- *Corresponding Author:
- Sayani Bhattacharyya
Department of Pharmaceutics, Krupanidhi College of Pharmacy, Bengaluru, Karnataka 560035, India
E-mail: sayanibh@gmail.com
| Date of Received | 28 May 2022 |
| Date of Revision | 23 January 2023 |
| Date of Acceptance | 06 September 2023 |
| Indian J Pharm Sci 2023;85(5):1208-1223 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Complex diseases require safe and effective delivery of drugs at the target site with improved physicochemical properties of drug mainly solubility and permeability. Pharmaceutical scientists have researched a vast number of controlled, targeted, and novel therapies using the concept of matrix release, nanotechnology, or a hybrid system. In recent years scientists and the pharmaceutical industry have shown a growing interest in mesoporous silica nanoparticles for the delivery of drugs. The mesoporous silica nanoparticles offer distinctive properties of pore size, pore volume, surface area, and functionalization. This technology offers high drug loading, magnificent biocompatibility, and surface functionalization to tune for their broad-spectrum applicability. Owing to this, research scientists have found its materiality for targeting drugs in cancer treatment and delivery of nucleic acids. The present review is a comprehensive collection of various aspects of mesoporous silica nanoparticles that includes the properties, types, synthesis, various methods for functionalization, drug loadings methods, and a briefing of their physicochemical evaluation. The review also highlights the application of these nanoparticles in the treatment of various diseases. The objective of this review is to enlighten the features of mesoporous silica nanoparticles and their biomedical applications.
Keywords
Mesoporous silica nanoparticles, mobil composition of matter No. 41, surface functionalization, drug loading methods, drug targeting
In the 21st century, nanotechnology has emerged as one of the key technologies in drug delivery. Modern nanotechnology has been considered a collaborative platform for research in association with the development of cutting-edge technologies[1]. Nanomaterial has been employed significantly in the healthcare sector because of its feature to hold, carry, protect and deliver therapeutic agents, particularly to the targeted tissue and provides safety by reducing dose size and frequency of administrations[2]. Many types of nanomaterials have shown potential effects on the delivery of the drug. Among them, the recent advances go for silica nanoparticles with mesopores referred to as Mesoporous Silica Nanoparticles (MSNPs).
They are the solid framework of Si-O bond with porous structure and large surface area. The unique feature of mesoporous silica offers high drug loading capacity and predetermined release of the drug. They are more stable and resistant to degradation and mechanical stress. Depending upon the drug and requirement it offers tuneable pore size. The presence of silanol group at the surface helps in easy functionalization of the drug and thereby controls the release of the drug[3,4]. The additional feature of mesoporous silica includes its availability in different morphologies like a rod, cage, fiber, silica nanosphere, film, and vesicles-like structure[5]. Mesoporous solids were introduced by Mobil Research and Development Corporation in the year 1992 and were fabricated using aluminosilicate by liquid-crystal template method and named Mobil Composition of Matter (MCM-41).
Mesoporous molecular sieves family are of three types MCM-41, MCM-48, and MCM-50 with hexagonal, cubic and lamellar shapes respectively. These unique properties of the mesoporous silica have been employed by the scientists to cargo various drugs and macromolecules like proteins[6], Deoxyribonucleic Acid (DNA)[7], micro Ribonucleic Acid (miRNA), small-interfering RNA(siRNA)[8], an Antisense Oligonucleotide (ASO)[9] in the form of nanospheres and nanotubes, generally formed using single-micelle epitaxial growth approach. These nanoparticles stay in the blood circulation for an extended period and show good internalization, and excellent cellular uptake properties[10].
The rapid uptake of MSNPS by the cell and their non-cytotoxic nature finds their way into the biomedical field. MSNPs less than 1 μm have become very popular in several biomedical applications like cell imaging, diagnosis, biosensing and intracellular drug, gene and protein delivery[9].
Hence this review focuses on the various aspects of mesoporous silica, its methods of synthesis, ways of functionalization and its application in the pharmaceutical field as a novel carrier for drug delivery, targeting and intracellular delivery of drugs.
Properties of Mesoporous Silica
Mesoporous silica, the promising novel vehicle for drugs, possesses its unique properties of chemical stability, surface functionality and biocompatibility due to the presence of salts, amines, alcohols, and types of surfactants used during processing[11]. The pore size and the pore volume depend on the amount of surfactant and precursor used in the processing of MSNPs. Cationic surfactant Cetyltrimethylammonium Bromide (CTAB) at high concentration, led to the formation of disordered mesostructured particles whereas, at low concentration of CTAB, the template required for the synthesis of the MSNPs was absent. Hence optimum concentration was suggested to get desired mesoporous structure[12]. Same observations were found with the use of tetraethoxysilane[13]. Uniform particle size with enlarged pore volume makes the MSNPs an ideal carrier for drug delivery. Varying the attributes like temperature, the concentration of surfactant, and the source of silica at the time of synthesis can help to accomplish the ideal properties of silica for improved loading of drugs.
