- *Corresponding Author:
- Amira M. Gamal-eldeen
Cancer Biology Laboratory, Nobel Project, Biochemistry Department, National Research Center, Dokki 12622, Cairo, Egypt
E-mail: aeldeen7@yahoo.com
| Date of Submission | 19 December 2006 |
| Date of Revision | 6 October 2007 |
| Date of Acceptance | 7 December 2007 |
| Indian J. Pharm. Sci., 2007, 69 (6): 805-811 |
Abstract
This work aimed to prove that simple chemical modification could provide new cancer chemopreventive and/or anticancer properties to the inactive extracted polysaccharide derived from Leucaena leucocephala . Polysaccharides were extracted from Leucaena leucocephala seeds and its 2,4-pentanedione-treated derivative (glycosylated form) was prepared, which is further sulphated to give sulphated glycosylated form. Estimation of their anti-initiation activity, modulation of carcinogen metabolism, was indicated by the inhibition cytochrome P450 1A (CYP1A) and the induction of glutathione-S-transferases (GSTs). Anti-proliferation activity was investigated by MTT assay against human hepatocarcinoma (HepG2), breast carcinoma (MCF-7) and lymphoblastic leukemia (1301). Apoptosis/necrosis and cell cycle were analyzed by flow cytometry. The results revealed that glycosylated form inhibited both CYP1A and GSTs, while sulphated glycosylated form not only inhibited CYP1A, but also induced the GSTs. Unlike GE, sulphated glycosylated form possessed a significant anti-proliferative activity against different cell lines. Analysis of HepG2 cell cycle phases demonstrated that glycosylated form led to a delay of G2/M-phase, while sulphated glycosylated form led to a concomitant arrest in S- and G2/M-phases. Investigation of apoptosis/necrosis ratio demonstrated that both of glycosylated form and sulphated glycosylated form induced HepG2 cell death by necrosis, but not apoptosis. Unmodified crude extract was neither active as cancer chemopreventive nor as anti-proliferative. In conclusion, chemical modification of Leucaena gum induced its cancer chemopreventive and anti-proliferative activities.
Keywords
Leucaena leucocephala, sulphated polysaccharides, HepG2, cell cycle, antiproliferation, chemoprevention, cytochrome P450 and glutathione-S-transferase
Leucaena leucocephala is a tropical plant belongs to Leguminosae and provides a useful source for fuel, protein, oil and commercial gum [1-3]. They have a total carbohydrate content of approximately 35% to 45%, with reducing sugars constituting 5.2% and an average degree of polymerization of 150 [4]. The highly viscous solutions of seed gum have the potential to be used as a laxative, in vegetable soups and in other food commercial products. L. leucocephala is reported to have few medicinal properties in contraception and abortion [2].
Galactomannans constitute the second most abundant storage polysaccharide in Leucaena sp. They are mainly also found in the endosperm cell wall of seeds from the other Leguminosae family [5]. The structure of these neutral polymers is relatively simple, consisting of a linear (1→4)-β-linked D-mannan backbone with single unit (1→6)-linked-α-D-galactopyranosyl side chains [6]. Galactomannans are relatively highly galactose substituted, where the mannose to galactose ratio [man/gal] between 1.1 and 3.5, which is varying with different species, crops, portions or fractions. Galactomannans properties depend on their chemical structure, such as chain length, availability of cis-OH groups, steric hindrance, substituents and degree of polymerization. In addition to these variations, hydrophilic properties, solubility; gelling and functional characteristics represent the basis of their different biological activities and numerous industrial applications such as pharmaceuticals, food processing and cosmetics [7,8]. Galactomannans have multiple side-chain galactose units that should readily interact with galactose-specific receptors (such as galectins on the tumor cell surface), modulate the tumor surface physiology and potentially affect delivery of drugs and functional molecules to the tumor [9]. They have biological activities including cancerchemopreventive, anticancer [10], immunostimmulation [11], antiviral [12], anticoagulant and antithrombotic [13] activities. Sulphated polysaccharides were also reported to have in vitro antiviral and anticoagulant activity, which was attributed to the negatively charged sulphate groups [14].
