- *Corresponding Author:
- A. K. Shah
National Facility for Drug Discovery through NCE’s Development and Instrumentation Support to SMPE’s, Department of Chemistry, Saurashtra University, Rajkot-360 005, India
E-mail: anamik_shah@yahoo.com
| Date of Submission | 9 August 2010 |
| Date of Revision | 4 July 2011 |
| Date of Acceptance | 15 July 2011 |
| Indian J Pharm Sci, 2011, 73 (4): 436-438 |
Abstract
A simple, precise, and accurate isocratic reversed-phase (RP) stability-indicating HPLC assay method was developed and validated for determination of Aripiprazole in bulk and solid pharmaceutical dosage form. A reversed-phase C8 (250×4.0 mm, 5 μm particle size) column for HPLC and C8 (50×2.1mm, 1.7 μm particle size) for UPLC method in isocratic mode was used. The mobile phase consists of acetonitrile: 20 mM ammonium acetate (90:10, v/v), flow rate was set at 1.0 ml/min and 0.250 ml/min for HPLC and UPLC, respectively and the detection was performed for both methods were at 240 nm. Further the validation of both developed method was performed and subsequently compared to prove its better applicability.
Keywords
Aripiprazole, high performance liquid chromatography, ultra performance liquid chromatography, validation
Aripiprazole is chemically identified as 7-[4-[4- (2, 3-dichlorophenyl) piperazin-1-yl] butoxy]-3, 4-dihydro-1H-quinolin-2-one, shown in fig. 1. More recently it received FDA approval for the treatment of acute manic and mixed episodes associated with bipolar disorder and as an adjunct for the treatment of depression [1]. Aripiprazole has been approved by the FDA for the treatment of acute manic and mixed episodes, in both pediatric patients aged 10-17 and in adults [2]. Several double-blind, placebocontrolled trials support this use [3-6]. In addition, it is often used as maintenance therapy, either on its own or in conjunction with a mood stabilizer such as lithium valproate. This use is also supported by a handful of studies [7,8]. The aim of the current study was to develop a validated stability-indicating high-performance liquid chromatographic (HPLC) assay method for determination of aripiprazole; additionally this method was transferred to UPLC, the latest technology in liquid chromatography to achieve the highest resolution, sensitivity and speed. Method validation was performed according to ICH guidelines [9,10] for both method and comparison of validation results were made to show its better applicability.
A high-performance thin-layer chromatographic (HPTLC) method has published for quantitation of aripiprazole in its formulation [11] but the HPLC method has many advantages over the HPTLC method for quantitation. Moreover, HPLC and UPLC is often the first choice of chromatographers compared to HPTLC. Few HPLC methods have reported in the literature for the determination of aripiprazole in bulk and pharmaceutical dosage form [11-15]. Some HPLC methods are also available for determination of aripiprazole from biological fluid [16-19]. The rapid quantization of aripiprazole in human plasma by HPLC/mass spectrometry has also reported to our knowledge [20]. Current work has advantage over the earlier in terms of speed and simplicity. Present work also deals with the forced degradation of aripiprazole under conditions such as acid hydrolysis, base hydrolysis, oxidation, and thermal and photolytic stress. Additionally this effort deals with the validation of the developed HPLC and UPLC method for the assay of aripiprazole from its bulk and dosage form.
HPLC grade acetonitrile (ACN) purchased from Fisher Scientific (Leicester, UK). Ammonium acetate (HPLC grade) was procured from Spectrochem Pvt. Ltd (India). Demineralized water was further purified by filtering through Milli-Q Elix-3 purification system (Millipore, Milford, MA, USA). Aripiprazole working standard was provided by Torrent Research Centre (Gandhinagar, India).
The chromatographic system (HPLC) used to perform development and validation of this assay method consisted of an LC-IOATvp binary pump, SPD-M10Avp photodiode array detector and Rheodyne manual injector Model 7725i with 20 μl loop (Shimadzu, Kyoto, Japan) connected to a multi-instrument data acquisition and processing system (Class- VP 6.13 SP2; Shimadzu) and the Waters Acquity UPLCTM System (Switzerland) was also used which includes Binary Solvent Manager, Sample Manager, PDA detector and Empower 2.0 version software for data acquisition.
