- *Corresponding Author:
- M. V. Raghav
Department of Pharmacology, Bangalore Medical College and Research Institute, Rajiv Gandhi University of Health Sciences, Bengaluru 560002, India
E-mail: raghav.m.ram@gmail.com
| Date of Received | 23 June 2022 |
| Date of Revision | 28 March 2023 |
| Date of Acceptance | 27 October 2023 |
| Indian J Pharm Sci 2023;85(5):1534-1538 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acne vulgaris is a chronic skin disease of multifactorial aetiology. Combination therapy may expedite treatment duration and minimise the adverse effects in turn improving patient compliance. The study was done to compare efficacy and tolerability of topical monotherapy with 2.5 % benzoyl peroxide vs. topical combination therapy with 0.1 % adapalene and 2.5 % benzoyl peroxide. A comparative, randomized, open label, parallel group study was conducted after obtaining approval from the institutional ethics committee, 80 patients fulfilling the inclusion and exclusion criteria were enrolled for the study after obtaining informed consent or assent. Patients were randomly allocated into 2 equal study groups. Group A received topical benzoyl peroxide 2.5 % and group B received a combination of topical benzoyl peroxide 2.5 % and adapalene 0.1 %. They were followed up at 4th, 8th, 12th w. Reduction in non-inflammatory lesion count (49.3 % vs. 63.5 %), reduction in inflammatory lesion count (23.9 % vs. 29.2 %) and reduction in total lesion count (54.1 % vs. 67.7 %) was observed at the end of 12 w. Significantly greater mean percentage reduction of non-inflammatory, inflammatory and total acne lesion count was seen in group B than group A at the end of study by 12 w (p<0.001 for all types of lesion counts). The tolerability profile in both the groups was similar. Reduction in non-inflammatory and inflammatory lesions was more in group B compared to group A, whereas tolerability profile was indifferent.
Keywords
Acne vulgaris, benzoyl peroxide, adapalene, randomized controlled trial
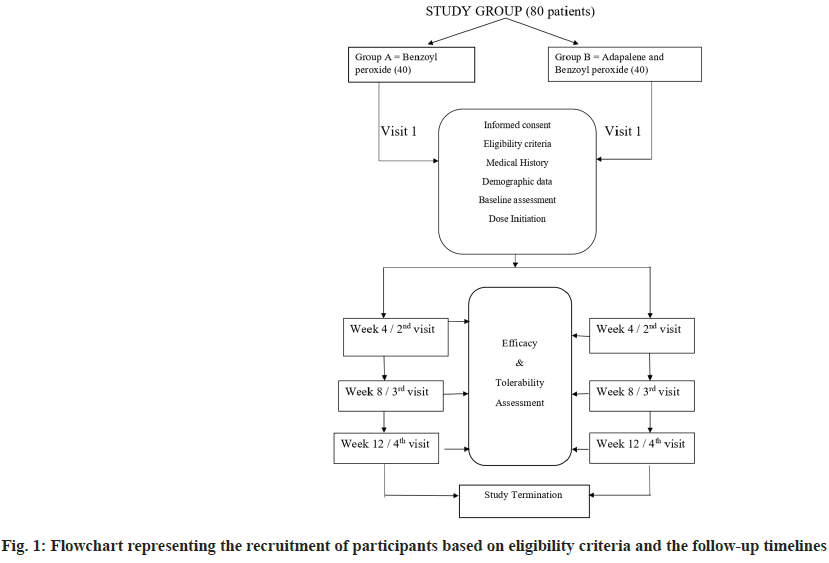
Acne vulgaris is a chronic inflammatory disorder of the pilo-sebaceous unit[1]. It affects approximately 90 % individuals between puberty and 30 y[2]. It is seen primarily in adolescents but can also be seen till 30 y of age. The peak prevalence and severity are between 14 to 17 y in girls and 16-19 y in boys[1]. Acne has significant physical and psychosocial impact on young population, resulting in scarring of face, emotional problems, withdrawal from society, anxiety and depression[3]. The herald of acne is micro-comedones which further progress to present as pleomorphic variety of lesions comedones, papules, pustules and nodules. Acne pathogenesis is multi-faceted where 4 relevant factors are follicular epidermal hyperproliferation, excess sebum production, inflammation and presence and activity of Propionibacterium acnes (P. acnes)[4]. Aim of the treatment is to reduce the severity of skin lesions and prevent the recurrence thereby improving appearance. Selection of therapy depends on the grade and extent of disease as well as patient's choice and economical consideration[5]. Topical treatment is usually used for mild to moderate acne vulgaris. Combination therapies help to target the multiple pathways of development of acne lesions making them more efficacious improving patient compliance and minimizing the adverse effects[6]. Benzoyl Peroxide (BPO) preparations are among the common topical medications that is being used for years without development of resistance[7]. BPO has anti-microbial, bactericidal, anti-inflammatory and keratolytic activity. It is lipophilic molecule and easily penetrates pilosebaceous follicles. The anti-bacterial activity is through generation of reactive oxygen species in sebaceous follicles and reduction of P. acnes and resistance is said to not develop to its action[5]. It is available in concentration ranging in strength from 2.5 % to 10 % and lower concentration are found to be equally efficacious in killing P. acnes with less side effects like local irritation, scaling and erythema[1]. Topical retinoids target follicular keratinocytes and prevent