In a recent work, we successfully modified the guar gum structure, in a way that improve and develop its immunomodulatory, antiinflammatory, antiproliferative, cancer chemopreventive properties by C-glycosylation and sulphation [15]. The present investigation aimed to prove that simple chemical modification such as 2,4-pentanedione treatment and additional sulphation of L. leucocephala polysaccharide-protein complex could provide new cancer chemopreventive and/or anticancer properties to the inactive crude extract. The ultimate goal is to use those derivatives as alternatives of L. leucocephala polysaccharide-protein complex in health food industries, to provide potential cancer chemopreventive and/or anticancer properties for highrisk populations.
Materials and Methods
Extraction of water-soluble polysaccharide
L. leucocephala gel complex were successively extracted from milled seed (5 g), with distilled water (200 ml for 3 times) at 100º under reflux for 1h as previously described [16]. The combined extracts were clarified by centrifugation and removing the contaminated protein by 20% of trichloroacetic acid precipitation and finally dialyzed against distilled water for 2 days. The retentate was concentrated to small volume and then precipitated with 3 volumes of ethanol. The resulting precipitate was isolated by centrifugation, washed by acetone then lyophilized.
Chemical modifi cation
According to the previously reported methodology [17], a solution of polysaccharide-protein complex (200 mg), NaHCO3 (200 mg) and pentane-2,4-dione (300 µl) in 20ml of water was stirred at 100º for 20 h, The solution was diluted and neutralized with Dowex 50 resin (H+ form). The resin was filtered off and the retentate was mixed with 100 mg NaBH4. The reaction mixture was stirred for 24 h at room temperature and the excess of NaBH4 was neutralized with glacial acetic acid. The solution was dialyzed against distilled water for 2 days and then lyophilized. The sulphation of the dried of 2,4-pentanedionetreated polysaccharide-protein complex (GE) was performed as follows: 0.3 g GE was suspended in 2 ml dry formamide and the mixture was stirred at room temperature for 24 h in order to disperse it into the solvent. A solution was prepared by dropping 5 ml of HClSO3 in 20 ml of formamide under cooling in an ice-water bath and then added to the GE-mixture. The reaction was cooled in ice, neutralized by 30% NaOH solution and dialyzed against distilled water for 48h and then lyophilized.
Compositional analysis
L. leucocephala extract, GE and the sulphated-GE (SGE) were submitted to many compositional analysis tests, including: estimation of total sugars [18] and total protein [19]. The sugar composition was determined after complete hydrolysis with H2SO4 (2 mol/l) at 100º for 8 h. The mixture was neutralized with BaCO3, centrifuged, filtered, neutralized with Dowex 50 resin (H+ form) and concentrated. The hydrolysates were spotted in Whatmann No.1 paper and subjected to chromatography in butanol:acetone:water, 4:5:1, for 24 h. The chromatogram was visualized by spraying with aniline phthalate [20]. The sulphate content was carried out by hydrolysis of SGE with HCl [21] and librated sulphate ions were determined by the BaCl2 turbidimetric method [22].
Cell culture
Several human cell lines were used through out this work including: hepatocarcinoma (HepG2) and breast carcinoma (MCF-7) and lymphoblastic leukemia (1301). Cells were routinely cultured in DMEM (Dulbeco’s Modified Eagle’s Medium) supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, containing 100 units/ml penicillin G sodium, 100 units/ml streptomycin sulphate and 250 ng/ml amphotericin B. Cells were maintained at sub-confluency at 37º in humidified air containing 5% CO2. For sub-culturing, monolayer cells were harvested after trypsin/EDTA treatment at 37°. Tested extracts were dissolved in phenol red-free medium. All cell culture material was obtained from Cambrex BioScience (Copenhagen, Denmark). All chemicals were from Sigma/Aldrich, USA, except mentioned. All experiments were repeated four times, unless mentioned.
Cytochrome P450 1A activity
Cytochrome P450 1A (Cyp1A) activity was determined by measuring the rate of dealkylation of 3-cyano-7- ethoxycoumarin (CEC) to the fluorescent 3-cyano- 7-hydroxycoumarin based on a previously reported method [23] that was modified by [24]. Homogenates from cultured Hep G2 cells induced with β-naphthoflavone were used as a source of Cyp1A. A final concentration of sample (1µg /ml) was used. CEC conversion rate was measured kinetically by microplate fluorescence reader (FluoStarOptima, BMG, UK). Inhibition of Cyp1A activity was compared with the initial fluorescence of a complete reaction mixture with cell homogenate and buffer instead of samples.
