the main component is deposited in the liver when the liver is damaged and repaired in response to various chronic stimuli [31]. Numerous evidences indicate that HSC activation is the central link in the formation and development of LF. Once HSCs in the body are activated, the activated HSCs migrate, proliferate and differentiate in the liver injury area and produce large amounts of ECM and cytokines [32]. The central link in the formation of LF and the PI3K/Akt signaling pathway has a very important regulatory role in the occurrence and development of LF [28,33,34]. It is involved in cell proliferation, differentiation and regulation of various cell functions such as apoptosis. Therefore, regulating the PI3K/Akt signaling pathway is of great significance against LF. Inflammation plays an important role in the activation of HSC. HSC can be differentiated from static to active form by inflammatory mediators or growth factors. Activated HSC can further up-regulate the expression of pro-inflammatory factors IL-6, IL-34 and TNF-α, forming a vicious circle between fibrosis and inflammation and promoting the progress of LF [35]. Therefore, the inflammatory response is involved in the occurrence and development of LF and played an important role in the process.
Based on the research ideas of multi-component and multi-target action, this study applied network pharmacology technology to analyze the target of ZYZGD anti-hepatic fibrosis, build an interaction network of drug targets and use ZYZGD active ingredients to verify the key nodes of the network. And verify that ZYZGD plays a hepatoprotective mechanism against LF. In this study, 119 potential active ingredients of ZYZGD were screened through network pharmacology, mainly including flavonoids, polyphenols, alkaloids, etc., and further analysis of the targets of potential active compounds and LFrelated genes were compared and the potential active compounds of ZYZGD were found there are 142 overlapping target genes with LF and these 142 genes may be the target of ZYZGD’s anti-LF effect. The ZYZGD anti-hepatic fibrosis target interaction network was constructed based on the STRING interaction network database. The analysis found that Epidermal Growth Factor (EGF), Estimated Glomerular Filtration Rate (EGFR), IL-6, etc. are the key targets of the network. These targets may play an important role in the process of ZYZGD’s anti-LF effect. GO enrichment analysis results show that ZYZGD anti-LF involves the response to drugs, the response to hypoxia and so on. In order to explore the potential pathways of ZYZGD against LF, the KEGG pathway was analyzed in this study and a total of 117 signal transduction pathways were analyzed. Among the 30 related pathways with the highest correlation between ZYZGD and LF, the PI3K/ Akt signaling pathway plays a very important role in the occurrence and development of LF. This pathway can reduce the deposition of ECM by inhibiting the PI3K/ Akt signaling pathway and inhibit the proliferation of HSCs and promote their apoptosis, so as to achieve the purpose of treatment. The PI3K/Akt signaling pathway is an important signaling pathway in the formation of LF. This pathway interferes with the process of LF by regulating the activation and apoptosis of HSCs. The proliferation of HSCs plays a key role in the formation of LF. The key to treatment is to inhibit HSC proliferation and promote its apoptosis. From the experimental results of a large number of literatures, it can be known that the PI3K/Akt signaling pathway is involved in the process of LF and is involved in mediating the proliferation and apoptosis of HSCs.
