- *Corresponding Author:
- S. E. Alayasin
Medical Biotechnology Division, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
E-mail: Seyedahmadaleyasin@gmail.com
| Date of Submission | 24 March 2018 |
| Date of Revision | 19 October 2018 |
| Date of Acceptance | 07 March 2019 |
| Indian J Pharm Sci 2019;81(3):527-532 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this investigation, the role of autophagy and apoptosis in Solanum nigrum fruit extract-induced cytotoxicity in MDA-MB-468, a triple negative breast cancer cell line was studied. Breast cancer cells were treated with varying concentrations (1 to 5 mg/ml) of Solanum nigrum extract for 24 and 48 h. Autophagy was detected using acridine orange staining and real-time PCR for Lc3 and Beclin1 genes expression. In addition, cell apoptosis was measured by double staining, DNA fragmentation and annexin V-fluorescein isothiocyanate binding assays. The expression levels of apoptosis-related genes Bcl2 and Bax were determined by real-time PCR. Different concentrations of Solanum nigrum extract showed cytotoxic effect on MDA-MB-468 cells. The expression levels of autophagy-related genes, Lc3 and Beclin1 increased in cells treated with 1.5 mg/ml of Solanum nigrum extract. Upregulation of Bax and downregulation of Bcl2 was detected after treatment with 5 mg/ml of Solanum nigrum extract. These findings indicated that Solanum nigrum extract induced cell death in MDA-MB-468 cells by two distinct mechanisms, apoptosis and autophagy, and these findings further suggest a possibility that this extract could be used to treat triple negative breast cancer.
Keywords
Apoptosis, autophagy, breast cancer, Solanum nigrum
Breast cancer is the most common cancer in women worldwide [1]. Triple negative breast cancer (TNBC) is an aggressive form of tumor. This represents a cancer that does not respond to most available treatments and usually escapes adjuvant therapy. In patients with TNBC the therapy included endocrine therapy while HER-2 targeted agents are ineffective. Hence, only a few treatment options are available for treatment [2]. Recently, increased attention is on antitumor herbs for cancer cure with fewer side effects compared to chemical agents. Solanum nigrum (SN) is a herb with anticancer effects such as destruction of tumor cell membrane, induction of apoptosis by NF-kappaB, caspase activation, nitric oxide production and inhibition of angiogenesis [3]. In the Indian traditional medicine, SN is used as hepatoprotective agent, and the fruit of SN is used as a nerve tonic in the Mexican medicine. The extract of SN contains carbohydrates, hydrolysable tannins, flavonoids, saponins, sterols and alkaloids [4]. Moreover, it has been used in traditional oriental medicines for treating various kinds of tumors and is believed to have various biological activities [5]. For example, SN has been used to cure hepatic cancer for a long time in oriental medicine [6]. A previous study reported that the extract of the whole plant of SN activated cell death in hepatoma cells through autophagy and apoptosis [7].
One of the hallmarks of breast cancer is resistance of tumor cells to cell death. Therefore, remedies based on the induction of programmed cell death attracted greater attention for cancer treatment [8]. Autophagy and apoptosis are two forms of programmed cell death, which are essential for cellular homeostasis. Autophagy, the type II cell death, is a physiological mechanism involving degradation of intracellular damaged proteins and organelles into membrane vacuoles. It is thought to be involved in many physiological and pathophysiological processes, including antiaging mechanisms, differentiation and development, immunity, and elimination of microorganisms [9-13]. Induction of apoptosis leads to characteristic cellular changes leading to death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation and DNA fragmentation [14]. Normally, autophagy and apoptosis have a tumorsuppressor role. Autophagy performs this role via degradation of oncogenic molecules thus preventing development of cancers, while apoptosis prevents the survival of cancer cells. Consequently, defective autophagy or apoptosis results in uncontrolled cell proliferation, such as cancer. Complete understanding of these defective activities are important for developing new anticancer agents [15-20].