Glutathione-S-transferases activity
Glutathione-S-transferases (GSTs) activity was measured [25] basing on the GSTs-catalyzed reaction between GSH and 1- chloro-2,4-dinitrobenzene (CDNB), which acts as an electrophilic substrate for GSTs. In brief, HepG2 cells (1×106 cells) were treated with 5 µg/ml galactomannans. In a kinetic analysis, the absorbance was assessed at 340 nm. GSTs were calculated by this equation: (Slopesample-Slopebuffer)/mg Protein and expressed as the percentage of control. Data were normalized to the cellular protein content, which was measured by bicinchoninic acid assay [26].
Cytotoxicity assay
Antiproliferative activity against various tumor cell lines was estimated by the 3-(4,5-dimethyl-2-thiazolyl)- 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, which is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells [28]. Different cell lines (5×104 cells/well) were incubated with gradual sample concentrations for 24 h at 37º in a serum free medium and then submitted to MTT assay. The relative cell viability was expressed as the mean percentage of viable cells comparing to untreated cells and the half maximal growth inhibitory concentration (IC50) was calculated.
Cell cycle analysis
HepG2 cells (5×105) were collected after treatment with galactomannans (20 µg/ml), washed twice with PBS, re-suspended in 300 µl of PBS and fixed with 4 ml of ice-cold 70% ethanol. Cells were centrifuged and the cell pellets were then re-suspended in 1 ml of propidium iodide (PI)/Triton X-100 staining solution (0.1% Triton X-100 in PBS, 0.2 mg/ml RNase A and 10 µg/ml PI) and incubated for 30 min at room temperature. The stained cells were analyzed by flow cytometry.
Apoptosis assay
Annexin V is a protein that binds to phosphatidylserine (PS) residues on the cell surface of apoptotic, but not normal cells. The binding of PS with annexin V was assayed using FITC-conjugated Annexin V and PI double staining using Apoptest Kit (DakoCytomation, UK). Treated and untreated cells were collected by trypsinization, washed with PBS, spooled with floating cells and submitted to the kit procedure and then submitted to flow cytometric analysis.
Statistical analysis
The results of statistical analysis are presented as the mean±SD and were compared by ANOVA followed by Tukey’s test. P values less than 0.05 were considered significant
Results
Chemical modifi cation of protein-polysaccharide complex
The native polymer was isolated from local leguminous seed (Leucaena sp.) by extraction with boiling water and we investigated its chemical composition in a previous work28, where the chemical composition of E and chromatographic examination of its hydrolysates showed the presence the major amounts of glucose (Gluc), Man, Gal and minor amount of arabinose and uronic acids28. In the present work, the chemical composition of GE and SGE revealed that the total carbohydrate content was 76.2% and 42.3%, respectively, in addition to the presence of small amount of protein 4.8% and 4.4%, respectively. The chromatographic examination of acid hydrolysates of GE and SGE showed difference in their relative monosaccharide constituents as 33.7: 11.7: 54.7% w/w and 42.7: 32.4: 24.8% w/w of man:gal:gluc, respectively and that the sulphate was detected only in SGE with 53.2%.