The PI3K/Akt signaling pathway plays a very important role in regulating cell differentiation, infiltration and metastasis and it can promote cell proliferation and avoid apoptosis [36,37]. PI3K is a key factor in the PI3K/Akt signaling pathway, which can specifically catalyze the phosphorylation of the 3-hydroxyl of ester acyl inositol to produce inositol lipid substances with the function of second messenger. After PI3K binds to tyrosine residue receptors, PI3K is activated to generate phosphorylated phospholipid product at position 3, which makes Akt translocate from the cytoplasm to the cell membrane under the action of Phosphoinositide-Dependent Kinase (PDK). After the Akt phosphorylation, it positively regulates the cell cycle, promotes cell cycle transition, HSCs proliferation and strengthens HSCs collagen synthesis [38]. By inhibiting the PI3K/ Akt signaling pathway, it can significantly reduce the proliferation of HSCs, the expression of type I collagen messenger Ribonucleic Acid (mRNA) and the secretion of type I collagen [39]. Therefore, based on the prediction of network pharmacology, this study explored the mechanism of ZYZGD’s anti-rat LF from the PI3K/ Akt signaling pathway. Furthermore,CCl4 was used to prepare rat LF models and the changes of PI3K/Akt signal pathway in rat liver tissues were observed, in order to explore the protective effect and mechanism of ZYZGD on the liver ofCCl4 induced hepatic fibrosis rats. After 6 w of modeling byCCl4, the rats in this experimental model group, histopathological observations showed that all rats in the other groups except the control group had liver damage and fibrosis, the serum ALT and AST levels were significantly increased and the liver cells were seriously damaged. After treatment with different doses of ZYZGD and colchicine, the above serum biochemical indicators and liver tissue pathological damage and fibrosis have been improved to varying degrees. At the same time, ZYZGD can reverse the increase in serum lipid peroxidation product MDA level and the decrease in SOD level in rats caused by CCl4, showing a dose-dependent manner.
The high-dose group has the best effect. CCl4-induced liver damage can cause the increase of pro-inflammatory cytokines TNF-α, IL-1β and IL-6. The continuous stimulation of these cytokines can further aggravate liver damage, form a vicious circle and lead to the development of LF. Compared with the model group, different doses of ZYZGD can significantly reduce the levels of TNF-α, IL-1β and IL-6. The results show that ZYZGD can play an anti-hepatic fibrosis effect by reducing the expression of pro-inflammatory cytokines through anti-oxidation. Western blot results showed that the protein levels of p-PI3K and p-Akt in the liver tissue of the model group were significantly higher than those of the control group. After intervention by ZYZGD, the phosphorylation levels of PI3K and Akt protein in the liver tissue of rats in the ZYZGD high and medium dose groups were significantly lower than those in the model group. And a number of studies have shown that the proliferation, activation and collagen synthesis of HSCs can be inhibited by regulating the PI3K/Akt signaling pathway, so as to achieve antifibrosis effects. The results suggest that when ZYZGD acts on the PI3K/Akt signaling pathway, it may also be involved in inhibiting the proliferation of HSCs closely related to this pathway or promoting their apoptosis, thereby inhibiting the process of LF.
In summary, this study initially revealed that ZYZGD can inhibit the development of CCl4-induced LF not only regulating anti-oxidation, inhibiting the expression of pro-inflammatory factors but also blocking the transmission of PI3K-Akt signaling pathway. It may be through blocking PI3K/Akt signaling pathway to inhibit HSCs activation and accelerate collagen degradation to improve LF. Subsequent in vitro experiments will be conducted to explore the relationship between PI3K/ Akt pathway and LF. The further verification of the prediction results of network pharmacology is of great significance for the next step in further research on the mechanism of ZYZGD’s anti-LF. Because the interaction of the signaling pathway network is very complex, it is necessary to explore the interaction between the PI3K/Akt signaling pathway and other pathways to further clarify the mechanism of ZYZGD’s anti-LF.
Author’s contributions:
Wei Liang Zhu conceived and designed the study and submitted the manuscript. Wei Liang Zhu and Jian Yue Ying performed the experiments. Hou Xiang Qin acquired and analyzed the data. Wei Liang Zhu and Jian Yue Ying prepared the manuscript. All authors read and approved the final manuscript.
Funding:
This work was supported by General scientific research project of Zhejiang Provincial Department of Education (Y201941280).
Ethical approval:
This study was approved by the Committee for Animal Research of Zhejiang University.
Conflict of interests:
The authors declare no conflict of interests.
References
- Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 2019;65:37-55.
[Crossref] [Google Scholar] [PubMed]
- Berg G, Barchuk M, Miksztowicz V. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: An update of extracellular matrix remodeling. Cells 2019;8(2):158.