In the present investigation the ability of the methanol extract of SN to induce programmed cell death in MDAMB- 468, a TNBC cell line was studied. Mechanism of cell death was evaluated, which indicated that the SN extract (SNE) not only initiated apoptosis but also caused cell death through autophagocytosis. The results obtained provided evidence that suggested that this herbal medicine could be of use in TNBC therapy.
Shade-dried SN fruits were powdered (100 g), mixed with 1 l of 70 % methanol in a screw-capped flask and shaken at room temperature for 3 d to obtain methanol extract. The methanol extract was subsequently filtered through Whatman No. 3 filter paper and centrifuged at 10 000 g for 20 min. The supernatant was concentrated in a rotary evaporator (B465, Switzerland) and lyophilized to obtain 2.78 g of SNE.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to determine viability of MDA-MB-468 after exposure to 0.1, 1.5, 2.5, 3.5 and 5 mg/ml of SNE [21]. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS; Bioidea, USA) and antibiotics. For MTT assay, 5×103 cells were seeded in a 96-well plate and were allowed to attach overnight at 37°. Plate was treated with 0.1, 1.5, 2.5, 3.5 and 5 mg/ml of SNE that left for 24 and 48 h. Then 20 μl of MTT solution was added to growth medium of plate. The supernatants were carefully aspirated after 4-6 h and 100 μl dimethyl sulfoxide was added for dissolving formazan crystals. A microplate reader (Bio-Rad, USA) was used to determine the absorbance values at 580 nm.
Acridine orange (AO) is a cell-permeable dye used for monitoring autophagy by labelling acidic vesicular organelles. MDA-MB-468 cells treated with 0.1 and 1.5 mg/ml of SNE for 48 h and untreated cells were stained with 1 μg/ml of AO for 15 min and fluorescent micrographs were taken using an inverted fluorescence microscope.
AO/ethidium bromide (EB) double staining was performed as previously described [22]. MDA-MB-468 cells were treated with 5 mg/ml of SNE for 48 h and were stained with 4 μg/ml AO and 4 μg/ml EB for 15 min along with untreated cells. The stained cells were examined under the fluorescence microscope in both red and green channels. Viable cells have uniform bright green nuclei with normal structure, whereas early apoptotic cells show green condensed nuclei. Late apoptotic cells exhibit orange to red nuclei with condensed chromatin, whereas necrotic cells have orange to red nuclei with normal chromatin levels.
Apoptosis in MDA-MB-468 cells was detected using DNA fragmentation assay. This assay involved extraction of DNA from a lysed cell homogenate followed by agarose gel electrophoresis. MDAMB- 468 cells were cultured in a 6-well plate in DMEM supplemented with 10 % FBS and antibiotic, placed at 37° and 5 % CO2 in an incubator. After 6 h, the cells were treated with 0, 3 and 5 mg/ml of SNE for 48 h. Wells were washed with PBS buffer and cells were detached from the plates with trypsin-EDTA (Gibco, Germany). The cell pellets were placed in Falcon tubes and 500 μl of lysis buffer was added. About 10 μl of proteinase K (Fermentas, Life Sciences) 20 mg/ml were added, followed by incubation at 56° overnight. The next day, 40 μl of 5 M NaCl was added, mixed completely and incubated at 4° for 10 min. After centrifugation at 12 000 rpm for 20 min, their upper layer was transferred to a fresh microtube and 1 ml of cold ethanol 100 % (stored in –20°) was added. The procedure was continued by incubation at –20° for 10 min, followed by centrifugation for 15 min at 12 000 rpm. The upper ethanol phase was removed completely and 1 ml of 70 % ethanol (kept at 4°) was added and mixed well. Next, the samples were centrifuged again for 10 min at 12 000 rpm, followed by removing ethanol completely. After drying the samples at room temperature, the pellets were dissolved in 100 μl distilled, deionized, sterile water. The DNA samples diluted with the 6X DNA loading dye (supplied with the ladder) were subjected to 1.5 % agarose submarine electrophoresis in company with DNA ladder marker (Fermentas, Life Sciences, 1 kb DNA Ladder). Finally, the fragmented DNAs bands were visualized by UV transilluminator following EB staining.