The special characteristics of MSNPs include tuneable pore size in the range of 2-50 nm, large pore volume (>0.9 cm3/g, and high surface area (>700 m2/g). MSNPs attain to be homogenously distributed in water and are stable to heat, pH, mechanical hydrolysis and stress[14]. The internal and external face of the MSNPs can be desirably functionalized through chemical linkages with stimuli-responsive or luminescent materials for smart and targeted drug delivery[15].
The distinctive properties of MSNPs are associated with various benefits for drug delivery and are elaborated in Table 1. The quantity of bioactive moieties that can be encapsulated depends upon the volume of the pore. Normally the pore size is lesser than 15 nm and the surface area is 1000 m2/g with a total pore volume of 1-2 cm3/g. Varying quantities and types of surfactants can alter the total surface area of the MSNPs[16].
| Properties of MSNPs | Benefits associated | References |
|---|---|---|
| Pore volume | Enhance drug loading capacity | [16] |
| Modification in drug release profile | [17] | |
| Pore size | Amplification of inner surface area | [18] |
| Drug carrier efficiency | ||
| Particle size | Boost endocytosis in plants and animals with decreased cytotoxicity | [19] |
| Bio compatibility | Stability under various biological environments | [20] |
| Nontoxic and biodegradable | [21] | |
| Stability | Corrosion-resistant | [22] |
| Stable on chemical, mechanical and thermal stress | [23] |
Table 1: Properties of MSNPs for drug delivery.
Types of Silica
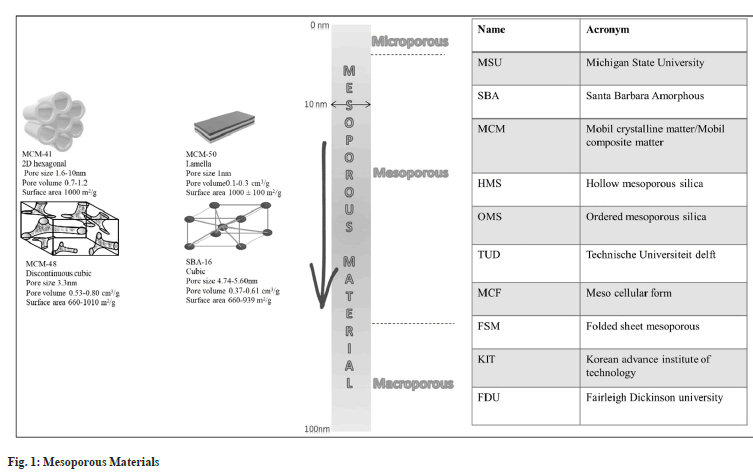
Based on the pore diameter, the classification of silica is presented in Table 2[17]. As per the discovery of various researchers, the many types of marketed mesoporous silica are presented in fig. 1. All the different varieties show variation in their structure and porosity.
| S no | Porous materials | Diameter |
|---|---|---|
| 1 | Microporous | <20 Å |
| 2 | Mesoporous | 20-500 Å |
| 3 | Macroporous | >50 Å |
Table 2: Types of Silica-Based on Pore Size.
Synthesis of MSNPs
The synthesis of the mesoscopic substance started in the year 1970. In the year 1992, Mobil research prepared the mesoporous solid using aluminosilicate gels employing liquid-crystal template mechanism. It was named Mobil Crystalline Materials or MCM[18].
The first-ever report related to the fabrication of monodisperse silica nanospheres was given by Stober et al. (1968). This practice is very well known and successfully employed in the synthesis of MSNPs. This method is based on the hydrolysis and condensation process. Many researchers modified the Stober method to fabricate particles of different shapes and sizes. Few of the approaches are discussed here.