Antiinitiating activity
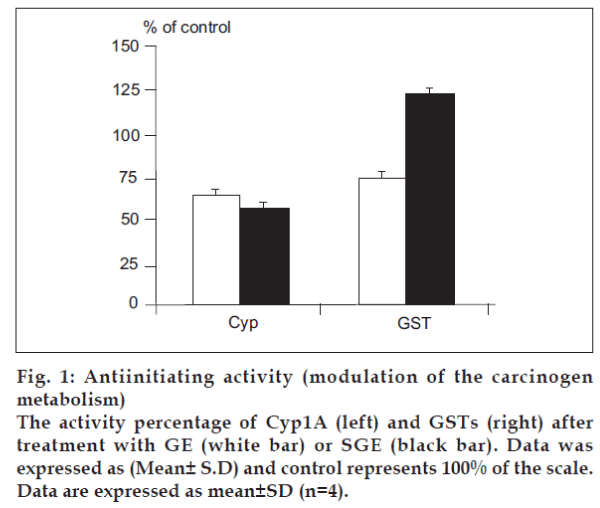
To identify the cancer chemopreventive properties, specifically antiinitiation, of different galactomannans, we used cell- and enzyme-based in vitro assays with markers relevant for measuring the inhibition of carcinogenesis cascade during the initiation stage. We investigated the influence of GE and SGE, compared to the crude unmodified extract, on the modulation of carcinogen metabolism, i.e, the carcinogen activation by the Phase 1 enzyme Cyp1A and the carcinogendetoxification by the Phase 2 enzymes GSTs. In Cyp1A assay, both of GE and SGE were recognized as potent inhibitors of Cyp1A activity in vitro (P<0.01), with a percentage of inhibition of 36.52% and 43.58%, respectively (fig. 1, left), while the crude extract revealed a non-significant inhibition of 5.3%, as examined also at the fixed final concentration (1 µg/ml). At a different concentration (5 µg/ml), which is relatively save non toxic dose, the phase 2 enzyme GSTs was dramatically inhibited by GE treatment (P<0.05), while GSTs activity was significantly induced by SGE (P<0.01) compared to the GSTs activity of the control (40.75 nmole/mg protein) (fig. 1, right). On the other hand, the crude extract led to 8.52% induction in GSTs (P>0.05).
Antiproliferative activity
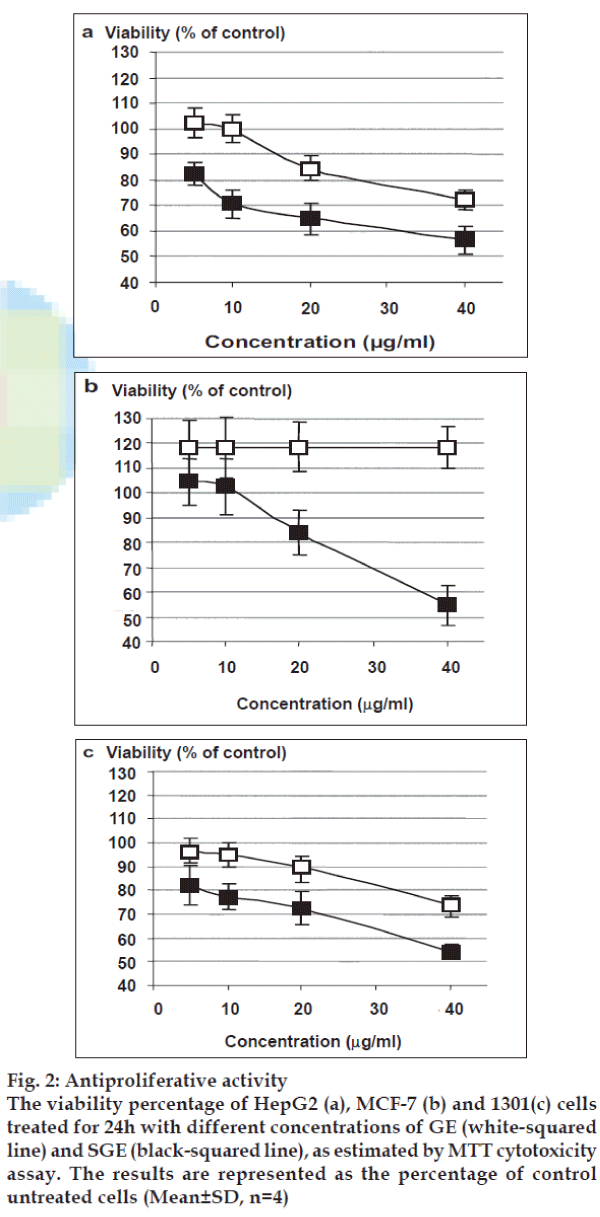
Treatment of different cell lines with gradual doses of native extract resulted in unchangeable proliferation. As shown in fig. 2a, the treatment of HepG2 cells with GE at 40µg/ml inhibited the HepG2 cells growth by 27.7%, while the treatment with SGE indicated a remarkable dose-dependent growth inhibition with a calculated IC50 value of 43.15 μg/ml. In MCF-7 cells, as shown in fig. 2b, GE led to unchangeable static growth rate with relatively higher growth baseline than untreated cells. On the other hand, SGE inhibited the cell growth of MCF-7 cells in a dose-dependent pattern with IC50 value of 47.36 μg/ml. Only SGE showed a remarkable inhibition of 1301 cell growth with IC50 value of 46.40 μg/ml (fig. 2c).
