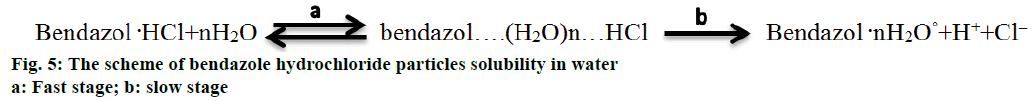- *Corresponding Author:
- E. V. Uspenskaya
Department of Pharmaceutical and Toxicological Chemistry, Medical Institute
RUDN University, Miklukho-Maklaya 8, Moscow, 117198, Russia
E-mail: uspenskaya75@mail.ru
| Date of Submission | 11 November 2016 |
| Date of Revision | 24 June 2017 |
| Date of Acceptance | 12 February 2018 |
| Indian J Pharm Sci 2018;80(2):318-324 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Kinetic analysis by means of low-angle laser light scattering has been used for the investigation into active pharmaceutical ingredients solubility depending on the content of heavy isotope of hydrogen 12H (D) in water. Rate constants of active pharmaceutical ingredient solubility differ approximately by ~1.5 times for the two types of water with different deuterium/protium ratios, natural deionized high-ohmic water (D/H=140 ppm) and deuterium-depleted water (D/H= 4 ppm). Results are to be utilized in order to control the quality of pharmaceutical substances with the purpose of dissolution quantitative evaluation, as well as with the aim of tackling the issue of new active pharmaceutical ingredients' low solubility while developing drugs.
Keywords
Active pharmaceutical ingredient, laser diffraction, low-angle laser light scattering, deuteriumdepleted water, kinetic isotope effect
Solubility is the key quality parameter reflecting not only physical and chemical properties of active pharmaceutical ingredients (APIs), but also stability of their manufacturing process. Solubility defines API purity and is the primary analysis while controlling the quality of ingredients and pharmaceutical products based on them [1]. Solubility is influenced by hydrophilicity or hydrophobicity of particles as well as polymorphism of API crystals. In the presence of various crystal forms of APIs, the solubility test can be selected as a method for determination of polymorphism of pharmaceutical substance [2,3]. The recovery rate of API is a matter of numerous researches. However, solubility preceding the recovery of API is one of the key factors, which influence drug efficiency and safety [1]. For this reason, the target-specific development of new drugs with predetermined features (drug design) must be based on the solubility of candidate substances data as well [4]. In vitro determination of API solubility is of importance in conducting preliminary trials during the development of an Investigational New Drug (IND) or during a New Drug Application (NDA) process. Therefore, the proposed new methodology for studying the solubility kinetics of APIs using high-tech equipment on the basis of low-angle laser light scattering (LALLS) may be of interest from the point of view of conducting "pilot studies" before a complete study of the bioequivalence. New drug delivery systems are developed with an aim to resolve solubility problems, prevent external environmental issues on the drug such as photo degradation and pH changes, make the drug more lipid soluble so that it can easily cross lipid barriers in the body and achieve expected concentration at the desired location for maximum therapeutic effect. Solubility, dissolution, and permeability of APIs in the gastrointestinal tract are the key features, which regulate the speed, absorption of drugs and as a result, their bioactivity [5]. On these grounds, the examined physical and chemical properties of APIs are of primary importance while assessing bioequivalence of generic to the original drugs and identifying in vitroin vivo correlation as well [6]. However, currently the assessment of API solubility is carried out by means of visual inspection in the pharmaceutical analysis, the terms are descriptive and volume is approximate (Table 1) [7].
| Descriptive term | Approximate volume of solvent in ml per g of solute |
|---|---|
| Very soluble | <1 |
| Freely soluble | 1 to 10 |
| Soluble | 10 to 30 |
| Sparingly soluble | 30 to 100 |
| Slightly soluble | 100 to 1000 |
| Very slightly soluble | 1000 to 10000 |
| Practically insoluble | >10000 |
Descriptive term, approximate volume of solvent in millilitres per gram of solute
Table 1: Solubility criteria
Solubility is a heterogeneous process dependent not only on the properties of the solid, but also on the character of the solvent. It is understood that water represents a blend of molecules having heterogeneous isotropic weight including water (H2O), hydrogen-deuterium oxide and heavy water (D2O). Waters having different ratios of D/H possess dissimilar physical, chemical and spectral features [8]. The thermodynamic heterogeneity of water isotopologues results in differences in the kinetics of chemical as well as biochemical reactions due to the kinetic isotope effect [9]. As it has been proved, deuterium-depleted water displays antidotal features under the individual and combined influence of pharmaceutical substances as well as excipients, it decreases the hazard of γ-ray radiation [10,11].