Sol-gel method:
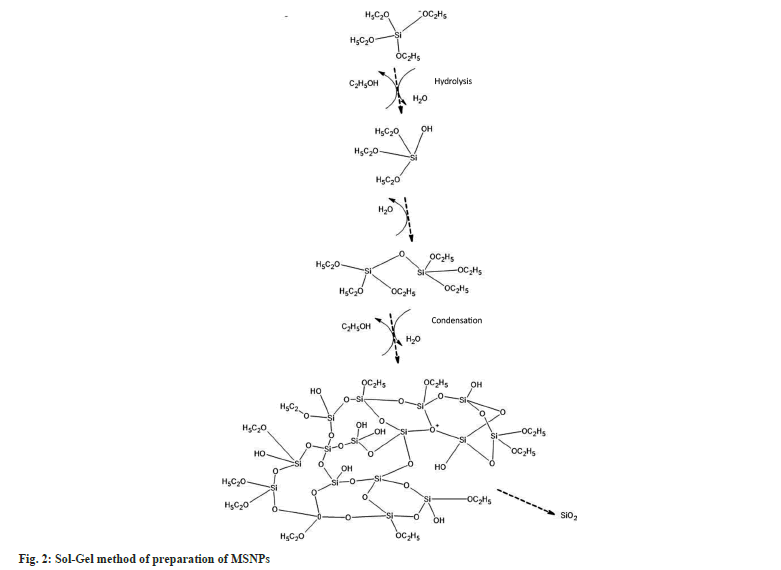
Most of the MSNPs are formed by the sol-gel procedure, popularly called modified Stober’s method. This method employs hydrolysis and condensation of tetraethyl orthosilicate the precursor of colloidal silica, in a controlled catalytic condition.
Acid and base catalysts are involved in this process. The hydrolysis of the alkoxide group mainly depends upon the reaction condition and the molar ratio of Si/H2O. The basic pH condition favors the rate of hydrolysis. Condensation helps in the hydrolysis reaction. Hydrolysis and subsequent condensation lead to crosslinking. A chain structure is formed by multiple condensations which get into the network-like structure of the gel. The sol-gel process is used with the modification to obtain the particles of the desired size and with enhanced properties[19]. The process of hydrolysis and condensation during the sol-gel method is expressed in fig. 2.
Evaporation Induced Self-Assembly (EISA) method:
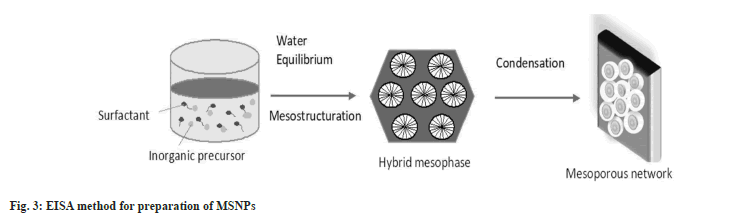
EISA method was first coined by Mahoney et al.[20]. This process serves as an alternate method for the preparation of mesoporous materials and patterned thin films. The process involves the following steps. Formation of initial micellar dispersion of structuring agents i.e., ionic or non-ionic surfactant, and inorganic precursor in ethanol and water; fast solvent evaporation to achieve inorganic encapsulation and film formation, equilibrium of water content in the film with atmosphere, hybrid mesophase formation and stabilization, and finally condensation to harden the network structure.
A wide variety of available structure-directing agents make this method suitable for growing ordered mesoporous transition metal oxides for photovoltaics and sensing properties[21]. The most commonly used structuring agents are amphiphilic block copolymers and pluronic surfactants. Rapid synthesis of the mesoporous nanoparticles is one of the major advantages offered by the EISA method compared to other hydrothermal techniques[22]. The EISA method is represented in fig. 3.
Microwave-assisted technique:
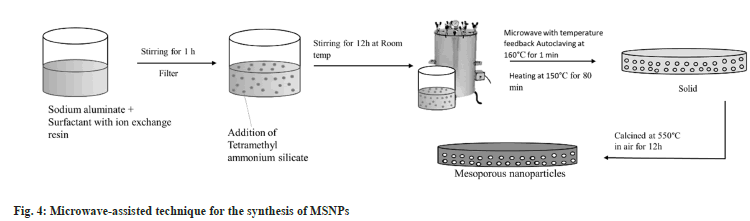
Microwave-assisted technique results in an inexpensive synthesis of MSNPs. It is a hydrothermal synthesis of mesoporous nanoparticles where heating assists nucleation. Advantages of this method include a reduction in synthesis time and particle size and faster polymerization compared to the conventional convection heating method. The swelling rate of the material is found to be much higher compared to a material prepared by conventional heating.
Microwave-assisted production of the molecular sieve is an emerging technique that is often employed in the scientific research domain. Several merits of this method over other general methods are speedy heating to the crystallization temperature, speedy supersaturation by the rapid dissolution of precipitated gels, decreased crystallization time, high localized heating[23], uniform nucleation during crystal growth due to uniform heating. A thermostable hexagonal molecular sieve of MCM -41 was prepared by this technique by Wu et al.[24] and is expressed in fig. 4.
