- *Corresponding Author:
- Lakshmi Sivasubramanian
Department of Chemistry, Pharmaceutical Chemistry Unit, Vellore Institute of Technology, Deemed University, Vellore–632 014. India
E-mail: lakshmiss@hotmail.com
| Date of Submission | 9 February 2005 |
| Date of Revision | 12 May 2005 |
| Date of Acceptance | 16 March 2006 |
| Indian J Pharm Sci, 2006, 68 (2):240-242 |
Abstract
Two simple, precise and accurate methods for simultaneous estimation of valdecoxib and tizanidine in combined dosage form, have been described. Method 1 involves formation of Q-absorbance equation at 239.6 (isoabsorptive point) and at 241 nm, while method 2 involves formation of simultaneous equation at 241 and 229 nm, using methanol as solvent. Both the methods were validated, and the results were compared statistically. They were found to be precise, accurate, and specific. The proposed methods were successfully applied to estimation of valdecoxib and tizanidine in combined tablet formulation.
Valdecoxib (VAL) is chemically a diaryl substituted isoxazole. The chemical formula is 4-(5-methyl-3-phenyl-4-isoxazoyl) benzene sulfonamide [1,2]. It is a non-steroidal antiinflammatory agent, a selective inhibitor of cyclooxygenase–2 (COX–2) indicated for oral administration, for the treatment of Osteoarthritis and Rheumatoid arthritis [3]. Valdecoxib is official only in the Martindale Extra Pharmacopoeia. A survey of literature reveals that valdecoxib is estimated by Solid phase LC in urine samples [4]. Tizanidine hydrochloride (TZN), 5– Chloro-N-(4,5-dihydro-1-H-imidazol-2-yl)-2,1,3– benzothiadiazol–4–amine is an α adrenergic receptor agonist, which is a centrally active skeletal muscle relaxant, and is chemically different from other muscle relaxants. [5] In addition to its muscle relaxant properties and central analgesic effect, TZN also has gastroprotective effect [6]. TZN is an imidazoline derivative, and not official in any of the Pharmacopoeia. Several analytical methods for the estimation of tizanidine using HPLC [7], isocratic SFC [8], colorimetry [9,10], and UV spectrophotometry [11], have been reported. Reddy et al. [12] reported simultaneous spectrophotometric estimation of tizanidine and nimesulide from combined dosage forms, using methanol as a solvent. Moreover, the literature survey revealed that so far, no method has been reported for estimation of TZN and VAL in combined dosage forms, hence we attempted to develop a simple, accurate, and economic analytical method. This paper describes two simple UV spectrophotometric methods for simultaneous estimation of TZN and VAL in tablets, using methanol as solvent.
An UV/Vis double beam spectrophotometer, Shimadzu model-1601, with 1 cm matched quartz cells was used. TZN standard stock solution (100 μg/ml) was prepared by weighing a 25 mg portion of TZN (Blue Cross Laboratories Ltd., Mumbai) standard, it was transferred to a 25 ml volumetric flask, and volume made to 25 ml with methanol. From this solution, an aliquot of 2.5 ml was withdrawn, and it was diluted to 25 ml with methanol. VAL standard stock solution (100 μg/ml) was prepared by weighing a 25 mg portion of VAL standard in to a 25 ml volumetric flask,and volume was made up to 25 ml with methanol. From this solution, an aliquot of 2.5 ml was withdrawn, and it was diluted up to 25 ml using methanol.
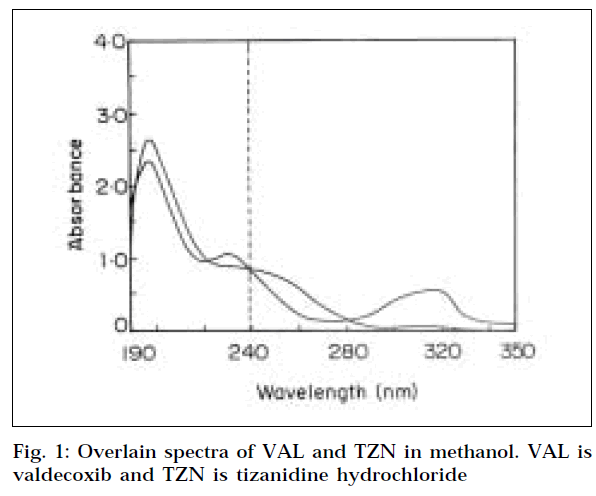
For selection of analytical wavelength for the Q-absorbance method (Method 1), the stock solutions of VAL and TZN were separately diluted in methanol, to get concentrations of 10 μg/ml each, and scanned in the wavelength range of 200-400 nm. From the overlain spectra of both drugs, (Fig. 1) wavelengths 239.6 nm (isoabsorptive point) and 241 nm (λmax of VAL) were selected for the formation of Q-absorbance equation. For calibration curves, stock solutions of VAL and TZN were appropriately diluted to obtain concentration range of 5-30 μg/ml for each drug. The absorbance of VAL measured at 241 nm and 239.6 nm, and calibration curves were plotted. Similarly the absorbance of TZN measured at 239.6 nm and 241 nm, and calibration curves were plotted. The absorptivities (A1%, 1 cm) of each drug at both the wavelengths were also determined.