In order to examine the kinetics of the solubility of APIs in waters having a varied quantity of isotopes of hydrogen 12H(D), the present paper utilizes the method of LALLS originally introduced into the United States Pharmacopeia in the year of 2000 [12]. The given method is aimed at controlling the quality of APIs and finished pharmaceutical products in terms of ''particle size distribution''. The research method of the kinetics of the solubility of APIs based on the laser obscuration analysis in the course of time by means of LALLS-method was developed by the Department of Pharmaceutical and Toxicological Chemistry, representatives of Peoples' Friendship, University of Russia [13]. The utilization of this method aimed at determining API size as a research tool for ingredients solubility examination has considerable benefits in comparison with the pharmacopoeial solubility test. In the first instance, this method gives an opportunity to assess objectively API solubility on the ground of changes in integral characteristics of dispersity in the course of time; aside from this, it allows the execution of solubility quantitative assessment, i.e. the calculation of the rate constant k (с-1).
The purpose of this research paper is to study the solubility kinetics of APIs of various pharmaceutical and chemical groups by means of laser diffraction in waters having a different quantitative content of isotope of hydrogen 12H(D).
Materials and Methods
Bendazole hydrochloride procured from Second Pharma Co., Ltd., China series Р111106, served as a model for examining the solubility of APIs in water with different quantitative content of isotopes of hydrogen 12H(D). Lactose monohydrate manufactured by DMV-Fonterra Exipients GmbH & Co. KG, Nörten- Hardenberg, Germany was utilized as an excipient and an additive agent in pharmaceutical formulations, in particular, while manufacturing pharmaceutical forms of high and ultrahigh dilution (Table 2). This is explained by its high stability, low hygroscopicity as well as avaliability [14,15].
 |
 |
| 2-benzyl -1Н- benzimidazole hydrochloride | О-β-D-galactopyranosyl-(1→4)-α-D-glucopyranose monohydrate |
| White or white with a slightly grayish or yellowish shade crystalline powder | White or almost white crystalline powder |
| Sparingly soluble in water[14] | Freely but slowly soluble in water[7] |
Table 2: Structure and some physical and chemical features of active pharmaceutical ingredients
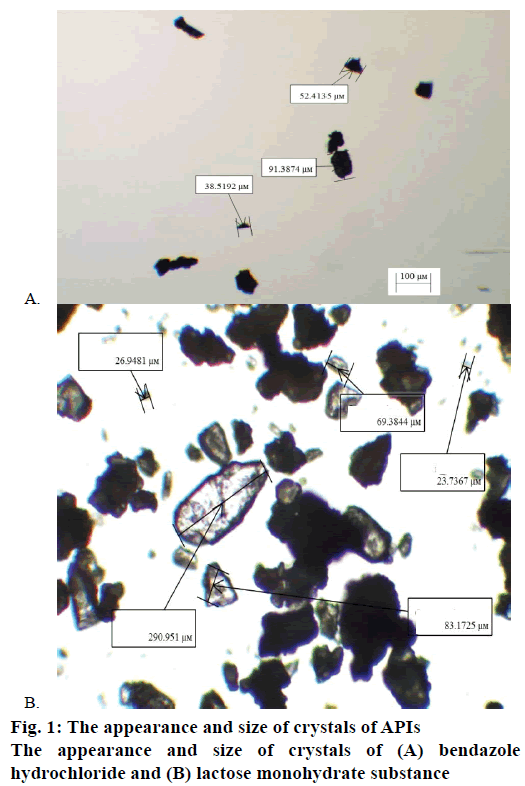
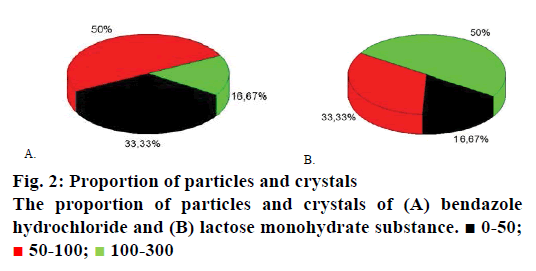
The sizes of particles of the examined substances were controlled by means of light-microscopical method utilizing the microscope type Altami BIO 2 with objective magnification per 4× (Figure 1A and B). The size of lactose monohydrate particles ranges from 20 to 300 μm; particles sized less than 50 μm comprise around 17 %. The particle size of bendazole hydrochloride does not exceed 250 μm, having more particles sized from 50-100 μm. The proportion of API particles crystals is presented in Figure 2A and B.