Chromatographic analysis was performed on a Phenominex Luna C8 (2) (250×4.6mm id, 5 μm particle size) column for HPLC analysis while Acquity BEH C8 (50×2.1 mm id, 1.7 μm particle size) was used for UPLC analysis. To prepare the buffer solution, 1.52 g ammonium acetate (HPLC grade) was dissolved in 1000 ml HPLC grade water. The mobile phase consisted of acetonitrile: 20 mM ammonium acetate buffer (90:10, v/v).The mobile phase has filtered through a 0.22 μm Nylon membrane (Millipore Pvt. Ltd, Bangalore, India) and degassed in an ultrasonic bath (Spincotech Pvt. Ltd, Mumbai, India). The flow rate was adjusted to 1.0 ml/ min for HPLC and 0.250 ml/min in UPLC method. The injection volume was 20 μl for HPLC and 5 μl for UPLC method. The detection was performed at 240 nm for both methods. The total analysis time was 10.0 min and 3.0 min for HPLC and UPLC analysis, respectively.
A mixture of acetonitrile and water (1:1) used as sample diluent for all sample preparations. Stock solution of Aripiprazole was prepared by dissolving accurately 100 mg of drug in 100 ml sample diluent. Further pipette out 10 ml of this solution and dilute it up to 100 ml with sample diluent. This mixture contains 0.1 mg/ml of aripiprazole standard solution.
To prepare sample, twenty commercially available 10.0 mg label claimed tablets of aripiprazole have weighed and the average weight of a tablet was determined. From these, 5 tablets were weighed and transferred into a 500 ml volumetric flask and added 50 ml acetonitrile followed by sonication of minimum 30 min with intermittent shaking. Then the solution has brought back to room temperature and diluted to volume with acetonitrile. The sample has filtered through a 0.22 μn nylon syringe filter and the available concentration was 0.1 mg/ml of analyte.
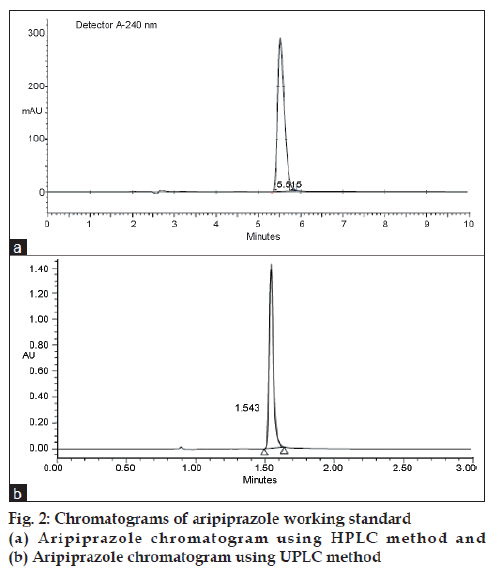
Mobile phase A consist of 20 mM ammonium acetate in HPLC grade water and mobile phase B consist of acetonitrile (HPLC grade). The Isocratic mode set at 10:90 (buffer:acetonitrile, v/v) for both HPLC and UPLC analysis. The selectivity of the aripiprazole can be altered by changing the pH of the buffer. The pre method validation experiments were performed to avoid uncertainty at the time of method validation; the chromatograms of the analyte using HPLC method and UPLC method are given in fig. 2.
The accuracy experiment was performed by recovery study at three level 150%, 100% and 50% of sample concentration. The recovery was found between 99-101% for HPLC and 99-100% for UPLC analysis which is under the acceptance criteria to the ICH guideline Q2(A).
The method precision was assessed using multiple preparations of a single sample. Six different preparations of aripiprazole were analyzed in triplicate on the same day. Fresh solutions were prepared and analyzed on each of two successive days for Intermediate Precision Study. The % assay value was calculated for each sample preparation using the peak area of chromatogram. The relative standard deviation values obtained for the assay value of aripiprazole using HPLC and UPLC methods were not more than 2.0%.