Fig. 2: Antiproliferative activity
The viability percentage of HepG2 (a), MCF-7 (b) and 1301(c) cells
treated for 24h with different concentrations of GE (white-squared
line) and SGE (black-squared line), as estimated by MTT cytotoxicity
assay. The results are represented as the percentage of control
untreated cells (Mean±SD, n=4)
Analysis of cell cycle phases and apoptosis of HepG2 cells
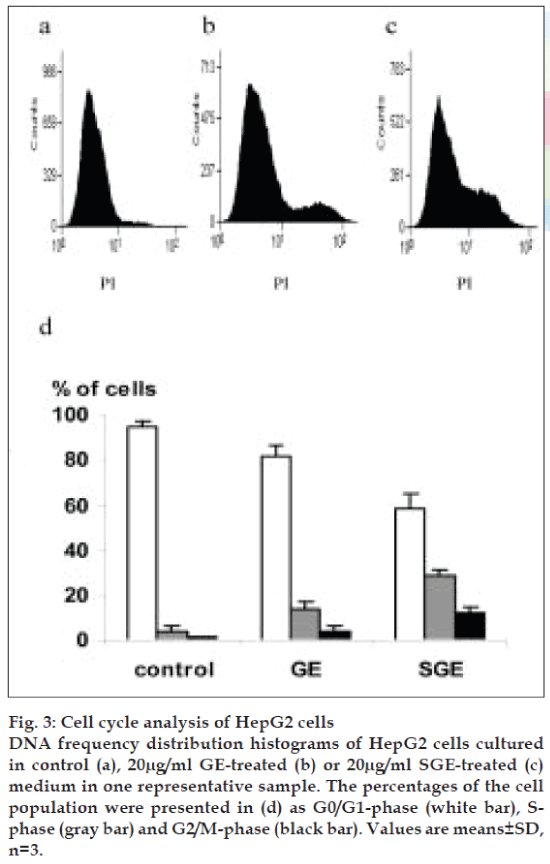
To explore the antiproliferative property of GE and SGE against HepG2 cells, we studied the cell cycle phases. Treatment of HepG2 cells with 20 µg/ml of GE indicating a predominated growth arrest at the S- and G2/M-phases (P<0.01) (fig. 3b and 3d), as compared with control cells (fig. 3a and 3d), where the S-phase progression of HepG2 cells was considerably delayed. The treatment with 20 µg/ml of SGE induced G2/M arrest of HepG2 cells and S-phase arrest, where a large proportion of cells were accumulated in S- and G2/M-phase (P<0.05) (fig. 3c and 3d).
Fig. 3: Cell cycle analysis of HepG2 cells DNA frequency distribution histograms of HepG2 cells cultured in control (a), 20µg/ml GE-treated (b) or 20µg/ml SGE-treated (c) medium in one representative sample. The percentages of the cell population were presented in (d) as G0/G1-phase (white bar), Sphase (gray bar) and G2/M-phase (black bar). Values are means±SD, n=3.