Water for study
Deionized high-ohmic water (specific electrical conduction no more than 5 μS/cm at 25°) received by means of the purification of pyretogenous-distilled water employing the system Milli-Q (Millipore U.K.). Deuterium-depleted water (manufactured by Light Water), obtained by means of liquid hydrogen low temperature vacuum rectification [16]. According to the data of mass spectroscopy the protium water contained 4 ppm (D/H) of deuterium in comparison with 140±0.9 ррm for the deionised high-ohmic water.
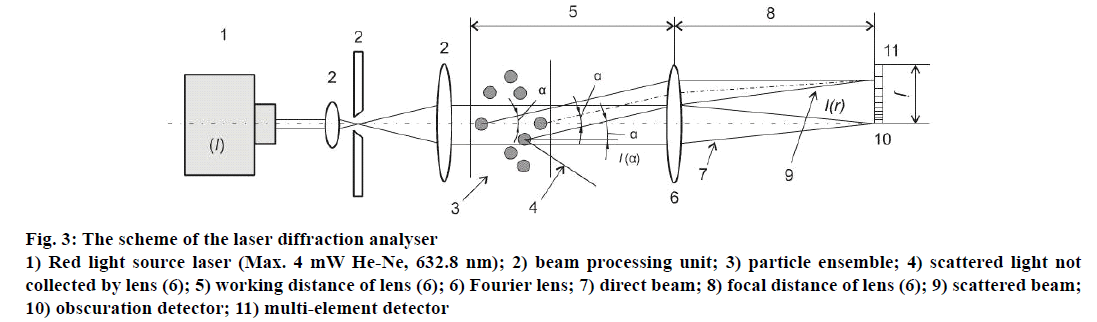
ParticleSizer (Malvern 3600 Ec) was utilized in order to define the size of particles within the range of 1 to 180 μm. The scheme of the laser diffraction analyser is illustrated in Figure 3. A capacity cell manufactured of quartz glass was used; the cell capacity was 3.5 ml, the necessary quantity for the measurement of the sample material was 3 ml. The capacity cell equipped with the actuator helped to keep the particles in suspension in the process of their measurement and prevented from their agglomeration; the speed of the actuator was regulated manually.
Figure 3: The scheme of the laser diffraction analyser
1) Red light source laser (Max. 4 mW He-Ne, 632.8 nm); 2) beam processing unit; 3) particle ensemble; 4) scattered light not
collected by lens (6); 5) working distance of lens (6); 6) Fourier lens; 7) direct beam; 8) focal distance of lens (6); 9) scattered beam;
10) obscuration detector; 11) multi-element detector
Laser diffraction spectroscopy is based on the analysis of the intensity of angular dispersion of the monochromatic electromagnetic plane wave on particles of aerosol or suspended material. The scattering indicatrix changes due to the relationship of πd/λ, where λ-electromagnetic wavelength, d-size of the particle. In order to define the presence of the particle in the examined disperse system, one employs the parameter of laser obscuration characterizing the loss of light intensity while introducing the particle into the measurement cell as a result of such processes as reflection, absorption and diffraction. Mathematically, laser obscuration may be presented by the following formula, obscuration (%) = (1-I/Io)×100, where, I is the light intensity measured by the detector while the particle is inside the measurement cell, Io is the light intensity measured by the detector without the particle in the measurement cell.