In addition, apoptosis in MDA-MB-468 cells was detected using annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Biolegend, San Diego, CA) following the manufacturer’s instructions. Briefly, cells were placed in 6-well culture dishes (4×105cells/well) for 24 h prior to the addition of SNE (5 mg/ml). Following 48 h incubation with SNE, the percent apoptotic cells was determined by the annexin V-FITC/PI assay. The cells were harvested, washed with cold phosphatebuffered saline and resuspended in binding buffer. The cells were treated with annexin V-FITC conjugate and incubated for 15 min at room temperature in dark. The cells were then stained with PI (5 μg/ml) and analysed by flow cytometry (Accuri C6 Flow Cytometer, Ann Arbor, MI) within 1 h following the staining. The data acquisition and analysis were performed using BD Accuri C6 Flow software and a minimum of 10 000 cells per sample was analysed.
Expression levels of two autophagy-related genes Lc3 and Beclin1 were determined in MDA-MB-468 cells treated with 0.1 or 1.5 mg/ml of SNE and incubated for 24 or 48 h. After harvesting, total RNA was extracted according to the manufacturer’s instruction (Qiagen, USA). The purity and concentration of the isolated RNA was checked by a NanoDrop instrument (Thermo Scientific, USA). In the next step, cDNA was synthesized (Fermentas, Germany). Real-time PCR for Lc3, Beclin1 and β-actin genes were carried out using designed specific primer sequences of Lc3-F, 5′ AAACGCATTTGCCATCAC 3′ Lc3-R, 5′ GACCTTCAGCAGTTTACAG 3′, Beclin1-F, 5′ ATGCAGGTGAGCTTCGTGTG 3′ Beclin1-R, 5′ CTGGGCTGTGGTAAGTAATGGA 3′, β-actin-F, 5′ AGACGCAGGATGGCATGGG 3′ β-actin-R, 5′ GAGACCTTCAACACCCCAGCC 3′. For Real-time PCR, a SYBR Green PCR kit was used (Takara, Japan). PCR was carried out in a volume 10 μl containing 5 μl of SYBR Premix, 1 μl of cDNA, 0.5 μl of forward and reverse primers and 3 μl of double-distilled water. Quantitative Real-time PCR assay was performed using a Rotor Gene 6000 thermal cycler (Corbett Life Science, Australia) according to the following conditions: 95° for 5 min, and 45 cycles (15 s at 95°, 1 min at 60° for Lc3, 62° for Beclin1 and 63° for β-actin). The data were analysed with a normalized gene expression method (ΔΔCt). ΔCt values were determined as the difference between the Ct of target and the Ct of reference gene (β-actin gene). The β-actin gene was used as a reference for normalization.
Expression levels of two apoptosis-related genes Bcl2 and Bax were determined in MDA-MB-468 cell line treated with 3 and 5 mg/ml SNE in different incubation periods 24 and 48 h. Total RNA was extracted from these cells using RNA extraction kit (Qiagen, USA). In the next step, RNA was converted to cDNA (Fermentas, Germany) and stored at –20°. Real-time PCR was carried out using the primers: Bcl2-F, 5′ TGTGGATGACTGAGTACCTGAACC 3′ Bcl2-R, 5′ CAGCCAGGAGAAATCAAACAGAG 3′, Bax-F 5′ TTGCTTCAGGGTTTCATCCAG 3′ Bax-R 5′ AGCTTCTTGGTGGACGCATC 3′. Real-time PCR was performed according to the procedure described in the above section. Quantitative real-time PCR assay was performed as described but with the following conditions: 95° for 5 min, and 45 cycles (20 s at 95°, 1 min at 63° for Bcl2 and 62° for Bax).
