- *Corresponding Author:
- H. Wang
Harbin Medical University (Daqing),Daqing, China
E-mail: liaohuidi3yy@126.com
| This article was originally published in a special issue, |
| "Clinical and Experimental Studies on Drug and Intervention Repurposing in China" |
| Indian J Pharm Sci 2019:81(4)spl issue1;191-196 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to explore the antifatigue effect of different doses of tea polyphenols on exercising rats, male Sprague Dawley rats were divided into 5 groups and each group was subjected to different treatments. The antifatigue effect of tea polyphenols on rats was evaluated by analyzing changes in body weight, exhaustion time and levels of internal factors in each group. The results showed that tea polyphenols can reduce the body weight of rats. In the analysis of exhaustive exercise time of rats, it was found that the time to exhaustion in low-dose tea polyphenols group was less than that of middle-dose tea polyphenols group, which is in turn less than that of high-dose tea polyphenols group. Therefore, tea polyphenols appear to effectively improve the endurance of rats antifatigue effect. In the analysis of serum creatine kinase, lactic acid, lactate dehydrogenase and blood urea nitrogen levels in rats of each group, it was found that the levels of creatine kinase, lactic acid, lactate dehydrogenase and blood urea nitrogen levels in rats increase significantly after exhaustion, but the levels of factors in rats decrease after intragastric administration of tea polyphenols. Moreover, the higher the dose of tea polyphenols was, the lower were the levels of creatine kinase, lactic acid, lactate dehydrogenase and blood urea nitrogen levels in rats, which was close to the blank control group. When the levels of IL-1β, TNF-α and IL-10 in serum of rats were analyzed, it was found that after exhaustion, the levels of IL-1β were not significantly increased, while the levels of TNF-α and IL-10 were significantly increased. After intragastric administration of tea polyphenols in rats, TNF-α factor decreased with increasing doses, but the effect on IL-10 was not obvious. Therefore, through this study, it was found that after exercise, rats can be given a certain dose of tea polyphenols to reduce their fatigue and improve ability to recovery. Although there are some shortcomings in the research process, it still provides a reliable basis for the study of the impact of fatigue resistance after exercise.
Keywords
Exercise intervention, tea polyphenols, anti-fatigue, enzyme-linked immunosorbent assay
In the process of modern society, with the improvement of people's living standards, people are not only concerned about food, clothing and warmth, but also pursuing quality with the progress of science and technology. Health has become the focus of people's most attention. Especially in today's age-striking era, national physical health has become one of the conditions to measure a country's comprehensive strength level[1]. Working people who exercise after a long day of sedentary work are likely to produce exercise-induced fatigue, which is a phenomenon that the physiological function of the human body changes due to exercise, thus temporarily reducing the ability to exercise[2,3]. If a person is in a state of fatigue, it can be found that they are mentally depressed, and unable to concentrate, while resistance will also decline and a series of symptoms will appear[4]. A large number of studies have shown that long-term regular exercise can promote physical health, delay aging, increase disease resistance, and thus achieve the health function[5]. Overload exercise or acute exercise can lead to inflammation. Inflammatory cell infiltration and release of inflammatory mediators can serve as trigger points of cascade reaction, promote the further expansion of inflammation and cause irreparable damage to cells, which may be an important cause of exhaustive exercise-induced injury to the body[6,7]. Therefore, research in to antifatigue, especially the resistance of athletes to fatigue in sports training, is a very critical.