Research method
Weighed portion of ingredients for the solubility studies were selected on the basis of the data on their pharmacopoeial solubility (Table 1). The exact weighed portions of ingredients taken for the examination was 0.2 g of lactose monohydrate and 0.03 g of bendazole hydrochloride (corresponds to solubility 1 g in 100 ml) in the bulk solution 3 ml. The weighed portion of ingredients was placed to a capacitive cell with a magnetic stirrer adding 3 ml of solvent measured by means of an autopipette with a capacity of 500-5000 μl. The measurement of laser obscuration was started while adding water to the cell and continued with 20 s intervals up to complete solubility of the substance, which was recorded up to the end of change in time of the examined parameter. Repeated measurements were held with the analysis of three representative samples of one series. The examination of the base solution darkening, presented by the solubility medium (Mili-Q water or deuterium-depleted water), was examined preliminary. The examination was carried out at 21±1°. The water samples were used out of newly opened phials and were not stored for more than 24 h.
Statistical analysis
All the results were expressed as the mean±standard deviation (SD). In order to determine the kinetics of pharmaceutical substance solubility each sample was measured three times. Statistical analysis was performed by Student’s t-test. All analyses were conducted at the 95 % confident level, p<0.05 was assumed as the statistically significant difference between the experimental points.
Results and Discussion
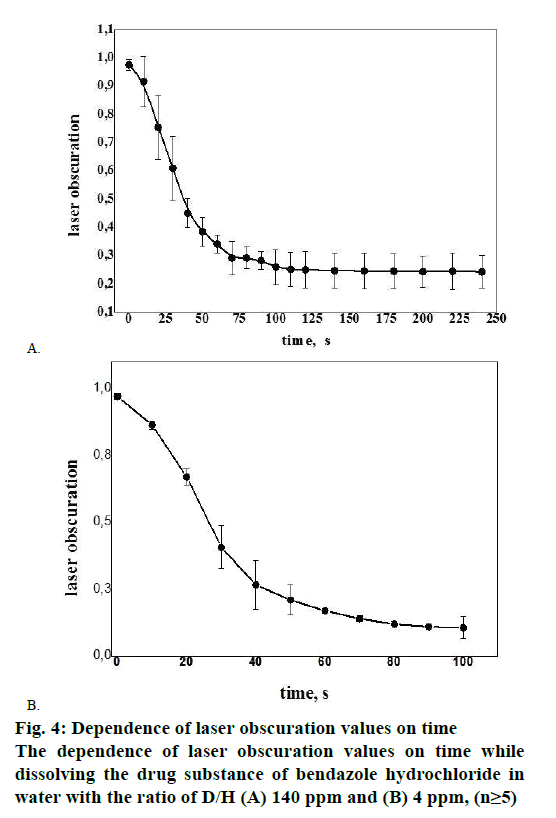
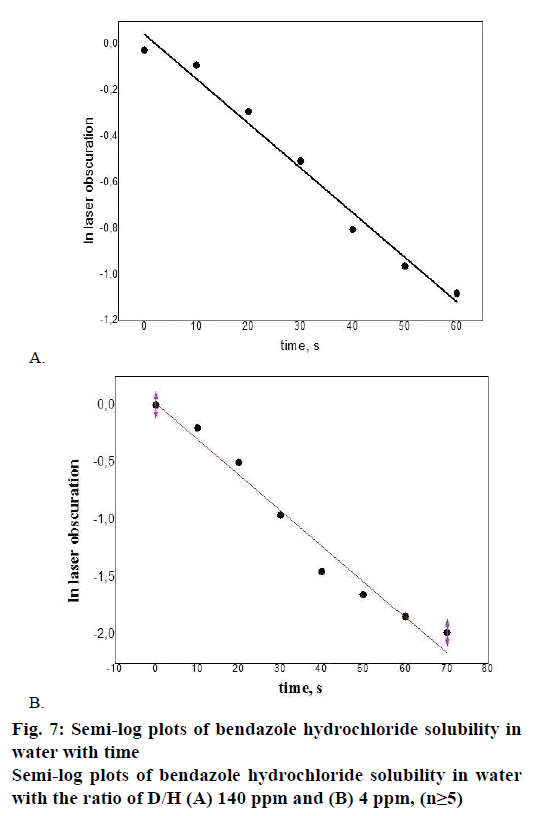
While examining the solubility of bendazole hydrochloride series Р111106, exponential relationship was revealed between the value of laser obscuration and the period of time in water environment having the ratio D/H corresponding 140 ppm and 4 ppm (Figure 4A and B). Figure 4A and B illustrate the fact that bendazole hydrochloride solubility kinetics in water represents a two-stage process: a sharp change in the tested parameter of bendazole hydrochloride substance solubility in water with the ratio of D/H 140 ppm (Figure 4A) during first 80 s of the disintegration process changes from a gradual decrease in laser obscuration value to a plateau in 90-100 s, which was recorded as complete solubility of the substance.