The results were considered statistically significant at p<0.05 and this was assessed using Student’s t-test between treatment and untreated groups. The experiments were performed in triplicate and data is presented as the mean±SD from three independent experiments.
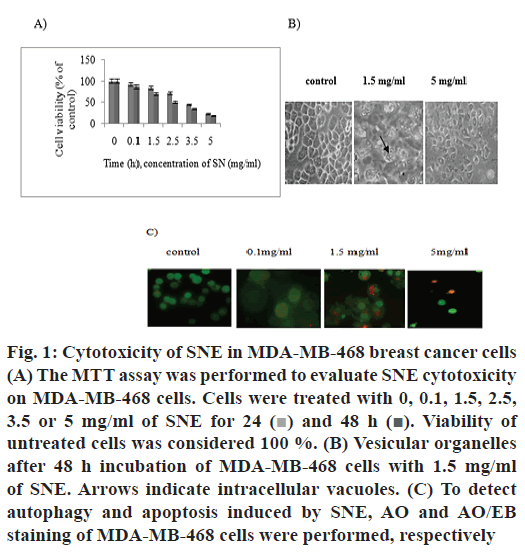
Different concentrations of SNE showed cytotoxic effects on MDA-MB-468 cancer cell line. The percent surviving cells after incubating for 48 h with 0.1, 1.5, 2.5, 3.5 and 5 mg/ml of SNE were 87, 70, 50, 35 and 18, respectively (fig. 1A). Therefore, cell viability decreased with increasing concentrations of SNE after 48 h. The IC50 concentration of SNE on MDA-MB- 468 cells was found to be 2.3 mg/ml. The MTT assay indicated that treatment with SNE reduced viability of MDA-MB-468 cell line in a dose and time-dependent manner.
Figure 1: Cytotoxicity of SNE in MDA-MB-468 breast cancer cells
(A) The MTT assay was performed to evaluate SNE cytotoxicity
on MDA-MB-468 cells. Cells were treated with 0, 0.1, 1.5, 2.5,
3.5 or 5 mg/ml of SNE for 24  and 48 h
and 48 h  Viability of
untreated cells was considered 100 %. (B) Vesicular organelles
after 48 h incubation of MDA-MB-468 cells with 1.5 mg/ml
of SNE. Arrows indicate intracellular vacuoles. (C) To detect
autophagy and apoptosis induced by SNE, AO and AO/EB
staining of MDA-MB-468 cells were performed, respectively
Viability of
untreated cells was considered 100 %. (B) Vesicular organelles
after 48 h incubation of MDA-MB-468 cells with 1.5 mg/ml
of SNE. Arrows indicate intracellular vacuoles. (C) To detect
autophagy and apoptosis induced by SNE, AO and AO/EB
staining of MDA-MB-468 cells were performed, respectively
MDA-MB-468 cells treated with 1.5 mg/ml of SNE exhibited large membranous vacuoles in the cytoplasm, which is a characteristic feature of cells undergoing autophagy (fig. 1B). Therefore, AO staining was used to confirm autophagy cell death induced by SNE. Results obtained showed cells after treatment with 1.5 mg/ml of SNE for 48 h developed cytoplasmic red vacuoles (fig. 1C). Moreover, AO/EB staining was used to detect other modes of cell death induced by SNE. As shown in fig. 1C, SNE could also induce apoptosis.