Ultrasonic synthesis:
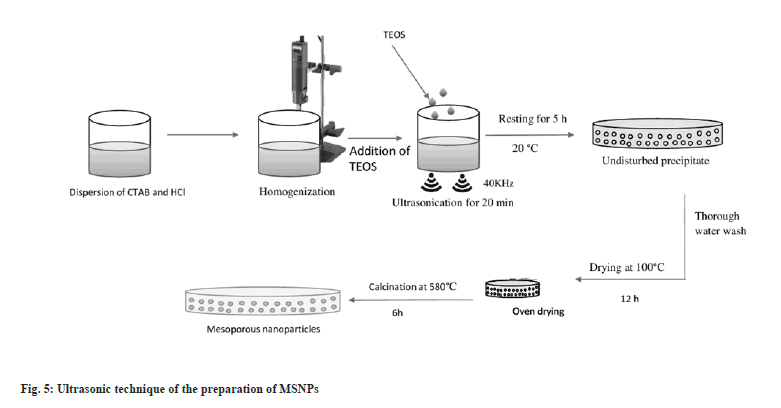
In 2004, Run et al. established an ultrasonic synthesis method for the preparation of MSNPs[25]. The ultrasonic synthesis produces well-ordered hexagonal mesostructures with a large surface area of over 1100 m2/g, primary pore size in the range of 22-30 Å, and pore volume around 1 cm3/g. The main advantage of this synthesis process is that the time for processing is drastically reduced to minutes[26]. The synthesis of the mesoporous silica by the ultrasonic process is reported by Run et al.[25] and the process is presented in the fig. 5. A briefing of the merits and demerits of all the synthetic processes is presented in Table 3.
| Type | Merit | Demerit |
|---|---|---|
| Sol-gel | Easy | Tedious |
| Process control over particle size and structure | Time consuming | |
| Labour-intensive | ||
| Evaporation-induced self-assembly | Broad material encapsulated | Decreased controllability of pore size and pore structure |
| Microwave-assisted technique | Increased yield | Less adjustable in pore size and pore structure |
| Time saving | Complicated preparation | |
| Ultrasonic | Cost-effective | Less structural uniformity |
| Time saving | Decreased yield |
Table 3: Merits and Demerits of the Synthesis processes of MSNPS.
Functionalization of MSNPs
The functionalization of the internal and external surfaces of mesoporous silica is an additional beneficial feature of its versatile applicability. Functionalization ushers to the development of new physical and chemical properties which renders it suitable for broad-spectrum applicability in drug delivery, sensor, catalytic, etc., and other applications. MSNPs are also used to offer intelligent gated release with response to stimuli like pH, enzyme, temperature, redox potential, etc., to minimize the side effects of the drugs. The different ways of functionalization are described below.
Functionalized MSNPs for temperature response:
Temperature is a few of the very in-demand stimuli often used practically to control the response of the MSNPs delivery system in biomedical applications. They are designed to remain intact at normal body temperature in the blood circulation and get activated when the desired temperature is reached at the site of the tumor.
Poly (N-isopropyl Acrylamide) (PNIPAM) stands is one of the very popular and well-studied polymers which is used in making the system temperature responsive[27]. At lower critical solution temperature (32-34°), PNIPAM is water-soluble but transforms into a coiled structure at higher temperatures and hydrophobicity increases. This property is utilized to transfer the active substance at the site of action.
The active molecules are entrapped into the pores of the MSNPs and are covered by the soluble conjugated PNIPAM at a Lower Critical Solution Temperature (LCST). As the surrounding temperature increases the soluble PNIPAM undergoes conformational changes and opens the pores in the surrounding environment and helps to release the active molecules in the surrounding area. This technique is mainly employed in the MSNPs with large pores size with short-chain lengths of the conjugated polymer[28].