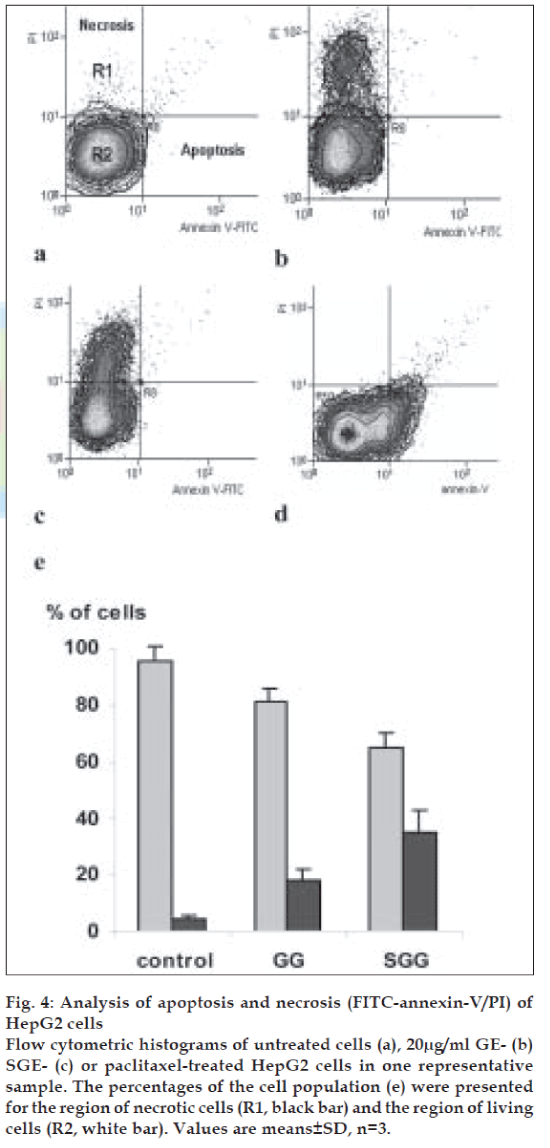
Surprisingly, both of GE and SGE induced necrosis but not apoptosis, as indicated by a cell population shift towards PI axes as shown in fig. 4b and 4c respectively, as compared to the control pattern (fig. 4a) and to the cell population shift of the positive control (paclitaxel, 700 nM for 6h, (fig. 4d). As shown in fig. 4e, GE induced necrosis to a population of 18.21% of cells and SGE to a population of 34.54% of cells. In parallel experiments, the treatment of HepG2 cells with crude extract resulted in a normal pattern of cell cycle stages and apoptosis/necrosis ratio similar to the control untreated cells (data are not shown).
Fig. 4: Analysis of apoptosis and necrosis (FITC-annexin-V/PI) of HepG2 cells Flow cytometric histograms of untreated cells (a), 20µg/ml GE- (b) SGE- (c) or paclitaxel-treated HepG2 cells in one representative sample. The percentages of the cell population (e) were presented for the region of necrotic cells (R1, black bar) and the region of living cells (R2, white bar). Values are means±SD, n=3.
Discussion
It is known that polysaccharides may have one terminal reducing glycosyl residue per linear polymer chain. This terminus provides a selective and convenient site for direct covalent attachment of molecules with β-dicarbonyl compounds such as pentane-2,4-dione. Knoevenagel-type condensation involves the reaction of the glycosyl residue with β-dicarbonyl compounds in presence of NaHCO3. Therefore, the reaction of L. leucocephala polysaccharides with pentane-2,4-dione in presence of NaHCO3 gave rise into C-glycosidic 2-propanone of the polysaccharide chain [17,29-31], which afforded C- glycosidic 2-propanol of corresponding polymer by, reducing agent, Na BH4.
Modulation of enzymes involved in metabolic activation (Cyp1A); and detoxification, conjugation and excretion of carcinogens (GST) is one of the bestinvestigated mechanisms of cancer chemopreventive agents [32]. Besides detoxifying electrophilic xenobiotics, such as chemical carcinogens, environmental pollutants and antitumor agents, GSTs inactivate endogenous alpha, beta-unsaturated aldehydes, quinones, epoxides and hydroperoxides formed as secondary metabolites during oxidative stress [33]. From our results of Cyp1A enzyme activity, it was obvious that the inhibitory activity of both GE and SGE was due to the C- glycosylation with no additional influence of the sulphation on these inhibitory properties, which was also not dependent on the original polysaccharide composition. However, in case of GSTs, the inhibitory property of GE is suggested due to carbohydrate or protein complex and the difference in mannose/ galactose ratios in GE and SGE, compared to the non-significant increase of GSTs by crude extract. The surprising induction of GSTs activity by SGE is likely to assume that additional sulphation of GE and/or the lower mannose/galactose ratio strongly enhanced its GSTs activity by a direct induction of GSTs expression and/or its indirect interaction with the glutathione cycle.
