- *Corresponding Author:
- W. R. Li
Institute of Clinical Pharmacology, Guangzhou University of Chinese Medicine, Guangzhou-510405, China
E‑mail: liwr@gzucm.edu.cn
| Date of Submission | 08 June 2013 |
| Date of Decision | 04 April 2014 |
| Date of Acceptance | 10 April 2014 |
| Indian J Pharm Sci 2014;76(3): 235-239 |
Abstract
Water, methanol and ethanol extracts of Evodia rutaecarpa were tested for antinociceptive activity, which were correlated with the contents of evodiamine, rutaecarpine and evodine. Determination of contents was achieved by chromatographic techniques. Extracts were evaluated for antinociceptive activities using hot-plate test; acetic acid-induced writhing test and formalin test. All three extracts of Evodia rutaecarpa showed antinociceptive activities but the ethanol extract exhibited better effect. The better antinociceptive activity appeared to be related to higher contents of evodiamine, rutaecarpine and evodine in ethanol extract of Evodia rutaecarpa.
Keywords
Evodia Rutaecarpa, evodiamine, rutaecarpine, evodine, antinociceptive activity
Evodia Rutaecarpa is commonly used and a “pungent, bitter and hot” natured drug in traditional Chinese medicine (known in Chinese as Wu Zhu Yu). It is used as an analgesic, antiemetic, antiinflammatory, and astringent agent in the Chinese Pharmacopoeia. The main alkaloids and terpene lactones with biological activity have been identified in ER including evodiamine, rutaecarpine and evodine (fig. 1). Pharmacological investigations have revealed that different extracts of E. rutaecarpa and its chemical constituents display a number of biological activities related to antinociception, antiinflammation and many more. Matsuda et al. [1] studied the antinociceptive effects of 70% methanol extract of ER, the results suggest that E. rutaecarpa possesses antinociceptive effects and proposed that it is possible to evaluate the quality of E. rutaecarpa using the antinociceptive effect and the content of evodiamine, rutaecarpine and limonin as indexes. Ko et al. [2]. revealed that the ethanol extract of E. rutaecarpa and its four bioactive components all exhibited antiinflammatory activities. The major virtue of E. rutaecarpa according to the herbal literature and books on the traditional Chinese system of medicine is its possible antinociceptive effect. Its antinociceptive effect could possibly be attributed to the presence of high concentration of alkaloids such as evodiamine, rutaecarpine and evodine.
It is normally used as aqueous extract of E. rutaecarpa in Chinese clinical application, but there have been a few reports on the aqueous extract and the antinociceptive activity difference between aqueous extract and alcohol extract remained to be clarified. In this paper we selected three classic pain models to study the antinociceptive effects of water, methanol and ethanol extracts of E. rutaecarpa and compared with contents of evodiamine, rutaecarpine and evodine in the three extracts.
Kunming mice with half male and female (Guangdong Medical Experimental Animal Center, Guangzhou, China) weighing 18 to 20 g were used for all the experiments. Animals were housed 5 per cage in a room maintained at 22±0.5° with an alternating 12 h light-dark cycle. Food and water were available ad libitum. Experiments were performed during the light phase of the cycle (10:00 to 17:00 h). These experiments were approved by the Animal Care and Use Committee, Guangzhou University of Chinese Medicine.
E. rutaecarpa was obtained from Kangmei Pharmaceutical Limited Company, Guangzhou, China. E. rutaecarpa originated in Sichuan province of China (lot number 110506851) and authenticated at Guangzhou University of Chinese Medicine as ripe fruit of E. rutaecarpa (Juss.) Benth. Fruits of E. rutaecarpa were subjected to size reduction to get coarse powder of desired particle size. The powdered material was soaked with water, 70% methanol or 70% ethanol for 1 h, refluxed for 2 h (80°) and dried into power (24 h) in freezer dryer (VisTis Advantage EL-85, SP Industries, Inc., Warminster, USA).The yield was 30.27, 36.09 and 36.52% w/w for water, methanol and ethanol extract, respectively.