The heterolytic process of bendazole hydrochloride particles solubility in water is accompanied by the formation of an activated complex, which could be presented as Figure 5. This scheme allows to consider the solubility of API crystals in water from the position of pseudo-first-order kinetics since the change in the concentration of water molecules in terms of water excess can be omitted. In the course of the heterolytic processes solvation layers undergo a considerable transformational change of particles initial condition of the dispersoid and the coat shell of the activated complex, consequently one can analyse the influence of the type of the solvent on the speed of solubility (Figure 4B). It is noticeable that the laser obscuration value decreases tenfold in the process of bendazole hydrochloride crystals solubility in deuterium-depleted water, whereas the reduction of the tested parameter accounts for a fourfold decrease in water having the ratio of D/Н= 140 ppm (Figure 4A). The sharpest change in the examined parameter of solubility in water having the ratio of D/Н= 4 ppm occurs during first 40 s, and reaches a plateau in 80 s. This may be indicative of more complete solubility of the substance in deuteriumdepleted water.
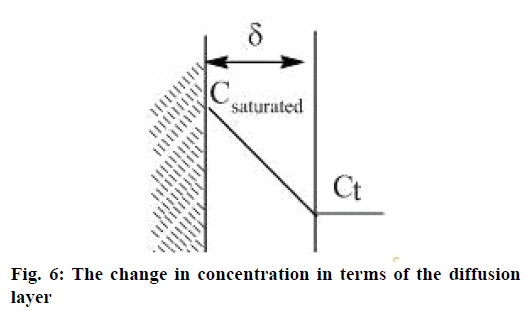
The obtained results reveal that the speed of the particles solubility of the disperse phase depends on the rate constant of the fastest stage of API solubility (Figure 4A). The heterolytic process of API solubility by diffusion, adsorption and desorption in accordance with Fick's law. In addition to Fick's diffusion theory revealed the solubility Eqn. [17]: dc/dt = kS×(Csaturated−Сt), where, dc/dt is the speed of the solution process; k is the rate constant of solubility depending on the temperature and type of substance; Csaturated is the concentration of the saturated solution; Сt is the concentration of the solution at a given period of time; S is the surface of the solid substance.
The two-stage process of API solubility becomes clearer: the speed of solubility at the beginning of the process when the remainder of values (Csaturated−Ct) is maximal in terms of the diffusion layer, and then it reaches gradually a plateau (Figure 6). Since k= D/δ, where D is diffusivity, δ is the width of the diffusion layer, solution mixing stimulates the increase of diffusivity and consequently the speed of solubility.
In order to make a quantitative assessment of API solubility in water having different ratios of D/Н, the results of the exponential curves (Figure 4A) were presented on the axes x = ln laser obscuration, y = t (Figure 7A and B; Table 3). The received Eqn., y= a+bx. The specific reaction rate was calculated by coefficient b of the straight-line equation as the slope of the x-axis: k = –tgα and tgα = −d(laser obscuration)/dt. The initial reaction rate is defined most precisely due to the fact that a kinetic curve is normally close to a straight line.