The relatively closed IC50 values of SGE against different types of tumor cells, concluding a nonspecific broad-spectrum anti-proliferative activity, regardless the cell type. Considering the high molecular weight of polysaccharides, the IC50 values (around 45µg/ml) represent low effective molar concentration of SGE. Our findings clarified that neither the crude extract nor GE exhibited significant antiproliferative properties. Necrosis is characterized by cell swelling, disruption and rapid cell membrane disintegration, leading to cellular content release, inflammatory response and lysis of intracellular organelles [34,35]. In contrast, apoptosis is a tightly regulated process controlled by a hierarchical set of molecules. During apoptosis the cells undergo nuclear and cytoplasmic shrinkage, the chromatin is condensed and partitioned into multiple fragments and the cells are finally broken into multiple membranesurrounded bodies (apoptotic bodies). In the present study, the GE- and SGE-inhibited cell growth of HepG2 cells was due to necrosis and was triggered by coincided disturbance in cell cycling suggesting that the observed necrosis is primary necrosis instead of apoptosis-derived secondary necrosis. Several possible mechanisms may explain necrosis, SGE and GE may deregulate the transcriptional protein p53, which can induce p21/WAF1 expression and consequently inhibits cyclin-dependent kinases for the control of both G1 and G2/M checkpoints [36,37]. They may inhibit caspases in such a manner that caspases inactivation may suppress apoptosis and lead cells into necrosis [38]. Many of the known cancer chemopreventive extracts, such as curcumin, showed anti-proliferative activity associated with cell cycle disturbance and growth arrest, but without associated apoptosis [39].
In a novel trial we successfully modify the structure of the extracted L. leucocephala polysaccharide by pentane-2,4-dione and by a further sulphation. Taken together, our results showed that the GE inhibited carcinogen metabolic activation and suppress the cancer cell growth. While the SGE showed a strong non-specific antiproliferative activity against different types of tumor cells and a promising antiinitiation property. In conclusion, these findings suggested that simple chemical modifications might convert the inactive extracted polysaccharide into a cancer chemopreventive and/or antiproliferative agent. A scope that may provide a broad spectrum of new probes, which exhibit cancer preventive properties derived from save nutritional resources. In vivo investigation of these probes may help in progression of a new anticancer food supplement of need in cancer risk areas.
Acknowledgements
We thank Prof. Mohammad A. Ali, Virology laboratory, NRC for his support, cooperation and supply of facilities. This work was funded by The National Research Center (NRC), Cairo, Egypt.
References
- Azeemoddin G, Rao SJM, Rao SDT. Amino acid composition of subabul (Leucaena leucocephala) seed kernel proteins. J Food Sci Technol 1988;25:158-62.
- Rushkin FR. Leucaena: Promising forage and tree crops for the tropics. 2nd ed. National Research Council, Washington, DC: National Academy Press; 1984.
- Rao SJM, Azeemoddin G. Recovery of lecithin and refining of subabul (Leucaena leucocephala) seed oil. J Oil Technol Assoc India 1988;20:16-7.
- Buckeridge MS, Dietrich SMC, Maluf AM. Galactomannan in seeds of different populations of Leucaena leucocephala. Rev Brasil Bot 1987;10:25-7.
- Reid JS. Cell wall storage carbohydrates in seeds: Biochemistry of the seed “gums” and “hemicelluloses”. Adv Bot Res 1985;11:125-5.
- Edwards ME, Marshall E, Gidley MJ, Reid GS. Transfer specificity of detergent-solubilized fenugreek galactomannan galactosyltransferase. Plant Physiol 2002;129:1391-7.
- Reid JSG, Edwards M. Galactomannans and other cell wall storage polysaccharides in seeds. In: Food polysaccharides and their applications. Stephen AM, editor. New York: Marcel Dekker; 1995.
- Shcherbukhin VD. Galactomannans of native flora. Appl Biochem Microbiol 1993;29:599-606.