their excess cornification and blockage of follicles. Adapalene is a synthetic retinoid having anti-comedogenic and anti- inflammatory properties. It is available as 0.1 % and 0.3 % topical cream or gel. Since the development of micro-comedones is the initial step in all acne lesions, adapalene inhibits micro-comedones formation leading to reduction in both non-inflammatory and inflammatory lesions. It is stable in sunlight and in presence of BPO and tends to be less irritating at 0.1 % concentration[8]. Its anti- inflammatory role is attributed to interference with polymorphonuclear leucocyte function and role in arachidonic acid metabolism. Adapalene and BPO have complementary mode of action and synergistic activity. The fixed dose combination of 2.5 % BPO+0.1 % adapalene was approved by United States Food and Drug Administration. Adapalene helps in effective penetration of BPO through its keratolytic activity that decreases the thickness of cornified layer of skin[9]. The combination therapy produces greater and faster results improving outcome and compliance. The limitations of currently available therapies are longer duration of treatment, relapse and emergence of tolerance which highlights the need for effective and safe treatment options. The present study focuses on an effective topical treatment option for mild to moderate acne vulgaris with adapalene as an add on therapy to BPO. A prospective comparative randomized, open label, parallel study design was conducted from November 2017 to May 2019 on out-patient basis at the department of dermatology. A sample size of 80 was arrived at using the formula for experimental design using Dreno et al.[3] data and a dropout rate of 15 % was taken into consideration. Patients willing to give informed consent/assent and willing to come for follow up, aged 12-30 y of either sex, patients with mild to moderate acne on face above jaw line (Indian Acne Alliance Grading) and women with negative urine pregnancy test result and agree to use an effective form of contraception for the duration of study (12 w) were enrolled as study participants. Exclusion criteria was patients not willing to give informed consent/assent and not willing to come for follow up, patients with severe acne on face (Indian Acne Alliance Grading), patients with acne on chest and back, other variants of acne; chloracne, oil acne, topical acne, mechanical acne, severe variants like acne conglobata and acne fulminans, drug induced acne, patients on any oral or topical medications for acne, if follow up disease progresses and necessitates systemic therapy, pregnant and lactating mothers, patients with known hypersensitivity to any of the components of the drug or vehicle or excipients, after obtaining approval and clearance from the institutional ethics committee (BMCRI/ PGs/289/2017-18, dated-03.10.2017). Patients fulfilling the eligibility criteria were enrolled for the study after obtaining informed consent or assent. The study design has been summarised in fig. 1. A self- designed informed consent form which explained the nature of the study was used. It was explained in the language understood by the patient. Written consent was taken on the form by the study subjects who were above 18 y of age and those who were below 18 y, written consent was taken on the assent form along with the parents or guardian in charge of the patient. Women of child bearing potential who had a negative urine pregnancy test result were enrolled in the study. Baseline evaluation of each patient was done at the first visit. Demographic data and personal history was obtained using study proforma with special attention to predisposition to acne. Dermatological evaluations including baseline clinical grading of acne severity was done as per Indian Acne Alliance. For each patient the number of acne lesions distributed all over the face were counted and further these were noted as either non-inflammatory or inflammatory acne lesions. Thus, the total acne lesion count in each patient was considered as 100 % at baseline and was entered in the study proforma. Treatment regimen was under the direction of treating dermatologist. 