In daily life, in fact, many traditional Chinese medicines have been reported to have significant antifatigue effect. In 2016, Liu et al. explored the effect of AACA treatment on the performance of mice under the action of Siebold et Zucc. The results showed that AACA has antifatigue effect and can improve the motor performance of mice. Therefore, Actinidia arguta can be used as an antifatigue dietary supplement in functional foods[8]. Ma et al. used male ICR mice as models in 2017 to explore the antifatigue effect of Changbai mountain Ginseng (CMG). The results showed that supplementation of CMG extract in vivo increases muscle quality, improves exercise performance and energy utilization, and reduces fatigue related parameters[9]. Miao et al. studied the antifatigue effect of Danggui Buxue decoction on mice in 2018 and found that various amino acids and TCA cycle in Danggui Buxue decoction have obvious effects on fatigue in mice[10]. Shen et al. studied the antifatigue effect of Sibao Cordyceps on a mice model in 2019 and found that it can inhibit the secretion of inflammatory cytokine IL-6 in mice and inhibit excessive lipid hydrolysis and lipid overuse[11]. Tea is one of the most popular beverages in the world and with a long history[12]. In the field of traditional Chinese medicine, there are many kinds of teas. Tea polyphenols (TPs) are rich in phenolic hydroxyl groups and have high stability and obvious antioxidant effect. Studies have shown TPs possessed antioxidant effect 20 times to that of vitamin C or vitamin E. It is also a good supporter of the permeability and stability of cell membranes, and has an obvious preventive and therapeutic effect on lipid peroxidation of cell membranes[13,14]. TPs have the functions of scavenging free radicals, antimutation, anticancer, antiatherosclerosis, lowering blood pressure and immune regulation. In recent years, many studies have shown that TP also have a strong antiinflammatory effect[15]. Therefore, the study of TPs, a relatively easyto- obtain component of traditional Chinese medicine, and its other medical effects has become the focus of attention of many researchers.
At present, many traditional Chinese medicines have been proved to have antifatigue effect during or after exercise, but little research has been done on the effect of TPs. Therefore, male Sprague Dawley (SD) rats were selected and divided into 5 groups. The rats were subjected to exercise intervention and were given TPs by intragastric administration. By observing and analyzing various indicators, the antifatigue effect on rats is analyzed, in order to provide evidence for the antifatigue ability after exercise.
In this study 40 male SD rats with an average body weight of 163.42±7.58 g were used. All rats were housed in cages with 4 rats in each case and were provided with standard rodent feed. The room temperature was kept at 22±2° and the relative humidity at 45-55 %, with natural light. The laboratory is disinfected with disinfectant every other day. Animal disposal and experimental procedures were in line with the national standards for laboratory animals. All animal experiment programs were approved by the Animal Management Committee.
The rats were allowed to adapt to an artificial 12 h light and 12 h dark environment. When the body weight reached 203.52±7.91 g, 8 rats were randomly grouped into a control group. The remaining 32 were used for development of the exercise model. The rats were given different doses of TPs 1 h ahead in darkness. All rats were given free access to food and water. Rats were weighed every 2 d and fed continuously for 2 w. Compared to the control group, there were significant differences in body weight (p<0.05), which indicated that the rat model induced by TPs was successfully established.
Forty male SD rats were randomly divided into a control group, a low dose TPs group (50 mg/kg, LTP group), medium dose TPs group (150 mg/kg, MTP group), high dose TPs group (300 mg/kg, HTP group) and a model control group, with 8 rats in each group. The control group and the model control group were given intragastric administration of normal saline. The LTP, MTP and HTP groups were given corresponding doses of TPs intragastrically, once a day, for 4 w.
The control group rats were exposed to no exercise intervention was. The other 4 groups of rats were allowed to swim for 20 min a day for 3 d, 4 d before the end of administration of TPs or saline. After each adaptive training, the rats were quickly dried with a towel and a hairdryer and put back to the cage to rest to prevent the rats from getting sick. Swimming was carried out in a large plastic bucket of a diameter of 60 cm and a height of 75 cm. The swimming area of each rat was above 300 cm2. The water depth was 60 cm, and the water temperature was 32±1°. At 9:00 am the next day after the last intragastric administration, the rats underwent a one-time exhaustive swimming exercise with 3 % body weight at the tail and the time for exhaustion was recorded. The exhaustion criterion was that the head of the rats cannot be exposed to the water for 10 s after sinking, and that the righting reflex cannot be completed after being pulled out and placed on the plane. They also show shortness of breath, large range and tiredness.