The absorbance and absorptivity values at the particular wavelengths were calculated and substituted in the following equation, to obtain the concentration. CVAL=(QM-QY) xA1/(QX-QY) xax1, CTZN=A1/ax1-CVAL where, CVAL, CTZN are concentration of VAL and TZN, respectively, A1 is the absorbance of sample at 239.6 nm, ax1 is the absorptivity of VAL at 239.6 nm, QX was obtained by using the equation, (absorptivity of VAL at 241 nm)/(absorptivity of VAL at 239.6 nm), QY was obtained from (absorptivity of TZN at 241 nm)/ (absorptivity of TZN at 239.6 nm) and QM from, (absorbance of sample at 241 nm)/(absorbance of sample at 239.6 nm).
For the selection of analytical wavelength in simultaneous equation method (Method 2), the spectra of VAL and TZN of the method 1 were used, and wavelength 229 and 241 nm (lmax of TZN and VAL, respectively) were selected for the formation of the simultaneous equations. For calibration curves, stock solutions of VAL and TZN were appropriately diluted to obtain VAL and TZN in the concentration range of 5-30 μg/ml. The absorbance of VAL and TZN were measured at 241 and 229 nm, and calibration curves were plotted. The absorptivities of both the drugs at both the wavelengths were determined.
The absorbance and the absorptivity values at the particular wavelengths were calculated and substituted in the following equation, to obtain the concentration. CVAL=(A1ax2-A2ax1)/(ax2ay1-ax1ay2), CTZN=(A2ay1-A1ay2)/ (ax2ay1-ax1ay2), where, CVAL, CTZN are concentration VAL and TZN, respectively, A1 is the absorbance of sample at 241 nm, A2 is the absorbance of sample at 229 nm, ax1 is the absorptivity of VAL at 241 nm and ax2 is the absorptivity of VAL at 229 nm, ay1 is the absorptivity of TZN at 241 nm and ay2 is the absorptivity of TZN at 229 nm.
For the estimation of drugs from the commercial formulations, twenty tablets of two brands Valeron (CFL Pharmaceuticals, Mumbai) and Zulu-V (Unichem, Mumbai) containing 2 mg of tizanidine hydrochloride and 50 mg of valdecoxib were weighed, and finely powdered. For methods 1 and 2, powder equivalent to 50 mg of VAL was accurately weighed. The mixture was then extracted with methanol, the extract was filtered, and the filtrate was appropriately diluted to get final concentration 1 μg/ml of TZN and 10 μg/ml of VAL. Absorbance of this solution was measured at appropriate wavelengths, and values were substituted in the respective formulae to obtain concentrations.
The overlain spectra of both the drugs showed that the peaks are well resolved, thus satisfying the criteria for obtaining maximum precision, based on absorbance ratios. [13] The criteria being the ratios, (A2/A1)/(ax2/ax1) and (ay2/ay1)/(A2/A1), should lie outside the range 0.1-2.0 for the precise determination of Y and X, respectively. Where A1, A2 represents the absorbance of the mixture at λ1 and λ2, ax1, and ax2 denote absorptivities of X at λ1 and λ2, and ay1, and ay2 denote absorptivities of Y at λ1 and λ2, respectively. In this context, the above criteria was found to be satisfied for VAL (X) and TZN (Y), where λ1 is 239.6 nm and λ2 241 nm for Q-absorbance method, and λ1 is 241 nm and λ2 is 229 nm for simultaneous equation method.
Three wavelengths that could serve as isoabsorptive points were 222 nm, 239.6 nm, and 280.2 nm, as determined by evaluation of overlain spectra. By comparing the absorptivity of both the drugs at these wavelengths, 239.6 nm was found suitable for the analysis, since both the drugs gave same absorptivity at this wavelength. The other wavelength i.e. the λmax of VAL selected at 241 nm. Hence 239.6 and 241 nm were selected for the formation of Q-absorbance equation.
In simultaneous equation method, two wavelengths i.e. λmax of both the drugs were required. The spectra of TZN showed two distinct peaks, one at around 229 nm, and other at 371 nm. The former was selected for analysis of TZN. The λmax of VAL was 241 nm, which was used for its estimation.