The linearity of both methods was evaluated by analyzing eight solutions in the concentration range between 40-160 μg/ml of the drug substance. The peak areas obtained from different concentrations of the drugs were used to calculate linear regression equations. These are y=31252x + 17480, y=32511x – 25877 for HPLC and UPLC method respectively. Additionally correlation coefficients of both methods were same as R2=0.999. The high values of the correlation coefficients were indicative of linear relationships between the analyte concentration and peak area.
The Limit of Detection (LOD) and Limit of Quantification (LOQ) were established by evaluating the minimum level at which the analyte could be readily detected and quantified accurately. The LOD and LOQ were 0.05 μg/ml and 0.1 μg/ml for HPLC method, while the UPLC method has given the higher sensitivity. The LOQ and LOD values were 0.05 and 0.01 μg/ml of analyte for UPLC method with signal to noise(S/N) ratio more than 3.3:1 for LOD and more than 10:1 for LOQ.
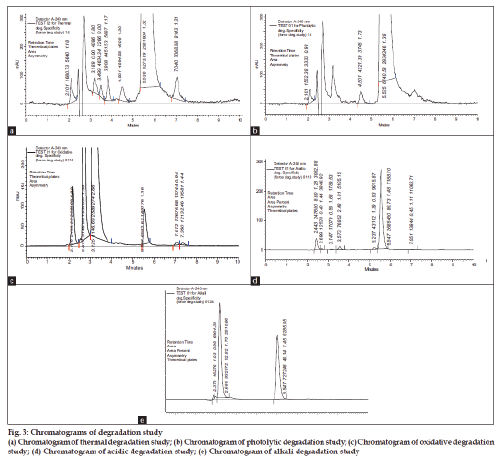
The evaluation of the specificity of the method was determined against a placebo. The interference of the claimed excipients present in the pharmaceutical dosage form was derived using a placebo solution. Further, the specificity of the method toward the drug was established by checking the interference of the degradation products in the drug quantitation for assay during the forced degradation study (fig. 3). The degradation was performed by applying the acidic, alkali, oxidative, thermal and photolytic stress to the sample and further the degradation was investigated using the above chromatographic method. The % degradation determined by the method (Table 1), which has also confirmed the information about the specificity of the method.
| Stress condition | Acidic | Alkali | Oxidative | Thermal | Photolytic | ||||
|---|---|---|---|---|---|---|---|---|---|
| % Degradation | 13.27 | 52.82 | 95.8 | 3.21 | 1.09 | ||||
Table 1: % Degradation of analyte by applying Forced degradation
The influence of five (k) chromatographic parameters on the separation was investigated. The parameters examined were the amount of acetoniltile in mobile phase, the amount of buffer solution in the mobile phase, different column lot, different flow rate and difference in pH of buffer. There was no significant impact observed on the results during this study, which suggest that both methods were highly robust.
The proposed HPLC and UPLC methods, shows a good sensitivity, resolution and selectivity in bulk drug as well in pharmaceutical dosage form. The method was shown to be selective, precise, sensitive and linear which was proven by the results of validation. Between the both proposed methods, the UPLC method is faster as it has only 3.0 min total analysis time; additionally the sensitivity was much higher as compare to HPLC method, which is proven in comparison of validation result (Table 2). The method can be used for the quantification of aripiprazole. It can be applicable for the analysis of drug substances and drug products in pharmaceutical dosage form also.
| Validation experiment | HPLC method | UPLC method |
|---|---|---|
| Specificity | No interference to analyte peak | No interference to analyte peak |
| LOQ | 0.1 µg/ml | 0.05 µg/ml |
| LOD | 0.05 µg/ml | 0.01 µg/ml |
| Linearity and range | ||
| Co-relation coefficient | R2=0.999 | R2=0.999 |
| Regression equation | Y=31252X+17480 | Y=32511X-25877 |
| Method precision (n=6) | %RSD=1.07 | %RSD=0.77 |
| Intermediate precision (n=6) | %RSD=0.23 | %RSD=0.98 |
| Accuracy (recovery %) | 99-101 | 99-100 |
| Robustness | Highly robust method | Highly robust method |
| Total analysis time | 10.0 min | 3.0 min |
Table 2: Comparison of hplc and uplc method By validation results
Acknowledgements
We are grateful to the National Facility for Drug Discovery through NCE’s development and Instrumentation support for Small Manufacturing Pharma Enterprises, Department of Chemistry, Saurashtra University, Rajkot for providing analytical facilities. We highly thankful to UGC, Govt.of India for providing financial support in terms of meritorious research fellowship and DST-DPRP for research assistantship. We are also thankful to the Torrent research Centre, India for providing working standards.