| Series number Р111106 | (k±Sr), s-1 | |
| Water having the ratio of D/Н=140 ppm | Water having the ratio of D/Н= 4 ppm | |
| (1.94±0.001)×10-2 | (3.10±0.002)×10-2 | |
Series number Р111106 and (k±Sr)×s-1. (n= 7)
Table 3: Solubility rate constants of bendazole hydrochloride in water having different contents of hydrogen isotopes
Thus, significant differences have been revealed in the substance bendazole hydrochloride solubility rate in deuterium-depleted water (D/H= 4 ppm) compared with Mili-Q water of natural isotopic composition (D/H= 140 ppm)- the rate has increased by 1.6 times. The obtained result provides evidence of a considerable role of variations in the isotopic composition of the solvent-water in accelerating the process of API dissolution.
The results of lactose monohydrate study showed that the process of API solubility in deuterium-depleted water proceeds with a larger speed rather than in the water containing deuterium D/H= 140 ppm. Thus, the process of lactose monohydrate solubility in Mili-Q water takes approximately 260 s, whereas the solubility of a similar sample in deuterium-depleted water occupies around 180 s. Statistically significant differences in the solubility values of the rate constants of lactose monohydrate in water having different ratios of D/Н have been revealed for intact lactose and lactose after water saturation in aerosol camera (Table 4) [18].
The statistical analysis of the results the calculated average value of the relative error (>10 %) in the process of the solubility rate constant measurement by means of laser obscuration is indicative of the absence of serious mistakes. The examined change in the rate constants of API solubility as a result of the isotopic replacement of deuterium for a deuterium-depleted water molecule is indicative of the realization of the kinetic isotope effect by the solvent. The quantitative measure of the kinetic isotope effect is the relation of the kinetic constant of API solubility in deuteriumdepleted water to the kinetic constant of dissolution in water containing heavy isotope (Table 4).
| Sample | Water having the ratio of D/Н= 140 ppm | Water having the ratio of D/Н= 4 ppm | kH/kD | ||
|---|---|---|---|---|---|
| (k±Sr), s-1 | ε, % | (k±Sr), s-1 | ε, % | ||
| Intact lactose | (9.7±0.35)×10-3 | 3.67 | (12.6±0.51)×10-3 | 4.07 | 1.3 |
| Lactose after water saturation in aerosol camera[18] | (7.9±0.2)×10-3 | 9.67 | (13.4±0.42)×10-3 | 3.17 | 1.7 |
Samples: Intact lactose and Lactose after water saturation in aerosol camera ratio D/H and rates of solubility contstans (k±Sr)×s-1, kH/kD − kinetic isotope effect, ε, % - relative error. (n= 11)
Table 4: The rate constants for the solubility of lactose monohydrate in water having different ratios of D/H
Thus, the research paper for the first time has made the assessment of pharmaceutical ingredients utilizing physical and chemical methods of analysis allowing to characterize this property of APIs not from the perspective of conventional terms but with the help of reliable numerical results. The analysis of the received rate constants of API solubility has shown that despite the difference in the conventional terms of bendazole hydrochloride solubility (slightly soluble in water) and lactose monohydrate (freely soluble in water), the rate constant of bendazole hydrochloride solubility exceeds the rate constant of lactose monohydrate solubility. Presumably this is connected with the peculiarities of the phase transition in bendazole hydrochloride solution with the appearance of more composition gradients (Csaturated−Ct) at the surface of the phase interface, and also the influence of the ionic strength of bendazole hydrochloride solution and the dispersity of an API (Figure 1).
For the first time the quantitative measure of the kinetic isotope effect connected with the isotopic nature of the solvent-water has been assessed. It is shown that irrespective of pharmacological and chemical properties of APIs, the rate of solubility in deuterium depleted water exceeds the speed of solubility in Mili-Q water by approximately 1.5 times. This feature will allow to increase the pharmacological availability of slightly soluble in water APIs ensuring a better release out of the pharmaceutical form by means of speed acceleration of pharmaceutical substances solubility.
Taking various samples of lactose as an example, it is demonstrated that it is possible to hold a withinrun manufacturing quality control of pharmaceutical substances on the ground of their water solubility analysis by means of laser obscuration. On retention of the stability of the manufacturing procedure of API production, no significant differences in the obtained kinetic constants of their dissolution will be observed.