- Sitanggang EJ, Antarianto RD, Jusman SW, Pawitan JA, Jusuf AA. Bone marrow stem cells anti-liver fibrosis potency: Inhibition of hepatic stellate cells activity and extracellular matrix deposition. Int J Stem Cells 2017;10(1):69.
- Latief U, Ahmad R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J Tradit Complement Med 2018;8(3):352-60.
- Li H. Advances in anti-hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J Ethnopharmacol 2020;251:112442.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Qiu X, Wang R, Wang D. 1H NMR-based metabolomics study of the dynamic effect of Xuefu Zhuyu capsules on coronary heart disease rats induced by high-fat diet, coronary artery ligation. J Pharm Biomed Anal 2021;195:113869.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Nie S, Yi M, Wu N, Wang W, Zhang Z, et al. UPLC-QTOF/MS-based metabolomics analysis of plasma reveals an effect of Xuefu Zhuyu capsules on blood-stasis syndrome in CHD rats. J Ethnopharmacol 2019;241:111908.
[Crossref] [Google Scholar] [PubMed]
- Hai-ming ZH. Effective observation on treating hepatitic cirrhosis with Xuefu Zhuyu decoction. J Prac Tradit Chin Int Med 2011.
- Ru QJ, Tang ZM, Zhang ZE, Zhu Q. Clinical observation on effect of Xuefu Zhuyu decoction in treating patients with liver fibrosis caused by chronic hepatitis B. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004;24(11):983-5.
[Google Scholar] [PubMed]
- Che JY, Ren DF, Peng SJ, Fang N, He J, Chen GM, et al. Effect of Zhuyu Zhenggan Decoction on hemodynamics and liver fibrosis in patients with hepatitis B and cirrhosis. J Chin Med Mat 2018;11(41):2697-9.
- Zhou YN, Sun MY, Mu YP, Yang T, Ning BB, Ren S, et al. Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl4 in mice. J Ethnopharmacol 2014;153(3):659-66.
[Crossref] [Google Scholar] [PubMed]
- Wang H, He F, Li SY, Xu JP, Cai WR, Liu S, et al. Pathological morphology study of Xuefu Zhuyu decoction on pulmonary vascular remodeling in rats. China J Tradit Chin Med Pharm 2012.
- Wang XH. Xuefu Zhuyu decoction against liver fibrosis and its influence on immune function and endocrine. Heilongjiang Med J 2016;15(3):213-4.
- Fan XH, Shi WZ, Cheng YX, Zou KJ, Yang XF. Effects of Xuefu Zhuyu decoction on antioxidant and drug-metabolizing enzymes in liver of rats. Zhongguo Zhong Yao Za Zhi 2014;39(22):4453-8.
[Google Scholar] [PubMed]
- Li G. The protective effect of Xuefu Zhuyu decoction on liver injury induced by D-galactosamine in mice. Shaanxi J Tradit Chin Med 2006;27(1):106-8.
- Song JW, Li SB. Study of Xuefu Zhuyu decoction on anti-hepatic fibrosis in rats. Chin J Integr Tradit West Med Liver Dis 2020;7(1):38-40.
- González JA, Barajas-Esparza L, Valadez-Vega C, Madrigal-Santillán E, Esquivel-Soto J, Esquivel-Chirino C, et al. The protective effect of antioxidants in alcohol liver damage. Liver Regen 2012.
- Moghadam AR, Tutunchi S, Namvaran-Abbas-Abad A, Yazdi M, Bonyadi F, Mohajeri D, et al. Pre-administration of turmeric prevents methotrexate-induced liver toxicity and oxidative stress. BMC Complement Altern Med 2015;15(1):246.
[Crossref] [Google Scholar] [PubMed]
- Brol MJ, Rösch F, Schierwagen R, Magdaleno F, Uschner FE, Manekeller S, et al. Combination of CCl4 with alcoholic and metabolic injuries mimics human liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2019;317(2):G182-94.