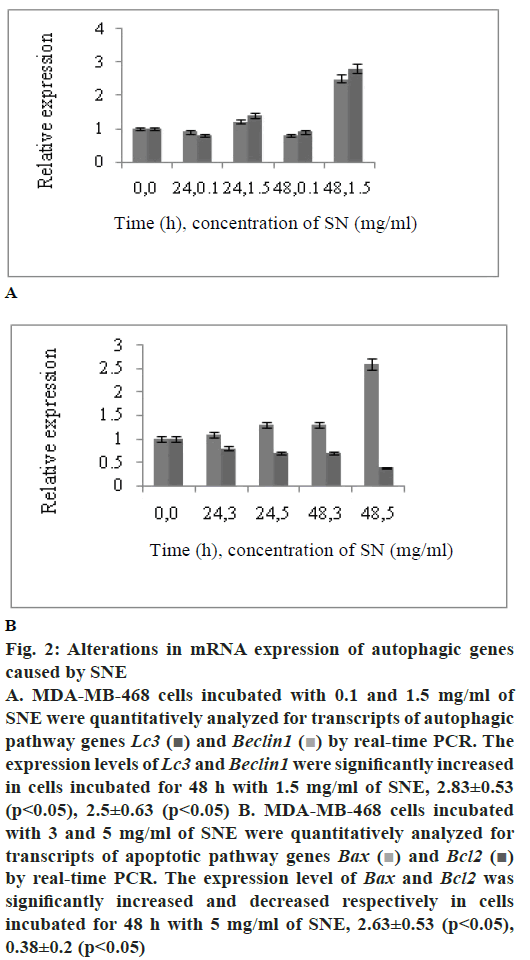
To determine whether SNE treatment reduced cell viability by activating programmed cell death in MDAMB- 468 cells, expression of two autophagy markers, Lc3 and Beclin1 and two apoptotic markers, Bax and Bcl2 were measured by real-time PCR. Fig. 2A shows that the mRNA levels of Lc3 and Beclin1 increased in MDA-MB-468 cell line treated with 1.5 mg/ml of SNE; these levels were 2.83±0.53 and 2.5±0.63 times higher than those in the untreated cells, respectively (p<0.05). Results shown in fig. 2B indicated upregulation of Bax relative to β-actin in MDA-MB-468 cells. A 2.6 fold increase in Bax transcript level was found in cells treated with 5 mg/ml of SNE compared to control cells. Bcl2 transcript level, the other apoptotic pathway genes, showed decline in SNE-treated MDA-MB- 468 cells.
Figure 2: Alterations in mRNA expression of autophagic genes
caused by SNE
A. MDA-MB-468 cells incubated with 0.1 and 1.5 mg/ml of
SNE were quantitatively analyzed for transcripts of autophagic
pathway genes Lc3 and Beclin1
and Beclin1  by real-time PCR. The
expression levels of Lc3 and Beclin1 were significantly increased
in cells incubated for 48 h with 1.5 mg/ml of SNE, 2.83±0.53
(p< 0.05), 2.5±0.63 (p< 0.05) B. MDA-MB-468 cells incubated
with 3 and 5 mg/ml of SNE were quantitatively analyzed for
transcripts of apoptotic pathway genes Bax
by real-time PCR. The
expression levels of Lc3 and Beclin1 were significantly increased
in cells incubated for 48 h with 1.5 mg/ml of SNE, 2.83±0.53
(p< 0.05), 2.5±0.63 (p< 0.05) B. MDA-MB-468 cells incubated
with 3 and 5 mg/ml of SNE were quantitatively analyzed for
transcripts of apoptotic pathway genes Bax 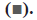 and Bcl2
and Bcl2 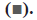 by real-time PCR. The expression level of Bax and Bcl2 was
significantly increased and decreased respectively in cells
incubated for 48 h with 5 mg/ml of SNE, 2.63±0.53 (p< 0.05),
0.38±0.2 (p< 0.05)
by real-time PCR. The expression level of Bax and Bcl2 was
significantly increased and decreased respectively in cells
incubated for 48 h with 5 mg/ml of SNE, 2.63±0.53 (p< 0.05),
0.38±0.2 (p< 0.05)
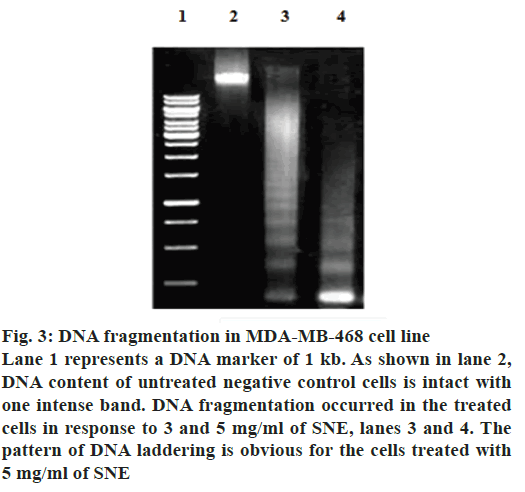
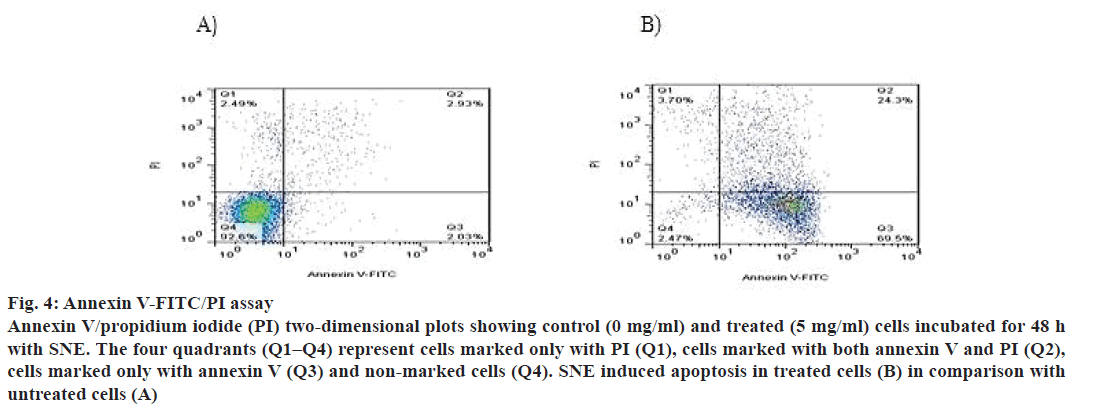