Functionalized MSNPs for light response:
Light has been extensively utilized in the formulation of nanomaterials and controls the flow of bioactive molecules. Some light-responsive materials, such as derivatives of Ortho Nitro Benzyl (ONB), derivatives of coumarin, thymine, derives of azobenzene (Azo), spiropyran are most commonly employed in the formation of the light-sensitive system. When these moieties are exposed to light, it undergoes structural changes which in turn initiate the release of the attached molecule from the surface of MSNPs. A few examples of the delivery of drugs are listed in Table 4.
| S.no | Example | Mechanism of action | References |
|---|---|---|---|
| 1 | ONB loaded in MSNPs | Release of bioactive molecule when exposed to the UV result | [89] |
| 2 | Ethyl dimethyl ammonium bromide with gold nanoparticles linked with MSNPs carrying doxorubicin | Activation of the system by UV radiation to release doxorubicin | [90,91] |
| 3 | Doxorubicin-loaded octadecyl-functionalized hollow MSNPs | Release of Doxorubicin in femtosecond at Near InfraRed (NIR) region at 800 nm | [7] |
| 4 | Combination of coumarin and ONB in a single system of MSNPs loaded with doxorubicin and short-hairpin RNA, functionalized with poly 2(N, N-dimethylaminoethyl)methacrylate) | Sequential release of Doxorubicin and short-hairpin RNA of biomolecules at 405 and 365 nm respectively | [92-95] |
Table 4: Drug delivery from MSNPs using light stimuli.
Functionalized MSNPs for magnetic field response:
Magnetic fields are considered speedy and specific methods to alter the response of magnetic materials. One of the most popular sources of the magnet is superparamagnetic ferric oxide nanoparticles (M-Fe-NPS) of Fe3O4 and γ-Fe2O3, used primarily in Magnetic Resonance Imaging (MRI), magnetic-mediated hyperthermia for transport of bioactive molecules, and cancer treatment[29-36].
M-Fe-NPs can be functionalized by different mechanisms either by entrapping into the core of the MSNPs or to cover on the surface of the MSNPs to create magnetic MSNPs (M-MSNPs)[33,37-40]. This has shown improvement in the controlled delivery of the release of various bioactive molecules, site-specific targeting and MRI.
Functionalized MSNPs respond to ultrasound:
Ultrasounds are considered as a promising stimulus to regulate the response of MSNPs to improve their performance in the delivery of bioactive molecules and imaging. Ultrasound provides properties like hyperthermia and cavitational effect in the functionalized MSNPs that aid in the release of the linked bioactive substance and transport.
Manzano et al.[41], studied the cellular internalization of Doxorubicin (DOX) in prostate cancer using this technology. A lack of cellular toxicity was reported before the application of ultrasound, which indicated the blocking of pores. Upon application of ultrasound, dose-dependence toxicity on the cancer cell was observed.
Functionalized MSNPs for pH response:
The pH of human biological fluid, the bloodstream is a very important character for drug absorption. Normally the pH of the fluid in healthy tissue is 7.4. A decrease in pH is observed due to the complications like tumors or inflammation. Lower pH is observed in cytosols (6.5-7.0) and lysosomes (4.5-5). Hence pH can be used to control the performance of the drug and targeting of functionalized MSNPs.
Lu et al. developed a smart nano valve with Poly (2-Diethylaminoethyl Methacrylate) (PDEAEMA) on the external surface of MSNPs[42].
Hydrophobic polymer like polyvinyl pyridine which is insoluble at physiological pH 7.4 can be used to cover the MSNPs, and help to withhold the release of the bioactive material. If the pH of the surrounding changes to 5 the protonation of the polymer occurs which makes it hydrophilic and water-soluble. This leads to the damage of the cover layer and result in the release of the bioactive moieties[43].
To control the release and performance of the bioactive molecules in the MSNPs other system employed are acid-labile functional groups that tend to be stable at neutral pH but show breakdown in acidic pH like at the site of tumor or cytoplasm. Different linkers employed for this are acetal[44,45], amide[46,47], schiff base[48,49] and cis-aconitic acid[46,50] etc. Thus, it enhances the circulation and deposition at the site with a decrease in systemic cytotoxicity.
Functionalized MSNPs for enzyme response:
Enzymes are the biological catalyst that is omnipresent in the living body and helps in enormous biochemical and metabolic reactions. There are various types of enzymes present in a variety of cells and various amounts. Hence this can be used in controlling the release of a particular drug at a particular site depending upon the desired enzyme, especially in chemotherapy. Thus, it helps to minimize the side effects due to the high specificity of the release of the drug depending upon the presence of the desired enzyme at the site. This technique can be used to functionalize various ligands superficially for the MSNPs[28].
DOX was loaded in the MSNPs and layered with oligo DNA to hinder the release of the loaded moieties during circulation. The conjugate in presence of telomerase enzyme in the cell cytoplasm released the drug for imaging[51] and chemotherapy application[52].
Reactive Oxygen Species (ROS):
A reactive oxygen species like H2O2 is very commonly produced by the metabolic reaction in the body and observed more in the person with certain pathophysiological conditions like Alzheimer's, heart failure, inflammation and aging. This allows using a signal in releasing and controlling the bioactive moieties from a system[53].