- Kim HS, Kacew S, Lee BM. in vitro chemopreventive effects of plant polysaccharides (aloe barbadensis Miller, Lentinus edodes, ganoderma Lucidum, Coriolus versicolor). Carcinogensis 1999;20:1637-40.
- Fujiki H, Suganuma M, Kurusu M, Okabe S, Imayoshi Y, Taniguchi S, et al. New TNF-alpha releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutat Res 2003;523-524:119-25.
- Ramesh HP, Yamaki K, Tsushida T. Effect of fenugreek (Trigonella foenum-graecum L.) galactomannan fractions on phagocytosis in rat macrophages and on proliferation and IgM secretion in HB4C5 cells. Carbohydrate Polymers 2002;50:79-83.
- Herold BC, Gerber SI, Polonsky T, Belval BJ, Shaklee PN, Holme K. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 1995;206:1108-16.
- Martinichen-Herrero JC, Carbonero ER, Sassaki GL, Gorin PA, Iacomini M. Anticoagulant and antithrombotic activities of a chemically sulphated galactogluco-mannan obtained from the lichen Cladonia ibitipocae. Int J Biol Macromol 2005;35:97-102.
- Ono L, Wollinger W, Rocco IM, Coimbra TL, Gorin PA, Sierakowski MR. in vitro and in vivo antiviral properties of sulphated galadctomannans against yellow fever virus BeH111 strain) and dengue 1 virus (Hawaii strain). Antiviral Res 2003;60:60201-8.
- Gamal-Eldeen AM, Amer H, Helmy WA. Cancer chemopreventive and antiinflammatory activities of chemically modified guar gum. Chem Biol Interact 2006;161:229-40.
- Anderson DMW, Howlett JF, McNab CG. The hydroxyproline content of gum exudates from several plant genera. Phytochemistry 1986;26:309-11.
- Graziani A, Amer H, Zamyatina A, Hofinger A, Kosma P. Synthesis of C-glycosides related to glycero-β-D-manno-heptoses. Tetrahedron. Asymmetry 2005;16:167-75.
- Dubois M, Gillls KA, Hamilton JK, Rebers PA, Smith F. Colourimetric method for determination of sugars and related substances. Anal Chem 1956;28:350-6.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin-Phenol Reagent. J Biol Chem 1952;193:265-75.
- Partridge SM. Aniline hydrogen phthalate as spraying reagent for chromatography of sugars. Nature 1949;164:443.
- Larsen B, Haug A, Painter JTJ. Sulphated Polysaccharides in brown algae I-isolation and preliminary characterization of three sulphated polysaccharides from Ascophylum nodosum. Acta Chem Scand 1966;20:219-30.
- Garrido ML. Determination of sulphur in plant material. Analyst 1962;89:61-6.
- Crespi CL, Miller VP, Penman BW. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal Biochem 1997;248:188-90.
- Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, et al. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res 2003;523-524:163-72.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130-9.
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic. Acid Anal Biochem 1985;150:76-85.
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203-10.
- Hussein MM, Helmy WA, Salem HM. Biological activities of some galactomannans and their sulfated derivatives. Phytochemistry 1998;48:479-84.
- Rodrigues F, Canac Y, Lubineau A. A convenient, one-step, synthesis of β-C-glycosidic ketones in aqueous media. Chem Commun 2000;:2049-50.
- Riemann I, Papadopoulos MA, Knorst M, Fessner WD. C-glycosides by aques condensation of 1, 3-diketones with unprotected sugars. Aust J Chem 2002;55:147-56.
- Wang Z, Shao H, Lacroix E, Wu SH, Jennings HJ, Zou W. Epimerization of 2’carboxyl-C-gkycosides via enolation, beta-elimination and intramolecular cyclo-addition. J Org Chem 2003;68:8097-105.
- Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett 1995;82-83:173-9.
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005;45:51-88.
- Wyllie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980;68:251-306.
- Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999;18:7719-30.
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993;75:817-25.
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 1993;366:701-4.
- Samali A, Nordgren H, Zhivotovsky B, Peterson E, Orrenius S. A comparative study of apoptosis and necrosis in HepG2 cells: Oxidant-induced caspase inactivation leads to necrosis. Biochem Biophys Res Commun 1999;255:6-11.
- Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 1997;130:576-84.