80 patients diagnosed with mild to moderate acne on face were allocated into 2 study groups A and B 40 each. They were randomly assigned to one of the 2 treatment schedules using computer generated random number table. Group A received topical Benzoyl peroxide 2.5 % and Group B received a combination of topical Benzoyl peroxide 2.5 % and adapalene 0.1 %. Patients were advised to wash their face and dry it well before applying the medication. 1 fingertip unit (approximately 0.5 g) of each study drug combination was applied by dotting it over the affected area of the face at bed time. The contact period was gradually increased. In the beginning if there was irritation with the drugs, initiation was done with short contact time of 15-30 min and gradually increased to leave it overnight. Caution was advised against application to nasal folds, periorbital and perioral areas. They were also informed that if there was intolerable irritation initially, they should wash it off with water and those patients were excluded from the study. Efficacy and tolerability assessment of the drugs was evaluated at 4th, 8th and at 12th w follow up by spot counting of acne lesions on face by the dermatologist. At baseline, the total number of lesions on face was taken as 100 %. Any reduction in the number of acne lesions at the follow ups was compared with baseline and expressed as percentage of improvement and graded and entered in the chart. Tolerability assessment each of the parameters-scaling, erythema, burning itching was assessed and graded by the dermatologist at 4th, 8th and 12th w follow up. Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on mean±SD (Min-Max) and results on categorical measurements are presented in numbers and percentages (%). Significance is assessed at 5 % level of significance. The assumptions on data were made-dependent variables should be normally distributed. Normality was tested using Shapiro Wilk test in frequency statistics. The samples showing not normal distribution were analysed accordingly. Samples drawn from the population should be random. Cases of the samples should be independent. Chi-square or Fisher exact (if the value of variable was <5 in any of the cells) test has been used to find the significance of study parameters on categorical data between two or more groups. The chi-square test for independence is used to determine the relationship between two variables of a sample. In this context independence means that the two factors are not related. The chi square test, when used with the standard approximation that a chi-square distribution is applicable, has the following assumptions. A random sampling of the data forms a fixed distribution or population. Out of 80 patients who were enrolled in the study, 76 patients completed the study. One patient in group A and 3 patients in group B did not report for the follow up. Group A had 1 patient loss to follow up at 12th w whereas group B had patient loss to follow up at 8 w and 2 patients had loss to follow up at 12th w. Hence, they were excluded from the study and analysis. Thus, for efficacy and tolerability assessment, 39 patients in group A and 37 patients in group B were included. In the present study, out of 80 patients who were randomized, most were between 16-20 y of age group (60 % in group A vs. 57.5 % in group B) followed by between 21-25 y (32.5 % in group A vs. 35 % in group B). The mean age of patients was 19.46±2.89 y. The mean age in group A was 20+3.06 y and in group B was 19.72±2.73 y. The summary of demographic parameters at baseline has been summarised in Table 1. The results showed that addition of adapalene 0.1 % to BPO 2.5 % produced clinically significant enhancement of efficacy of the topical treatment when compared to monotherapy. There was significantly greater mean reduction in non-inflammatory, inflammatory and total lesions after 12 w of treatment with topical 2.5 % BPO and 0.1 % adapalene combination therapy compared to monotherapy alone with topical 2.5 % BPO. The efficacy parameters are as tabulated in Table 2. There were no significant differences between the groups in producing improvement in acne