In one study phenylboronic acid conjugated MSNPs were used to cargo the loading molecules and sealed with diol molecules like human Immunoglobulin G and β-D-glucose-gold nanoparticles to prevent the premature diffusion of the loading molecules. When the system gets exposed to H2O2 environment it causes damage to phenyl borate ester bonds and results in conversion into phenol group and allows the release of the loading molecules[54].
Methods of Drug Loading
The drug loading into the pores of mesoporous silica can be done by two methods, the solvent-free method and the solvent-based method. The drug loading involves drug adsorption onto the inner and outer surfaces of the silica with various interaction forces like vander waal forces, electrostatic force, covalent and hydrogen bonding. The extent of drug loading in the mesopores is critically dependent on the surface area, the affinity between drug and silica, and pore volume. The simple, economic, ecological and industry-oriented drug loading techniques are highlighted in this review.
Solvent-Based Methods
Adsorption method:
This is very frequently adopted method for drug loading in the mesoporous material. In this method, the mesoporous silica is dispersed in a concentrated solution of the drug. The Si-OH (Silanol) group present in mesoporous material helps in the adsorption. The choice of the solvent plays a very important role in the amount of loading. This process is suitable for loading thermolabile drugs. Both hydrophilic and hydrophobic drugs can be used to load on mesoporous silica by this method. The critical processing factor for this method is to identify the optimum concentration of drug and a solvent to achieve high filling in the pores of silica[55]. This method is carried out in the following steps; In the 1st step, the mesoporous material is immersed/ incorporated into the concentrated drug solution, this leads to the filling of the pores of the mesoporous material through capillary action. In the 2nd step, the drug molecules diffuse into the mesopores and get adsorbed onto the pore walls of mesoporous material. In 3rd step, the recovery of the drug-loaded matrix is carried out from the solution by filtration and followed by rapid evaporation of the solvent. The amount of the adsorption can be tailored by adding different functional groups on the surface of the silica[56,57].
Solvent evaporation method:
This process involves a combined method of adsorption and rapid solvent evaporation. The dispersion of the drug and silica is made using an organic volatile solvent. The advantage of this method over the adsorption method is the conversion of the physical state of the drug which promotes localization of the drug and drug release[58].
Incipient wetness impregnation method:
In this method, the pore volume of mesoporous carriers is impregnated several times with the drug solution to completely occupy the pores of the carriers. The drug loading takes place with the capillary mechanism. After several impregnations, a quick wash with a small amount of the solvent should be carried out to remove the free drug from the external surface. The difficulty of this process is to control the uniformity of the drug distribution. Furthermore, there is a chance that the remaining drug recrystallizes on the external surface of mesoporous material after solvent evaporation[56].
Diffusion supported loading:
Potrzebowski et al. developed the most efficient economic technique of drug loading by this method, where the initial step consists of homogenization of the physical mixture of drug and mesoporous silica, followed by diffusion of vapors of ethanol through the solid mixture in a closed environment for 3 h at room temperature. During this diffusion process, the vapors of ethanol penetrate the whole volume of the solid mixture, causing local condensation and dissolution of the drug and transportation into the pores of silica[59]. This method enables to load multicomponents without any special equipment or experimentation conditions.
Liquid and Supercritical Carbon dioxide (SC-CO2) loading:
In this method, the drug loading is carried out by mixing both the drug and mesoporous material in a high-pressure reactor. Investigation on this method revealed that the temperature was maintained at 25° throughout the experiment. The reactor cell was filled with liquid Carbon dioxide (CO2). A high-pressure pump was used to pump additional CO2 to a final processing pressure i.e., 27.58 MPa[56]. The cell was depressurized rapidly by venting the CO2 at the end of the process or experiment. The SC-CO2 loading process followed the same above procedure but instead of heating the cell at 25°, it was heated up to 40°[56]. The final product was rendered free of residual solvent. It is an environmentally friendly, economic and fast technique to achieve high drug loading.
Solvent-Free Methods
Melt method:
The physical mixture of the drug and mesoporous silica is melted above the melting point of the drug. It reduces the time of drug loading into the porous matrix but is suitable for thermostable drugs with low molten viscosity as it affects the penetration over the mesopores[60].
Microwave irradiation method:
In this method, the drug and the mesoporous silica particles are heated at a constant temperature using a feedback system to protect the drug from degradation. It can use a variety of silica and radiation. The process can be carried out with or without water[61].