In the quiet state, the control group rats were anesthetized with 20 % urethane solution and then executed for blood collection. The other 4 groups of rats were anaesthetized with 20 % or immediately after exhaustion. The whole blood was taken from the abdominal aorta and placed in the common blood vessel for half an hour at room temperature for natural coagulation. The blood was centrifuged for 10 min at 4°, 3000 rpm/min. The upper serum was collected and stored in a refrigerator at -80°. It was used to determine the effects of creatine kinase (CK), LD, lactic dehydrogenase (LDH), blood urea nitrogen (BUN) and inflammatory factors such as IL-1β, TNF-α and IL-10.
The rats were weighed once a week during feeding and the time is set at 7 pm every Sunday. The last weighing was at 7 pm the day before the rats were sacrificed. Exhaustion time refers to the time when each rat reached exhaustion. CK, LD, LDH and BUN were detected using colorimetric methods following the instructions provided in the commercial kits.
Forty microliters of blood was sampled for direct determination. Before the test, the kit was placed at room temperature for 40 min, and all the reagents were shaken gently before use. Then, the standard was diluted and added to 5 tubes labelled as S1, S2, S3, S4 and S5. When IL-1β was determined, 240 ng/l standard solution was diluted to 7.5, 15, 30, 60, 120 ng/l with standard diluent. When TNF-α was determined, 1280 ng/l standard solution was diluted to 40, 80, 160, 320, 640 ng/l with standard diluent and 960 pg/ml standard application solution was diluted to 30, 60, 120, 240, 480 pg/ml with standard diluent when IL-10 is determined. In the blank wells, antiIL-1β antibody, antiTNF-α antibody, antiIL-10 antibody and streptavidin-HRP were labelled with biotin were not added, the other steps were the same and in the sample wells and standard wells were set, respectively. In each sample well, 40 μl of the sample was added, then 10 μl of antiIL-1β antibody and 50 μl of streptavidin- HRP were added respectively. In the standard well, different concentrations of standard substance 50 μl and streptavidin-HRP 50 μl were added. When adding samples, the samples were added at the bottom of the ELISA plate without touching the walls of the well and shaken gently to mix. After covering with a sealing film, the plate was incubated at 37° for 60 min. Concentrated detergent was diluted 1:20 with fresh double-distilled water, mixed and placed at room temperature for use. The sealing film was carefully removed and the liquid was discarded. After drying, each well was filled with detergent and it was kept for 30 s before discarding. These steps were repeated 5 times and the enzyme labels were patted dry on the absorbent paper. Each well was added with 50 μl of colour developer A, followed by 50 μl of colour developer B. After gently shaking and mixing, the reaction was allowed for 10 min at 37°. Finally, 50 μl terminator was added to each well to terminate the reaction. The OD of the ELISA plate was measured at 450 nm According to the standard curve, the factor content in the sample was calculated.
Statistical software SPSS22.0 was used to analyse the data. Paired T test was used to compare the data before and after the experiment. One-way ANOVA was used to compare the data between groups. Pearson correlation analysis was used to analyse the correlation. The results of the experiment were expressed by mean±SD. The significant level is p<0.05.
The body weights of rats are shown in Table 1 and initially the weight of rats in each group were all similar. During the next 4 w of intragastric administration of TPs, it was found that the weight of rats increased. The growth of rats in the control group and model control group was always higher than that of the TPs treated groups. With the increase of intervention time, the difference of body weight increased until the end of the experiment. Administration of low-dose or middledose or high-dose TPs, the body weight of rats did not show any significant changes between different doses of TPs. however, it was found that TPs administration reduced the weight of experimental group of rats, perhaps owing to their ability to prevent weight gain.