The proposed methods were successfully used to estimate the amount of VAL and TZN, present in two of the marketed tablet formulations containing VAL and TZN. The assay values for both the tablets were comparable with the corresponding labeled amounts, as shown in Table 1. The validation parameters of proposed methods are summarized in Table 1 and 2. On observing the validation parameters, both the methods were found to be precise accurate and specific. When compared to method 2, method 1 gave assay results close to 100%. Hence, the proposed methods can be employed for routine assay of tablets containing VAL and TZN.
| Parameters | Method 1 | Method 2 | ||
|---|---|---|---|---|
| VAL | TZN | VAL | TZN | |
| Linearity range (µg/ml) | 2.5-15 | 1-3 | 2.5-15 | 1-3 |
| Correlation coefficient (r2) | 0.999a | 0.997a | 0.999a | 0.997a |
| 0.999b | 0.997b | 0.998c | 0.999c | |
| Intercept | 0.00195a | 0.00867a | 0.00195a | 0.00867a |
| 0.00245b | 0.00951b | 0.00329c | 0.00375c | |
| Slope | 0.0607a | 0.0508a | 0.0607a | 0.0508a |
| 0.0605b | 0.0543b | 0.0572c | 0.0698c | |
| Accuracy (%) | 98.8-102 | 98.2-98.8 | 97.5-99.5 | 100.2-100.9 |
| Repeatability (RSD) | 0.472 | 0.0058 | 0.467 | 0.0051 |
| % Recovery | 98.5 ± 1.8 | 102 ± 0.3 | 99.5 ± 1.8 | 101 ± 0.2 |
Method 1 is Q-absorbance method while Method 2 is the Simultaneous equation method, RSD is the relative standard deviation while r2 is the correlation coefficient, a; at 241 nm, b; at 239.6 nm, c; at 229 nm.
Table 1: Summary Of Validation Parameters
| Method | Tablet A | Tablet B | ||||
|---|---|---|---|---|---|---|
| % VAL | % TZN | %VAL | % TZN | |||
| Method 1 | 98.5 ± 1.8 | 100 ± 0.3 | 98.2 ± 1.8 | 99.1 ± 0.5 | ||
| Method 2 | 98.4 ± 1.8 | 101 ± 0.2 | 99.0 ± 1.8 | 98.5 ± 1.2 | ||
Method 1 is Q-absorbance method while Method 2 is the Simultaneous equation method. Values for recovery are mean±SD for three determinations. Tablet A: Zulu-V (Unichem Ltd) and Tablet B: Valeron (CFL Pharmaceuticals), containing 2 mg tizanidine hydrochloride and 20 mg valdecoxib.
Table 2: Analysis of Commercial Formulations
Acknowledgements
The authors thank Blue Cross Laboratories Ltd., Mumbai, for the sample of pure tizanidine hydrochloride and Novartis India Ltd., Mumbai for the pure sample of Valdecoxib.
References
- Budavari, S., Eds., In; The Merck index, 12th Edn., Merck & Co., Inc., Whitehouse Station, NJ, 1994.
- Reynolds, J.E.F., Eds., In; Martindale: The Extra Pharmacopoeia, 30th Edn., The Pharmaceutical press, London, 1993, 90.
- Ormrod, D., Wellington, K. and Wagstaff, A.J., Drugs, 2002, 62, 2059.
- Zhang, J.Y., Fast, D.M. and Breau, A.P., J. Chromatogr. B.Anal. Technol. Biomed. Life. Sci., 2003, 785, 123.
- Sayers, A.C., Burki, H.R. and Eichenberger, E., Arzneim-Forsch-Drug-Res., 1980, 30, 793.
- Coward, D.M. and Emre, M., In; Muscle Spasms and Pain.,Carnforth Parthenon Publishing., Carnforth., UK., 1988, 101.
- Raman, B. and Patil, D., Indian Drugs, 2002, 39, 392.
- Bhoir, I.C., Raman, B., Sunderesan, M. and Bhagwat, A.M., Anal.Lett.,1998, 31, 1533.
- Amin, A.S. and El-Kousy, N., J. Drugs. Res., 2000, 23, 259.
- Sujatha, K., Chitra, K., Dhanushkha, S.H., Krishnan, M.V. and Janardhan, V., Indian Drugs, 2002, 39, 548.
- Murthy, T.K., Reddy, M.N. and Sankar, D.G., Indian J. Pharm.Sci., 2001, 63, 521.
- Murthy, T.K., Reddy, M.N. and Sankar, D.G., EasternPharmacist, 2001, 44, 121.
- Beckett, A.H. and Stenlake, J.B., In; Practical Pharmaceutical Chemistry, 4th Edn., Part-II, CBS Publisher, New Delhi, 1997, 275