The designed technique of the kinetic assessment of drug substances solubility by LALLS-method allows to compare the constants of APIs solubility in solutions with different isotopic composition. The ability to obtain quantity characteristics of solubility makes this technique suitable for the quality control and standardization of other pharmaceutical ingredients and allows to utilize it in addition to the already existing solubility pharmacopeia test. That's why solubility of API in waters with different D/H-relation is currently under investigation to improve the therapeutic efficacy and potency of the drug. Note, by varying the 2H/1H ratio, we can manage the pharmacokinetics of drugs in a fairly simple way.
Acknowledgements
The authors thank the company (Light Water) which produces deuterium-depleted water 4 ppm as an essential reagent for this research work.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Project 5-100.
References
- Sun J, Wang F, Sui Y, She Z, Zhai W, Wang C, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine 2012;7:5733-44.
- European Pharmacopoeia. 8th ed. Nördlingen: Druckerei C. H. Beck; 2013. p. 685.
- Griesser U, Burger A, Mereiter K. The polymorphic drug substances of the European Pharmacopoeia. Part 9. Physicochemical properties and crystal structure of acetazolamide crystal forms. J Pharm Sci 1997;86:352-8.
- Bhusal P, Harrison J, Sharma M, Jones DS, Hill AG, Svirskis D. Controlled release drug delivery systems to improve post-operative pharmacotherapy. Drug Deliv Transl Res 2016;6:441-51.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Paradkar A, Bakliwal S. Biopharmaceutics & Pharmacokinetics. 3rd ed. Pune: Nirali Prakashan; 2008.
- European Pharmacopoeia. 8th ed., Vol.1. Nördlingen: Druckerei C. H. Beck; 2013. p. 2333.
- Goncharuk VV, Lapshin VB, Burdeinaya TN, Pleteneva TV, Chernopyatko AS, Atamanenko ID, et al. Physicochemical properties and biological activity of the water depleted of heavy isotopes. J Water Chem Technol 2011;33:8-13.
- Levitskaya O, Syroeshkin A, Pleteneva T. Arrhenius kinetics as a bioactivity assessment criterion for drug substances and excipients. Pharm Chem J 2016;49:779-8.
- Kulikova E, Kriuchkova D, Severiukhin I, Gaevskiĭ VN, Ivanov AA. Radiomodifying properties of deuterium-depleted water with poor content of heavier isotopes of oxygen. Aviakosm Ekolog Med 2012;46:45-50.
- Avila DS, Somlyai G, Somlyai I, Aschner M. Anti-aging effects of deuterium depletion on Mn-induced toxicity in a C. elegans model. Toxicol Lett 2012;211:319-24.
- United States Pharmacopoeia/National Formulary. 24th ed. Rockville, Maryland: The United States Pharmacopeial Convention; 2013. p. 2149.
- Borisova U, Uvarova N. Kinetics study of solubility pharmaceutical substances by laser diffraction method // VI International Conference, Science Health 2015. San Francisco: Materials of Conference; 2015. p. 32-33.
- https://pubchem.ncbi.nlm.nih.gov/compound/Bendazol#section=Top.
- Hwang R, Peck G. A systematic evaluation of the compression and tablet characteristics of various types of lactose and dibasic calcium phosphate. Pharm Tech 2001;25:54-68.
- Alekseev I, Arkhipov E, Bondarenko S, Fedorchenko O, Ganzha V, Kravstov P, et al. Experimental results of hydrogen distillation at the deuterium removal unit of the MuCAP experiment. St. Petersburg Institute of Nuclear Physics, Russian Academy of Sciences, 2006.
- Larsson J. Method for measurement of solubility and dissolution rate of sparingly soluble drugs [dissertation]. Sweden: Department of Chemical Engineering, Lund University; 2009; p. 26.
- Tarasov SA, Zarubaev VV, Gorbunov EA, Sergeeva SA, Epstein OI. Activity of ultra-low doses of antibodies to gamma-interferon against lethal influenza A (H1N1) virus infection in mice. Antiviral Res 2012;93:219-24.
 0-50;
0-50;  50-100;
50-100;  100-300
100-300