| Group | 0 w | 1 w | 2 w | 3 w | 4 w |
|---|---|---|---|---|---|
| Control group | 164.32±10.51 | 203.52±7.91 | 245.84±12.51 | 291.37±16.09 | 348.94±20.17 |
| Low dose group | 167.17±11.21 | 189.46±8.19 | 229.57±13.08 | 276.43±14.53 | 325.62±19.48 |
| Medium dose group | 162.09±9.18 | 187.43±9.08 | 228.63±11.76 | 278.52±14.87 | 328.59±18.76 |
| High dose group | 164.07±8.52 | 188.53±10.15 | 229.09±11.67 | 275.86±13.08 | 328.75±19.47 |
| Model control group | 168.03±8.81 | 194.98±9.17 | 237.52±14.29 | 285.03±15.58 | 337.53±21.52 |
Table 1: Changes in body weights after gastric administration in rats
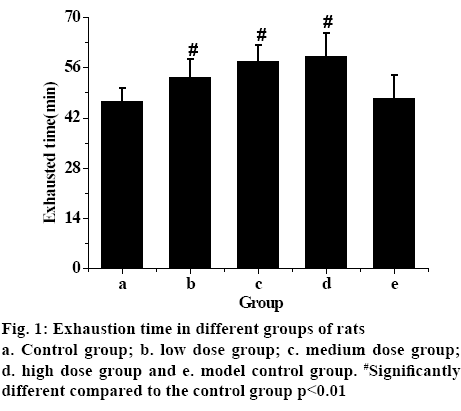
Statistical analysis of the data showed that the time pf the exhaustion of the model control group was the shortest, while the exhaustion time of the rats in groups that received different does of TPs increased. The exhaustion time of low-dose TP group was shorter than that of the medium-dose and higher-dose TP group. The exhaustion time of rats increases with the increasing TPs dosage, with significant statistical difference (p<0.01). Therefore, the experimental results showed that TPs can effectively improve the endurance of rats and exert antifatigue effect.
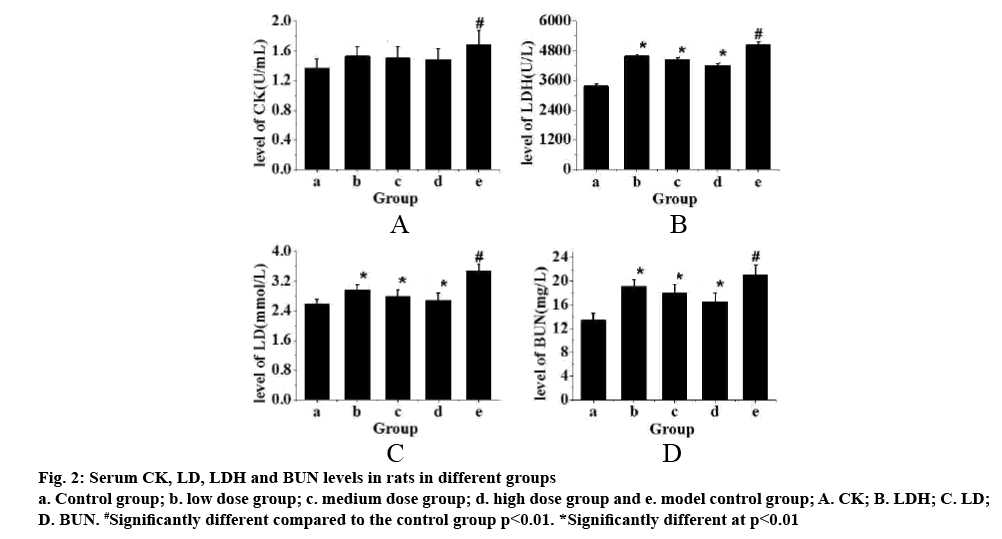
The serum levels of CK, LD, LDH and BUN in each group are shown in fig. 1. Fig. 2 showed that these factors in the control group were the lowest, but the levels of CK, LD, LDH and BUN in exercise-trained model control group were significantly higher than those in control group (p<0.01). After intragastric administration of TPs, the levels of each factor decreased significantly, especially in the high-dose TPs group. The expression levels of CK, LD, LDH and BUN were similar to those of the control group. Therefore, it is evident that the levels of CK, LD, LDH and BUN in rats increase significantly after exhaustion. However, administration of TPs, the levels of factors decreased. In addition, the higher the dose of TPs was, the lower the levels of CK, LD, LDH and BUN in rats, which was closer to that of the control group.