References
- Miranda H. FDA OKs Abilify for Depression. WebMD. Available from: http://www.webmd.com/depression/news/20071120/fda-oks-abilify-fordepression. [Last accessed on 2007 Nov 20].
- Patent and Exclusivity Search Results, Electronic Orange Book, US Food and Drug Administration. Available from: http://www. accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_ No=021436andProduct_No=001andtable1=OB_Rx.
- Keck PE, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160:1651-8.
- Sachs G, Sanchez R, Marcus R, Stock E, McQuade R, Carson W, et al. Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: A 3-week placebo-controlled study. J Psychopharmacol (Oxford) 2006;20:536-46.
- Vieta E, T’joen C, McQuade RD, Carson WH, Marcus RN, Sanchez R, et al. Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/ lithium monotherapy: A placebo-controlled study. Am J Psychiatry 2008;165:1316-25.
- Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, et al. Aripiprazole monotherapy in the treatment of acute bipolar I mania: A randomized, double-blind, placebo- and lithium-controlled study. J Affect Disord 2009;112:36-49.
- Keck PE, Calabrese JR, McIntyre RS, McQuade RD, Carson WH, Eudicone JM, et al. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: A 100-week, double-blind study versus placebo. J Clin Psychiatry 2007;68:1480-91.
- Keck PE, Calabrese JR, McQuade RD, Carson WH, Carlson BX, Rollin LM, et al. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry 2006;67:626-37.
- Stability Testing of New Drug Substances and Products Proceedings of the International Conference on Harmonization, International Federation of Pharmaceutical Manufacturers Association, Geneva, Switzerland 2003.
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline on Validation of Analytical Procedure-Methodology ICH, Geneva, Switzerland 1996.
- Rao DV, Shetty S, Satheesh K, Radhakrishnanand P, Himabindu V. A stability indicating RPLC method for aripiprazole. Indian J Anal Chem 2008;7:444-53.
- Vijaya kumar M, Muley PR. Determination of aripiprazole in bulk drug and solid dosage forms by RP-HPLC method. Indian Pharm 2005;4: 71-5.
- Koduri SV, Buchireddy SR, Madhusudhan G, Mukkanti K, Srinivasulu P. Stress degradation Studies on aripiprazole and development of a validated stability indicating LC method. Chromatographia 2008;68:635-40.
- Liu H, Jiang Y, Hao X. Determination of aripiprazole by nonaqueous reversed-phase high performance liquid chromatography. Se Pu 2005;23:563.
- Sastry BS, Gananadhamu S, Devala RG. RP-HPLC determination of aripiprazole in Pharmaceutical formulations. Asian J Chem 2009;21:6643-6.
- Kirschbaum KM, Muller MJ, Zernig G. Therapeutic monitoring of aripiprazole by HPLC with column-switching and spectrophotometric detection. Clin Chem 2005;51:1718-21.
- Lancelin F, Djebrani K, Tabaouti K, Kraoul L, Brovedani S, Paubel P, et al. Development and validation of a high-performance chromatography method using diode array detection for the simultaneous quantification of aripiprazole and dehydro-aripiprazole in human plasma. J Chromatogr B 2008;867:15-9.
- Musenga A, Saracino MA, Spinelli D, Rizzato E, Boncompagni G, Kenndler E, et al. Analysis of the recent antipsychotic aripiprazole in human plasma by capillary electrophoresis and high-performance liquid chromatography with diode array detection. Anal Chim Acta 2008;612:204-21.
- Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto G. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: Application to the pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2005;821:8-14.
- Zuo XC, Wang F, Xu P, Zhu RH, Li HD. LC–ESI–MS for Rapid and Sensitive Determination of Aripiprazole in Human Plasma. Chromatographia 2006,64:387-91.