Evodiamine was obtained from Chengdu Ruifensi Biotechnology Limited Company, Chengdu, China (lot number w-012-120418). Rutaecarpine and evodine were obtained from National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China (lot number 110801-201006, 110800-20040). Compound aminophenazone and barbital Injection was obtained from Tianjin Pharmaceutical Group, Tianjin, China (lot number 1105122) which contained 0.1 g aminophenazone per 2 ml. Methanol for HPLC was obtained from Beijing Dikma Science and Technology Limited Company, Beijing, China. Other analytical grade reagents were used for chemical analysis.
The contents of evodiamine, rutaecarpine and evodine in extracts were determined by HPLC consisted of Waters 515 pump and Waters 486 UV detector (Waters Corporation, Milford MA, USA). Chromatography conditions included column Synergi Hydro-RP C18 (Phenomenex, 4.6×250 mm); detection UV absorption at 230 nm; mobile phase: (A) methanol; (B) water; gradient elution (Time, A%): (0 min, 40%; 12 min, 75%; 26 min,75%; 30 min, 40%; 37 min, 40%) and flow rate was 1 ml/min; column temperature 30°; injection volume 20 μ1.
The hot-plate test was used to measure response latencies according to the method described by Almeida et al. [3]. with minor modifications. The mice were placed on an YLS-6B hot-plate maintained at 55±0.5° and the time between placement of the mouse on the platform and shaking or licking of the paws or jumping was recorded as the hot-plate latency. Mice with baseline latencies from 8 to 22 s were selected into the study. A significant increase of the latency was considered as indicative of analgesic activity. Twenty-four hours later and 60 min before the test, groups of animals was treated with water, methanol or ethanol extract of E. rutaecarpa 150 or 300 mg/kg p.o., respectively. Another group was given compound aminophenazone and barbital injection (150 mg/kg p.o.) while control animals received the same volume of 0.5% CMC-Na p.o. (20 ml/kg).
Antinociceptive effects by acetic acid-induced writhing test were performed by the method of Koster et al. [4]. Animals were divided into eight groups containing ten animals in each. Group I (normal) mice were fed with standard diet and were administered with an aqueous solution of 0.5% CMCNa (20 ml/kg p.o.). In Group II, mice were treated with compound aminophenazone and barbital injection (150 mg/kg p.o.). Group III and group IV mice were treated with water extract of E. rutaecarpa 150 and 300 mg/kg p.o., respectively. Group V and VI mice were treated with methanol extract of E. rutaecarpa 150 and 300 mg/kg p.o., respectively. Group VII and VIII mice were treated with ethanol extract of E. rutaecarpa 150 and 300 mg/kg p.o., respectively. All groups were treated once daily for a period of 7 days. Mice were given an intraperitoneal injection with 1% acetic acid (10 ml/kg) 60 min after the last administration. The number of abdominal constrictions produced in these animals was counted cumulatively for 15 min after the injection. Antinociceptive activity, indicated by the reduction in the mean of the number of abdominal constrictions in the test groups compared to the control group, was calculated as percent inhibition of abdominal constrictions (percent of inhibitory level) using the following formula: (mean of (control-test group)/control group×100 %).
The formalin test was carried out as described by Hunskaar and Hole [5] but with slight modifications. The design of grouping and drug administration was the same as acetic acid induced writhing test. Pain was induced by injecting 9 μl of 2% formalin in the subplantar region of the right hind paw 60 min after the last administration. The amount of time that the animal spent licking the injected paw, considered as an indicator of pain, was recorded for duration of 30 min in two phases, known as the early (0-10 min) and late (10-30 min) phases.