Figure 2: Serum CK, LD, LDH and BUN levels in rats in different groups a. Control group; b. low dose group; c. medium dose group; d. high dose group and e. model control group; A. CK; B. LDH; C. LD; D. BUN. #Significantly different compared to the control group p<0.01. *Significantly different at p<0.01
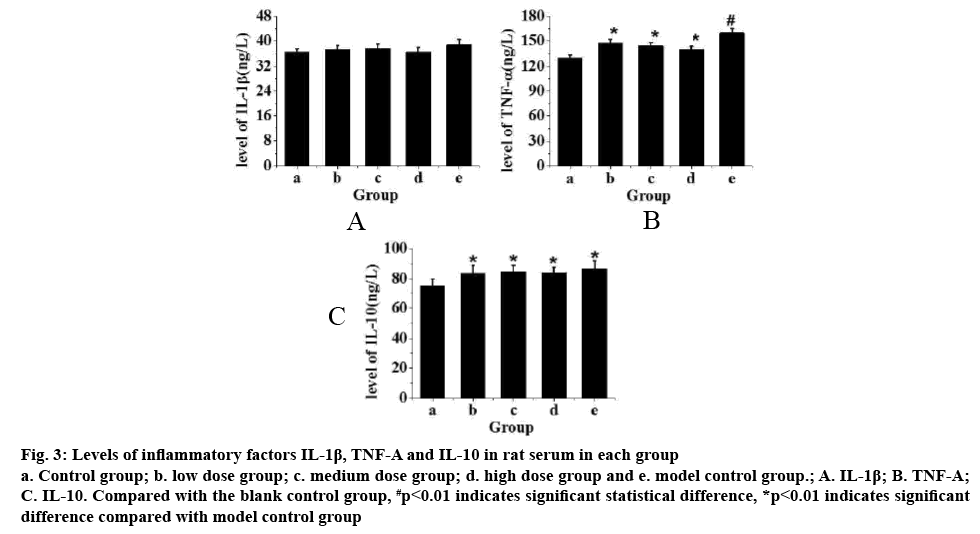
Fig. 3 showed the levels of IL-1β, TNF-α and IL-10 in rat serum. From fig. 3A, it can be seen that compared with the control group, the level of IL-1β in rats increased but not significantly after exercise training. From fig. 3B, it can be seen that compared to the control group, TNF-α in rats after exercise training increased significantly (p<0.01). After intragastric administration of TPs, it was found that the level TNF-α with increased doses of TPs (p<0.05). From fig. 3C, it can be seen that the level of IL-10 in rats increased significantly after exercise training compared to the control group (p<0.05), but there is no significant difference in the IL-10 levels in rats administered TPs. Therefore, after exhaustion, the increase of inflammatory factors IL- 1β in rats was not obvious, but the levels of TNF-α and IL-10 are significantly increased. After intragastric administration of TPs, TNF-α factor decreased with increased doses of TPs, but not the levels of IL-10.
Figure 3: Levels of inflammatory factors IL-1β, TNF-Α and IL-10 in rat serum in each group a. Control group; b. low dose group; c. medium dose group; d. high dose group and e. model control group.; A. IL-1β; B. TNF-Α; C. IL-10. Compared with the blank control group, #p<0.01 indicates significant statistical difference, *p<0.01 indicates significant difference compared with model control group
In order to explore the antifatigue ability of exercised rats after intragastric administration of different doses of TPs, male SD rats were divided into 5 groups. The rats in each group received different treatments. The antifatigue effects of TPs were observed after analyzing the changes of body weight, exhaustion time and levels of various factors in each group. By analyzing body weights, it was found that before the intervention with TPs, there was no significant difference in body weight among the groups. During the following 4 w of intragastric administration, it was found that the weight of rats in each group increased, and the weight gain of rats in control group and model control group was always higher than that of the TP intervention groups. With the increase of intervention time, the difference of body weight increases until the end of the experiment, with statistical difference.