In addition, apoptosis was measured by DNA fragmentation and annexin V-FITC/PI assays. DNA fragmentation can be analysed by the typical DNA ladder formation, for which DNA is extracted from the apoptotic cells and separated on an agarose gel. As shown in fig. 3, treatment with SNE resulted in degradation of chromosomal DNA into small internucleosomal fragments, a biochemical hallmark of cells undergoing apoptosis. Fig. 4 shows that the treatment of MDA-MB-468 cells with 5 mg/ml of SNE for 48 h induced alterations in plasma membrane features regarding patterns of phosphatidylserine exposure (annexin V+ cells), and plasma membrane permeability (PI+ cells). An increase of 21.37 % in the proportion of annexin V+ and PI+ cells were observed after treatment. Furthermore, there was a 67.47 % increase in the number of cells labelled only with annexin, a 90.13 % decrease in the number of unlabelled cells and a 1.2 % increase in the number of cells marked exclusively with PI.
Figure 3: DNA fragmentation in MDA-MB-468 cell line
Lane 1 represents a DNA marker of 1 kb. As shown in lane 2,
DNA content of untreated negative control cells is intact with
one intense band. DNA fragmentation occurred in the treated
cells in response to 3 and 5 mg/ml of SNE, lanes 3 and 4. The
pattern of DNA laddering is obvious for the cells treated with
5 mg/ml of SNE
Figure 4: Annexin V-FITC/PI assay
Annexin V/propidium iodide (PI) two-dimensional plots showing control (0 mg/ml) and treated (5 mg/ml) cells incubated for 48 h
with SNE. The four quadrants (Q1–Q4) represent cells marked only with PI (Q1), cells marked with both annexin V and PI (Q2),
cells marked only with annexin V (Q3) and non-marked cells (Q4). SNE induced apoptosis in treated cells (B) in comparison with
untreated cells (A)
SNE was commonly used in traditional medicine as antiinflammatory, hepatoprotective and more recently for anticancer effects [23]. It is believed to contain anticancer polysaccharides and polyphenolic components with fewer side effects for treatment of cancers [24]. Son et al. reported that SNE induced apoptosis in MCF7, the ER-positive breast cancer cell line [5]. To date, there is no report on the capacity of SNE to induce autophagy and apoptosis in TNBC. TNBC is an aggressive tumor with a poor prognosis and limited treatment options [2]. Hence, in this study, the effect of SNE was investigated on MDA-MB-468 as a TNBC cell line.