Co-milling method:
This technique involves the milling of drug and mesoporous silica using a planetary ball mill to achieve submicron particles and solid-state amorphization. It is a solvent-free system and depends on the proportion of drugs and silica. The method is scalable for industrial use but suffers from the drawback of nanocarriers' resistance to mechanical stress. Hence the morphological properties of the nanocarriers and the milling time are the critical process parameters[62].
The entrapment of the drug in the mesoporous materials is subjected to various physicochemical evaluations. Table 5 briefs out the various physicochemical characterization, its objective and methods employed.
| S.no | Types of characterization | Objectives | Methods employed |
|---|---|---|---|
| 1 | Thermal analysis | Physical state | Differential Scanning Calorimetry (DSC) |
| Drug location | Thermo Gravimetric Analysis (TGA) | ||
| Loading efficiency | |||
| 2 | Gas sorption | Pore size | Brunauer–Emmett–Teller (BET) |
| Pore volume | Barrett–Joyner–Halenda (BJH) models | ||
| Pore area | Computational Density functional theory (DFT) | ||
| 3 | Microscopy | Size | Transmission electron microscopy (TEM) |
| Morphology | Scanning electron microscopy (SEM) | ||
| Pore dimension | Stochastic optical reconstruction microscopy (STORM) | ||
| Atomic force microscopy (AFM) | |||
| 4 | Spectroscopy | Host-guest interactions | Solid State NMR |
| Surface chemistry | Raman Spectroscopy | ||
| Fourier Transform Infrared spectroscopy | |||
| 5 | X-ray scattering | Crystallinity | Powder X-Ray Diffraction |
| Physical state | |||
| 6 | Chromatography | Stability | High performance liquid chromatography (HPLC) |
| Loading efficiency | |||
| Drug release | |||
| 7 | MS | Elemental composition | Time-of-flight analyzer Secondary Ion Mass Spectroscopy (ToF-SIMS) |
| Stability | |||
| Drug release | |||
| 8 | Other methods | Size | Dynamic Light Scattering (DLS) |
| Surface charge | Zeta Potential | ||
| Wettability | Contact Angle by capillary penetration | ||
| Density | Gas Pycnometry | ||
| Binding affinity | Isothermal titration calorimetry (ITC) |
Table 5: Physicochemical characterization of MSNPs.
Application of MSPNs in Pharmaceutical Field
The unique properties of MSNPs like their tuneable pore size, thin pore wall, larger surface area, and nano range particles have to need widely used in the pharmaceutical field to enhance the effectivity of drug molecules by targeting, improving solubility, sustained-release, or as a biocompatible carrier for various drugs or nucleic acids. The applications of MSNPs in several sectors are discussed here briefly.
Drug delivery:
Certain drugs require to be delivered through a controlled release system to maintain an effective concentration of drug in the target tissue and enhanced bioavailability. Numerous approaches are available to sustain the effect of drugs. Out of these, mesoporous silica has attracted a significant amount of researchers’ interest. The mesoporous silica M41S family has been employed in the controlled release of the drug with enhanced drug adsorption attributes and predictable pre-determined release kinetics. Various studies showed that mesoporous silica can be employed in drug delivery for various diseases viz. bone/tendon tissue engineering, diabetes, inflammation, and cancer because of their unique acquired morphology[5].
The wide varieties of morphology and functionality make mesoporous silica a suitable carrier for passive and active drug targeting. The biodistribution property of the nanoparticles can be altered with tunable degradability[63]. The rapid renal clearance and uptake by the reticuloendothelial system of MSNPs can be easily corrected by functionalization of the outer surface with hydrophilic carriers like PEG or by zwitterionization. PEGylation increases stealth properties and was found to be effective in the prevention of adsorption onto nonspecific proteins in the body, thus improving the stability of the MSNPs[64]. Covalent grafting of Zwitterionic polymer of small moiety with mesoporous silica can be beneficial for the passive targeting of drug-loaded nanoparticles. This concept was successfully employed in the multifunctional nanodevice of ferric oxide that facilitated the co-delivery of siRNA and daunorubicin[65].
The internalization of the drug to the specific cell or cell organelles can be achieved by surface modification of the nanoparticles with the ligand, and mesoporous silica plays a suitable platform for active targeting with the various types of ligands like antibodies[66], proteins[67], peptides[68], aptamer[69], saccharides[70], small molecules, etc. MSNPs are also used to offer intelligent gated release with response to stimuli like pH, enzyme, temperature, redox potential, etc to minimize the side effects of the drugs.