For low-dose, middle-dose and high-dose TP groups, it was found that different doses of TPs exerted similar effects on the body weights of rats. Therefore, TPs can reduce the weight of rats. In the exhaustive exercise time analysis of rats, it was found that the exhaustion time after low-dose TP group was less than that of the medium-dose TP group which was less than that of high-dose TP group. Therefore, TPs can effectively improve the endurance of rats and show antifatigue effect. The levels of CK, LD, LDH and BUN in serum of rats in each group were analyzed. It was found that the levels of CK, LD, LDH and BUN increased significantly after exhaustion.
However, after intragastric administration of TPs, the level of these factors decreased. The higher the dose of TP was, the lower were the levels of CK, LD, LDH and BUN in rats, which was similar to those of the control group. When the levels of IL-1β, TNF-α and IL-10 in serum of rats were analyzed, it was found that the level of IL-1β did not increase significantly after exhaustion, but the levels of TNF-α and IL-10 increased significantly. After intragastric administration of TPs in rats, TNF-α factor decreased, but not the level of IL-10 factor. In conclusion, through the study of antifatigue ability of exercise rats after different doses of TPs, it was found that after exercise, rats can be given a certain dose of TPs to reduce fatigue and improve ability to recovery. However, there are also some shortcomings in the process of this study, such as the small size of the sample, so the sample size will be further increased in the later research process, so as to make the results more valuable for reference.
References
- Chang CW, Chen CY, Yen CC, Wu YT, Hsu MC. Repressed Exercise-Induced Hepcidin Levels after Danggui Buxue Tang Supplementation in Male Recreational Runners. Nutrients 2018;10(9):1318.
- Zhou Z, Yang J, Kong AN. Phytochemicals in traditional Chinese herbal medicine: cancer prevention and epigenetics mechanisms. Curr Pharmacol Rep 2017;3(2):77-91.
- Ashour ML, Youssef FS, Gad HA, Wink M. Inhibition of cytochrome P450 (CYP3A4) activity by extracts from 57 plants used in traditional Chinese medicine (TCM). Pharmacogn Mag 2017;13(50):300.
- Chen F, Wen Q, Jiang J, Li HL, Tan YF, Li YH, et al. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs. J Ethnopharmacol 2016;179:253-64.
- Cheng AJ, Place N, Westerblad H. Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb Perspect Med 2018;8(2):a029710.
- Garbincius JF, Merz LE, Cuttita AJ. Transgenic Expression of Dimethylarginine Dimethylaminohydrolase Attenuates Exercise-induced Fatigue in Duchenne Muscular Dystrophy Carrier Mice. Circ Res 2017;121(suppl_1):A415-A415.
- Proschinger S, Freese J. Neuroimmunological and Neuroenergetic Aspects in Exercise-Induced Fatigue. Exerc Immunol Rev 2019;25:8-19.
- Liu Y, Liu C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J Food Drug Anal 2016;24(4):738-45.
- Ma GD, Chiu CH, Hsu YJ, Hou CW, Chen YM, Huang CC. Changbai Mountain ginseng (Panax ginseng CA Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules 2017;22(2):237.
- Miao X, Xiao B, Shui S, Yang J, Huang R, Dong J. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J Pharm Biomed 2018;151:301-9.
- Shen Q, Miao CX, Zhang WL, Li YW, Chen QQ, Li XX, et al. SiBaoChongCao exhibited anti-fatigue activities and ameliorated cancer cachexia in mice. RSC Adv 2019;9(30):17440-17456.
- Li L, Guo D, Guo J, Song J, Wu Q, Bi H, et al. Thermosensitive in situ forming gels for ophthalmic delivery of tea polyphenols. J Drug Deliv Sci Technol 2018;46:243-50.
- Kumar V, Gupta P, Hassan MI. Mechanism and implications of traditional Chinese medicine in amyotrophic lateral sclerosis therapy. Mol Cell Proteomics 2019:1-17.
- Liu L, Wu X, Zhang B, Yang W, Li D, Dong Y, et al. Protective effects of tea polyphenols on exhaustive exercise-induced fatigue, inflammation and tissue damage. Food Nutr Res 2017;61(1):1333390.
- Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH. The effect of green tea and sour tea (Hibiscus sabdariffa L.) supplementation on oxidative stress and muscle damage in athletes. J Diet Suppl 2017;14(3):346-57.