This study showed a significant cytotoxic effect of SNE on breast cancer cells that was mediated via two mechanisms depending on the concentration of extract. The exposure of cells to a low concentration (1.5 mg/ml) of SNE resulted in autophagy. Autophagy is a physiological mechanism during which portions of cytoplasm are sequestered into double membrane vesicles, which then fuse with lysosomes to form autophagolysosomes. Lysosomal enzymes degrade the contents of the autophagolysosomes [25]. MDA-MB-468 cells treated with 1.5 mg/ml of SNE represented intracellular vacuoles consistent with the characteristics of autophagy when observed under the inverted microscope. Furthermore, SNE-treated cells were able to be stained with AO, a specific marker for autophagic vacuoles [22]. Confirmatory experiments performed with real-time PCR for genes expression analysis for Lc3 and Beclin1 showed that SNE increased the expression of LC3 and Beclin1.
A higher concentration of SN (5 mg/ml) induced apoptotic cell death in MDA-MB-468 cells as evidenced by increase in gene expression of Bax and decrease in Bcl2. The mechanisms of apoptosis involved two different pathways that include mitochondrial (intrinsic) and death receptor (extrinsic) pathway. The intrinsic pathway, implicated in the function of a majority of anticancer drugs, is regulated by the Bcl2 family proteins, including pro-apoptotic proteins, such as Bax, Bad and Bak, and antiapoptotic proteins including Bcl2, Bcl-xL and Bcl-w [26,27]. The activation of Bax and inhibition of Bcl2 resulted in release of cytochrome c through the outer mitochondrial membrane into the cytosol. Cytochrome c in cytosol interacts with apoptotic protease activating factor 1 and caspase-9 to form a complex called the apoptosome, which activated caspase-3 leading to cell death [28,29]. In addition, apoptosis was measured by DNA fragmentation. As DNA cleavage is a sign of apoptosis, the DNA laddering assay was conducted. The banding pattern between the treated and untreated cells suggested that the treated cells show DNA laddering compared to the large intact band seen with the untreated cells. Consistently, the annexin V/PI experiments performed with 5 mg/ml of SNE showed an increase in the number of cells marked positively for annexin V, revealing cells in early stage apoptosis (69.5 %). Simultaneously, there was an increase in double marked cells (annexin V+/PI+, 24.3 %), revealing the presence of cells undergoing late stage apoptosis or necrosis. These data agreed with studies of Lin et al., which showed that the water extract of the whole plant of SN induced apoptosis and autophagy in HepG2 cells. They concluded that cells treated with low concentration of SNE (1.5 mg/ml) result in autophagic cell death and cells treated with 2 and 5 mg/ml of SNE become apoptotic [7]. Moreover, another study analysed the cytotoxic effects of various concentrations of aqueous, methanol and ethanol extract of SN on leukemic cell lines (Jurkat and HL-60). It was reported that the methanol SNE was more effective than other extracts on Jurkat T cell line (IC50, 3 mg/ml) and HL-60 promyelocytic cell line (IC50, 4.8 mg/ml) [30].
In summary, the present investigation reported for the first time that SNE induced autophagic and apoptotic cell death in MDA-MB-468 cells, a TNBC cell line. The present data revealed that SNE has the potential to act as a new anticancer agent for TNBC. These results add SNE to the list of herbal medicine that possess autophagic and apoptotic effects on MDAMB- 468 cells. The present study showed that a low concentration (1.5 mg/ml) of SNE caused autophagy and a high concentration (5 mg/ml) of SNE caused apoptosis in MDA-MB-468 cells. The molecular basis for the concentration-dependent different effects of SNE needs further investigation.