[Crossref] [Google Scholar] [PubMed]
- Naji KM, Al-Shaibani ES, Alhadi FA, Al-Soudi SA, D’souza MR. Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement Altern Med 2017;17(1):114.
[Crossref] [Google Scholar] [PubMed]
- Jayakumar T, Ramesh E, Geraldine P. Antioxidant activity of the oyster mushroom, induced liver injury in rats. Food Chem Toxicol 2017;44(12):1989-96.
- Yang J, Li Y, Wang F, Wu C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem 2010;58(10):6525-31.
[Crossref] [Google Scholar] [PubMed]
- Wei L, Chen Q, Guo A, Fan J, Wang R, Zhang H. Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 2018;60:1-8.
[Crossref] [Google Scholar] [PubMed]
- Pouyandeh Ravan A, Ghasemi Basir HR, Taheri Azandaryani M, Azizi A, Goudarzi F. Role of Vaccinium arctostaphylos extract on CCl4-induced chronic liver fibrosis in rats. Comp Clin Pathol 2020;29(5):1051-60.
- Zhou Y, Wu R, Cai FF, Zhou WJ, Lu YY, Zhang H, et al. Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FOXO3 signaling pathways based on network pharmacology analysis. J Ethnopharmacol 2021;264:113021.
[Crossref] [Google Scholar] [PubMed]
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Xu HY, Zhang YQ, Qing YW, Zhao HY, Wang P, Liu F. Exploration on scientific connotation of TCM syndromes and recommended prescriptions against COVID-19 based on TCMTP V2.0. Zhongguo Zhong Yao Za Zhi 2020;45(7):1488-98.
[Crossref] [Google Scholar] [PubMed]
- Mi XJ, Hou JG, Jiang S, Liu Z, Tang S, Liu XX, et al. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem 2019;67(5):1392-401.
[Crossref] [Google Scholar] [PubMed]
- Moczydlowska J, Miltyk W, Hermanowicz A, Lebensztejn DM, Palka JA, Debek W. HIF-1 α as a key factor in bile duct ligation-induced liver fibrosis in rats. J Invest Surg 2017;30(1):41-6.
[Crossref] [Google Scholar] [PubMed]
- Gan F, Liu Q, Liu Y, Huang D, Pan C, Song S, et al. Lycium barbarumpolysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-kB signaling pathway expression in Wister rats. Life Sci 2018;192:205-12.
[Crossref] [Google Scholar] [PubMed]
- Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 2017;65(3):1039-43.
[Crossref] [Google Scholar] [PubMed]
- Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology 2019;70(4):1317-35.
[Crossref] [Google Scholar] [PubMed]
- Gong Z, Lin J, Zheng J, Wei L, Liu L, Peng Y, et al. Dahuang zhechong pill attenuates CCl4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. J Cell Biochem 2020;121(2):1431-40.
[Crossref] [Google Scholar] [PubMed]
- Huixian F. A review on intervening PI3K/Akt signal pathway against liver with TCM medicine. Clin J Chin Med 2018;72:72-9.
- Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in liver fibrosis. Semin Liver Dis 2017;37(02):119-27.
[Crossref] [Google Scholar] [PubMed]
- Nagai S, Kurebayashi Y, Koyasu S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci 2013;1280(1):30-4.
[Crossref] [Google Scholar] [PubMed]
- Khan MW, Keshavarzian A, Gounaris E, Melson JE, Cheon EC, Blatner NR, et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages and epithelial cells in colitis-induced cancer. Clin Cancer Res 2013;19(9):2342-54.
[Crossref] [Google Scholar] [PubMed]
- Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst 2015;11(7):1946-54.
[Crossref] [Google Scholar] [PubMed]
- Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol 2007;22:S79-84.
[Crossref] [Google Scholar] [PubMed]