at the end of 4 w, p=0.061. In Group A by the end of 4 w, 48.71 % patients reported fair improvement in acne lesions whereas 51.28 % had poor improvement. In Group B, 8.10 % patients had good improvement whereas 59.45 % has fair and 32.43 % had poor improvement. There was a significant difference between the groups in producing a fair improvement in acne at the end of 8 w, p<0.01. In Group A by the end of 8 w, 23.07 % patients had good improvement, 71.79 % reported fair improvement in acne lesions whereas 5.1 % had poor improvement. In group B 2.7 % had excellent improvement, 56.75 % patients had good improvement whereas 40.54 % has fair improvement. The current study was designed to compare the efficacy and tolerability of topical monotherapy of BPO 2.5 % (Group A) vs. addition of adapalene 0.1 % to BPO 2.5 % as FDC (Group B) in mild to moderate acne vulgaris on face. The results of the present study support the efficacy of both combinations of topical 2.5 % BPO with 0.1 % adapalene and monotherapy with 2.5 % BPO in the treatment of acne. In a study done by Korkut et al.[5], 105 patients were randomized into 3 groups of 35 in each group consisting of combination of 5 % BPO lotion+0.1 % adapalene gel, monotherapy of 5 % BPO lotion and monotherapy of 0.1 % adapalene gel and the overall mean age of patients was 18.44±3.75 y. In a study by Thiboutot et al.[9], 517 patients who were 12 y or older were randomized in a multicentric trial into 4 groups of 2:2:2:1 ratio with 2.5 % BPO, 0.1 % adapalene and their FDC having 140 patients in each group whereas the vehicle had 70 patients allocated. The mean age of patients was 16.4 y. A male preponderance was seen in this study (59.8 % males vs. 40.2 % females). Our study also showed a male preponderance (52.5 % males vs. 47.5 % females) in group A and (57.5 % males vs. 42.5 % females) in group B. This trend shows that both sexes are conscious about their cosmetic appearance. In our study, most patients were in the age group of 16- 20 y. Similar age distribution was observed in study done by Korkut et al.[5] and Thiboutot et al.[9]. Most of the patients were from urban background in our study. According to a study done by Gowda et al.[10] 50 % patients had Grade 2 (mild severity) and other 50 % had Grade 3 (moderate severity) acne. Acne severity at baseline in our study is comparable with this study. The study by Thiboutot et al.[9] included patients of mild, moderate and severe baseline acne severity according to IGA and 75 % patients in all groups were having moderate acne. Tan et al.[11] in their study comparing efficacy of adapalene 0.1 % with BPO 2.5 % combination vs. adapalene alone or BPO alone showed greater success with the combination therapy. They reported that the treatment from baseline to 12 w resulted in median percentage reduction in non-inflammatory lesion count as 58 % with adapalene/BPO combination and 45 % with BPO; inflammatory lesion count as 66 % with adapalene/BPO combination and 57 % with BPO; total lesion count as 59 % with adapalene/BPO combination and 46 % with BPO. Gold et al.[12] in their study showed median percentage reduction from baseline till end of 12 w of treatment in non- inflammatory lesions for adapalene/BPO combination group as 53.8 % and for BPO group as 44.1 %, inflammatory lesions for adapalene/BPO combination group as 62.1 % and for BPO group as 55.6 %. Topical formulations are the most widely used treatment for Acne vulgaris to target one or more steps in the pathogenesis of acne. It was concluded from the results of the present study that the combination of benzoyl peroxide with adapalene has a better efficacy than benzoyl peroxide alone in mild to moderate acne vulgaris. Participants of both the intervention groups had a significant improvement in acne lesions after 12th w of topical application and were satisfied with the therapy. No major adverse effects were observed in both groups. Hence, topical combination therapy of benzoyl peroxide with adapalene represents a potential alternative to first line combination therapies in mild to moderate acne vulgaris. Further large-scale studies are needed to establish the same.