Acknowledgements
This work was supported by National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
Conflicts of interest:
The authors declare that there is no conflict of interest.
Financial support and sponsorship:
Nil.
References
- Pedraza V, Gomez Capilla JA, Escaramis G, Gomez C, Torne P, Rivera MJ, et al. Gene expression signatures in breast cancer distinguish phenotype characteristics, histologic subtypes, and tumor invasiveness. Cancer 2010;116:486-96.
- Peddi PF, Ellis MJ, Ma C. Molecular Basis of Triple Negative Breast Cancer and Implications for Therapy. Int J Breast Cancer 2012;56:570-7.
- Jain R, Sharma A, Gupta S, Sarethy IP, Gabrani R. Solanum nigrum: current perspectives on therapeutic properties. Altern Med Rev 2011;16:78-85.
- Perez RM, Perez JA, Garcia LM, Sossa H. Neuro- pharmacological activity of Solanum nigrum fruit. J Ethanopharmacol 1998;62:43-8.
- Son YO, Kim J, Lim JC, Chung Y, Chung GH, Lee JC. Ripe fruit of Solanum nigrum L. inhibits cell growth and induces apoptosis in MCF-7 cells. Food Chem Toxicol 2003;41:1421-8.
- Sultana S, Perwaiz S, Iqbal M, Athar M. Crude extracts of hepatoprotective plants, Solanum nigrum and Cichorium intybus inhibit free radical-mediated DNA damage. J Ethnopharmacol 1995;45:189-92.
- Lin HM, Tseng HC, Wang CJ, Chyau CC, Liao KK, Peng PL, et al. Induction of Autophagy and Apoptosis by the Extract of Solanum nigrum Linn in HepG2 Cells. J Agric Food Chem 2007;55:3620-8.
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646-74.
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004;306:990-5.
- Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2004;2:301-14.
- Deretic V. Autophagy in Immunity and Cell-Autonomous Defense against Intracellular Microbes. Immunol Rev 2011;240:92-104.
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol 2007;7:767-77.
- Mizushima N. Autophagy: process and function. Genes Dev 2007;21:2861-73.
- Burgess DJ. Apoptosis: Refined and lethal. Nat Rev Cancer 2013;13:79-9.
- Fuldaand S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006;25:4798-811.
- Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev 2008;19:325-31.
- Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol 1997;6:133-42.
- Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 2009;9:501-7.
- Jin S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2006;2:80-4.
- Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Autophagy 2007;3:610-3.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int 2006;30:924-32.
- Heo KS, Lee SJ, Ko JH, Lim K, Lim KT. Glycoprotein isolated from Solanum nigrum L. inhibits the DNA-binding activities of NF-kappaB and AP-1, and increases the production of nitric oxide in TPA-stimulated MCF-7 cells. Toxicol In Vitro 2004;18:755-63.
- Wang HC, Wu DH, Chang YC, Li YJ, Wang CJ. Solanum nigrum Linn. Water Extract Inhibits Metastasis in Mouse Melanoma Cells in vitro and in vivo. J Agric Food Chem 2010;58:11913-23.
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004;6:463-77.
- Green DR, Evan GI. A matter of life and death. Cancer Cell 2002;1:19-30.
- Heath-Engel HM, Shore GC. Mitochondrial membrane dynamics, cristae remodeling and apoptosis. Biochim Biophys Acta 2006;1763:549-60.
- Byrneand GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol 2004;2:802-08.
- Giansanti V, Torriglia A, Scovassi AI. Conversation between apoptosis and autophagy: is it your turn or mine? Apoptosis 2011;16:321-33.
- Gabrani R, Jain R, Sharma A, Sarethy I, Dang S, Gupta S. Antiproliferative Effect of Solanum nigrum on Human Leukemic Cell Lines. Indian J Pharm Sci 2012;74:451-53.