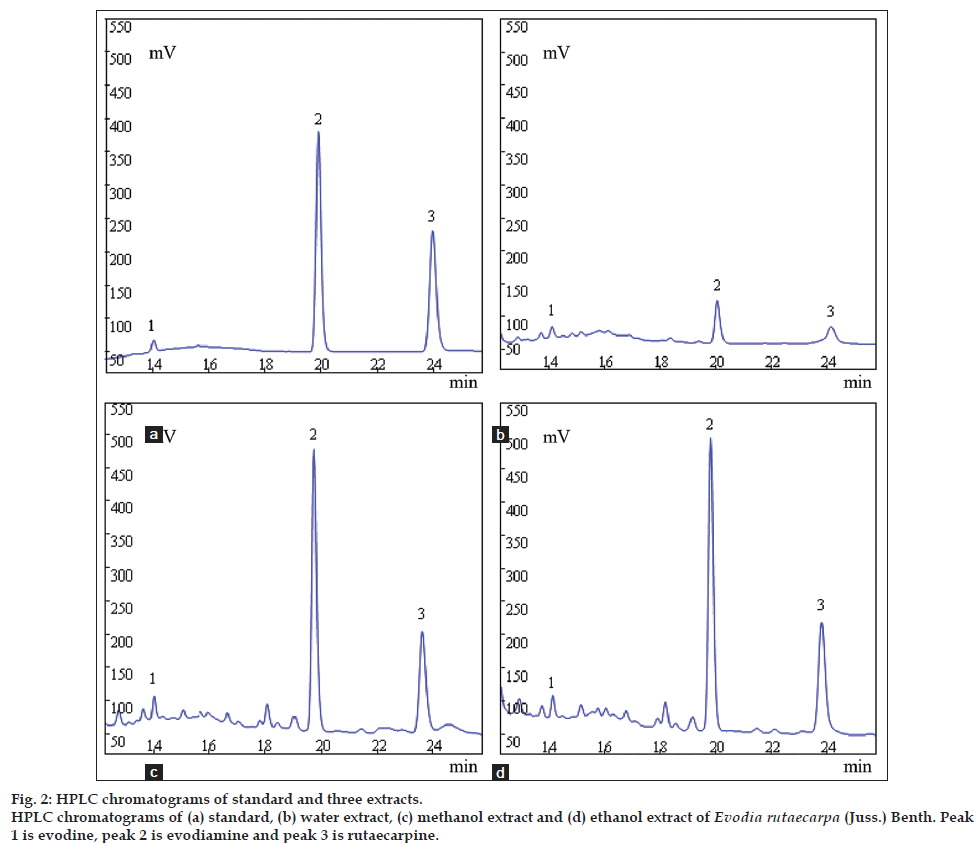
Chromatograms obtained from a standard solution and different extracts (fig. 2) reveal the selected marker constituents in standard and sample solutions were having same retention time. Spiking the sample solution with the standard compounds was also used to assist confirmation of peak identity. The method enables precise, sensitive and highly accurate quantification of evodiamine, rutaecarpine and evodine (method validation data not shown). When the method was used for analysis of ER, the contents of evodiamine, rutaecarpine and evodine showed in Table 1. Results revealed that alcohol was a better solvent to extract ER than water, the contents of evodiamine and rutaecarpine were about 20-30 times better extracted by alcohol than water and for evodine was about 1.6 times, respectively. There was no difference between methanol and ethanol.
| Extracts | Evodiamine % | Rutaecarpine % | Evodine % |
|---|---|---|---|
| Water | 0.009±0.001 | 0.004±0.001 | 0.747±0.024 |
| Methanol | 0.200±0.008* | 0.129±0.005* | 1.217±0.064* |
| Ethanol | 0.201±0.006* | 0.154±0.002* | 1.220±0.029* |
Values expressed as mean±SEM using three duplicated assay data. The results were analyzed using One‑way ANOVA followed by Dunnett’s multiple comparison tests. *P<0.05 when compared with water extract. There was no statistical significance between methanol and ethanol extracts of Evodia Rutaecarpa
Table 1: The Active Contents In Different Extracts Of Evodia Rutaecarpa
In hot plate test, oral administrations of different E. rutaecarpa extract were ineffective on the reaction to thermal stimuli in mice but compound aminophenazone and barbital injection (150 mg/kg p.o.) could raise the pain threshold (data not shown). These results were consistent with the report of Matsuda et al. [1].