| Group A | Group B | |
|---|---|---|
| Age in years (mean±SD) | 20±3.06 | 19.72±2.73 |
| Gender-Number of participants (%) | ||
| Male | 21 (52.5 %) | 23 (57.5 %) |
| Female | 19 (47.5 %) | 17 (42.5 %) |
| Acne severity-Number of participants (%) | ||
| Grade I (mild) | 17 (42.5 %) | 19 (47.5 %) |
| Grade II (moderate) | 23 (57.5 %) | 21 (52.5 %) |
Table 1: Summary of Demographic Parameters at Baseline in Patients of Acne Vulgaris
| Non-Inflammatory lesions | Inflammatory lesions | |||||
|---|---|---|---|---|---|---|
| Group A (Mean±SD) | Group B (Mean±SD) |
p-value | Group A (Mean±SD) |
Group B (Mean±SD) |
p-value | |
| 4 w | 21.06 | 31.77 | 0.02 | 35.99 | 38.1 | 0.18 |
| 8 w | 38.38 | 49.81 | 0.002 | 53.83 | 64.87 | 0.02 |
| 12 w | 49.96 | 64.88 | <0.001 | 70.59 | 77 | 0.03 |
| p-value | <0.001 | <0.001 | - | <0.001 | <0.001 | - |
Table 2: Mean Reduction (%) of Acne Lesions at 4th w, 8th w AND 12th w in between Group A (Benzoyl Peroxide) and Group B (Benzoyl Peroxide with Adapalene)
Conflict of interests:
The authors declared no conflict of interests.
References
- Griffiths CE, Barker J, Bleiker TO, Chalmers R, Creamer D. Rook's textbook of dermatology. 2016;4:42-78.
- Kubba R, Bajaj AK. Thappa DM, Sharma R, Vedamurthy M, Dhar S, et al. Acne in India: Guidelines for the management. Indian J Dermatol Venerol Leprol 2009;75(S1):50-52.
[Google Scholar] [PubMed]
- Dreno B. Tan J, Rivier M, Martel P, Bissonnette R. Adapalene 0.1%/benzoyl peroxide 2.5% gel reduces the risk of atrophic scar formation in moderate inflammatory acne: A split-face randomized controlled trial. J Eur Acad Dermatol Venerol 2017;31(4):737-42.
[Crossref] [Google Scholar] [PubMed]
- Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's dermatology in medicine. 2008;690-703.
- Korkut C, Piskin S. Benzoyl peroxide, adapalene and their combination in the treatment of acne vulgaris. J Dermatol 2005;32(3):169-73.
[Crossref] [Google Scholar] [PubMed]
- Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12-17 years with the fixed dose adapalene-benzoyl peroxide combination topical gel: Efficacy and safety. J Drugs Dermatol 2010;9(11):1395-401.
[Google Scholar] [PubMed]
- Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: A report from a global alliance to improve outcomes in acne. J Am Acad Dermatol 2003;49(1):S1-37.
[Crossref] [Google Scholar] [PubMed]
- Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: New perspectives on formulation and utilization. Dermatol Clin 2009;27(1):17-24.
[Crossref] [Google Scholar] [PubMed]
- Thiboutot DM, Weiss J, Bucko A, Eichenfield L, Jones T, Clark S, et al. Adapalene- benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: Results of a multicentre, randomized double-blind, controlled study. J Am Acad Dermatol 2007;57(5):791-99.
[Crossref] [Google Scholar] [PubMed]
- Gowda A, Sanmitha SK, Satish KS. Comparative study of clinical efficacy and side effects of adapalene 0.1% gel and benzoyl peroxide 2.5% gel as monotherapies and combination therapy in facial acne: Indian perspective. J Evol Med and Dent Sci 2014;3(14):3786-98.
- Tan J, Gollnick HP, Loesche C, Ma YM, Gold LS. Synergistic efficacy of adapalene 0.1%-benzoyl peroxide 2.5% in the treatment of 3855 acne vulgaris patients. J Dermatolog Treat 2011;22(4):197-205.
[Crossref] [Google Scholar] [PubMed]
- Gold LS, Tan J, Cruz-Santana A, Papp K, Poulin Y, Schlessinger J, et al. A north American study of adapalene-benzoyl peroxide combination gel in the treatment of acne. Cutis 2009;84(2):110-6.
[Google Scholar] [PubMed]