In acetic acid-induced writhing test, the water extract, methanol extract and ethanol extract (150 and 300 mg/kg, p.o.) demonstrated a significant (P<0.05) antinociceptive activity in the acetic acidinduced writhing test (Table 2) with the percentage of analgesia ranging between 35 to 56%. Though there was no statistical significance among different extracts of E. rutaecarpa, the ethanol extract of E. rutaecarpa (300 mg/kg) produced a higher percentage change (56.4) relative to other extracts. Compound aminophenazone and barbital injection at 150 mg/kg (p.o.) showed the most efficacious antinociceptive activity, inhibitory percentage up to 96%.
| Treatment | Number of writhes (15 min) | percent inhibition (%) |
|---|---|---|
| Normal | 25.0±4.5 | ‑ |
| Compound aminophenazone and | 1.30±0.6* | 96.0 |
| barbital injection (150 mg/kg) | ||
| Water extract of ER (150 mg/kg) | 16.2±3.3* | 35.2 |
| Water extract of ER (300 mg/kg) | 14.9±4.7* | 40.4 |
| Methanol extract of ER (150 mg/kg) | 13.8±4.4* | 44.8 |
| Methanol extract of ER (300 mg/kg) | 14.4±4.6* | 42.4 |
| Ethanol extract of ER (150 mg/kg) | 14.9±4.2* | 40.4 |
| Ethanol extract of ER (300 mg/kg) | 10.9±3.4* | 56.4 |
Values expressed as mean±SEM, n=10 animals in each group. The results were analyzed using One way ANOVA followed by Dunnett’s multiple comparison tests. *P<0.05 when compared with normal group. There was no statistical significance among different extracts of Evodia Rutaecarpa
Table 2: Effect Of Different Extract Of Evodia Rutaecarpa In Acetic Acid-Induced Writhingtest in Mice
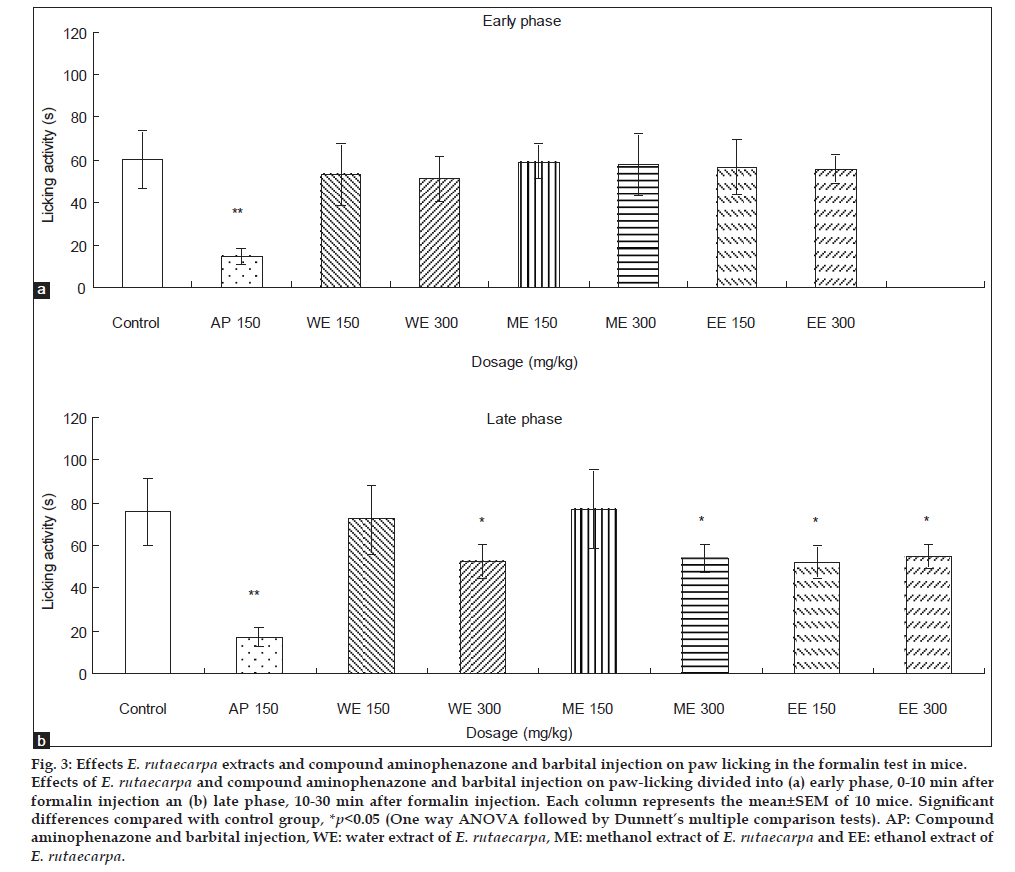
In formalin test, injection of formalin develops a biphasic licking response on the injected paw of mice. The early phase (neurogenic phase) occurs 0-10 min after injection and the late phase (inflammatory phase) occurs between 10 and 30 min after formalin injection. As shown in fig. 3, water, methanol and ethanol extracts of E. rutaecarpa at 300 mg/kg (p.o.) significantly reduced the time the mouse licked its stimulated paw in late testing phase when compared with control, ethanol extract of ER at 150 mg/kg (p.o.) also showed the effect. Compound aminophenazone and barbital injection at 150 mg/kg (p.o.) showed the effect in both phases. All 3 extracts exhibited antinociceptive activity in the inflammatory phase, while the ethanol extract was better than the others. It is likely that the E. rutaecarpa was able to produce greater activity due to the presence of higher contents of evodiamine, rutaecarpine and evodine.
Fig. 3: Effects E. rutaecarpa extracts and compound aminophenazone and barbital injection on paw licking in the formalin test in mice. Effects of E. rutaecarpa and compound aminophenazone and barbital injection on paw-licking divided into (a) early phase, 0-10 min after formalin injection an (b) late phase, 10-30 min after formalin injection. Each column represents the mean±SEM of 10 mice. Significant differences compared with control group, *p<0.05 (One way ANOVA followed by Dunnett’s multiple comparison tests). AP: Compound aminophenazone and barbital injection, WE: water extract of E. rutaecarpa, ME: methanol extract of E. rutaecarpa and EE: ethanol extract of E. rutaecarpa.
In conclusion, the results that the extract of E. rutaecarpa exhibited analgesic activities in acetic acid-induced pain model and the phase II of pain model mice induced by formalin, we could deduced that the type of analgesic activity of E. rutaecarpa is peripheral analgesia and the exact analgesic mechanisms of E. rutaecarpa need further study. Our observations confirm that water, methanol and ethanol extracts of E. rutaecarpa all showed antinociceptive activities and ethanol extract exhibited better effect. The better antinociceptive activity was involved with the higher contents of evodiamine, rutaecarpine and evodine in ethanol extract of Evodia Rutaecarpa.
Acknowledgements
The authors thank the “211” Project of Guangdong Province for financial support (Project principal: Professor Ningsheng WANG) and Professor Chenchen Zhu for identification of fruit of Evodia Rutaecarpa.
References
- Matsuda H, Wu JX, Tanaka T, Iinuma M, Kubo M. Antinociceptive activities of 70% methanol extract of evodiaefructus (fruit of Evodiarutaecarpa var. bodinieri) and its alkaloidal components. Biol Pharm Bull 1997;20:243-8.
- Ko HC, Wang YH, Liou KT, Chen CM, Chen CH, Wang WY, et al. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodiarutaecarpa and its bioactive components on neutrophils and microglial cells.Eur J Pharmacol 2007;555:211-7.
- Almeida JR, Araújo EC, Ribeiro LA, de Lima JT, Nunes XP, Lúcio AS, et al. Antinociceptive activity of ethanol extract from Duguetiachrysocarpa Maas (Annonaceae). ScientificWorldJournal 2012;2012:859210.
- Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Fed Proc 1959;18:412.
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 1987;30:103-4.