MSNPs in in vitro drug release:
The pore properties of mesoporous silica mainly the pore size, pore volume and geometry affect the drug loading and hence drug release from it. Once a drug molecule is loaded into a confined space cannot recrystallize and can retain the amorphous state of the drug. Depending on the pore size, drug loading and drug release vary[71]. As the pore size decreases, drug loading minimizes and slows the release of drugs from the tightly packed mesopores. mesopores. The drug loading in mesoporous silica occurs through the adsorption of the drug on its active surface. Adsorption occurs in different layers and is greatly affected by the method of preparation and time of incubation. The extent of drug loading in the monolayer can be calculated by Dening and Talyor equation[72].
Theoretical drug load at monolayer adsorption (% g/g)=SSA×Mw×1020/SAM×NA
Where SSA: Specific Surface Area of mesoporous silica in (m2/g); Mw: Molecular weight of the drug in g/mol; SAM: Maximum projected contact surface area of single molecule; NA: Avogadro’s Number (6.022×1023)
Pore channels and shape are also critical in drug loading, along with ordered channels exhibiting good drug loading and faster release of the drug[73]. Uniform hollow hexagonal mesopores were found to have greater drug loading and offer faster release[74,75]. The silica surface can be functionalized to control the release of the drug. Scientific research revealed that the drug release from mesoporous silica exhibit biphasic release characteristics with an initial burst, followed by sustained release. The dissolution of amorphous drug from mesoporous silica leads to the formation of a supersaturated solution which can be an effective means of improving the bioavailability of poorly soluble drugs. Improvement of solubility of ibuprofen, naproxen, erythromycin, amikacin, vancomycin, and griseofulvin to enhance the bioavailability was studied.
MSNPs as carriers for intracellular delivery of nucleic acids:
Different synthetic nucleic acids, like DNA, miRNA, siRNA, and ASO, are considered significant for modulating endogenous gene expression because of their high specificity and comparatively low toxicity. Numerous gene-targeted therapeutics with synthetic nucleic acid as a prodrug has been adopted for the treatment of many diseases like cardiovascular disease, inflammation, infection, and cancer. The negative charge on the nucleic acids makes it difficult to cross the cellular membrane possess the bigger challenges in delivery. The biocompatible MSNPs can be used as a carrier to pack the nucleic acids and deliver them into the cell and regulate the target gene expression for therapeutic effect[7].
Second-generation PAMAM(G2-PAMAM) has been reported very effective in the delivery of DNA. The DNA is complexed on the external surface of the G2-PAMAM. G2-PAMAM is loaded on the Isocyanate Propyl-functionalized MSN(ICP-MSN) suspension. After the internalization of the complex, the DNA is released from the surface and diffuses into the cytoplasm of the cell[76].
MSNPs in colon cancer:
The use of the capped MSNPs in the treatment of colon cancer was found to be very effective for oral delivery of drugs. The ability of the MSNPs to increase the solubility of the poor soluble anti-cancer drugs and efficient targeting and prevention from the premature release of the drug makes it suitable as a carrier of drugs for the treatment of colon cancer.
A nanodevice was developed with Santa Barbara Amorphous (SBA)-15 type MSNPs functionalized with Phenylacetic Acid (PAA) to trigger the release at specific pH. DOX was loaded in the mesoporous silica. Mesoporous PAA act as a gatekeeper for DOX to release at the target site. The nanodevice could control the premature release under gastric conditions while promoting the release at colonic condition pH 7.6. The system exhibited advantages like high drug loading capacity, excellent compatibility and pH-triggered response with improved solubility of DOX molecules in the colonic environment[77].
Kumar et al.[78] employed MCM-41 to increase the efficacy of the anticancer drug 5-Fluorouracil (5-FU) for the treatment of colon cancer, which triggered to release the drug at the desired enzyme system. Functionalisation was done using the natural polymer of guar gum to cargo the drug in the mesoporous channels. Degradation of the natural polymer (guar gum) in presence of colonic enzyme functioned as a gatekeeper to release the drug at the site. This investigation shows another use of MSN built system based on guar gum capping as an efficient enzyme responsive carrier[78].
MSNPs in breast cancer:
MSN has been studied to increase the solubility of the drug employed for breast cancer. Anti-cancer drugs like paclitaxel and curcumin have poor aqueous solubility and permeability which accounts for their limited bioavailability and adverse effect. To improve solubility and stability for the antitumor drugs paclitaxel and curcumin lipid bilayer-coated MSNPs were employed through the intravenous route for the treatment of breast cancer[79,80].
A pH-responsive MSN carrier of anastrozole was found to have a better-sustained drug release profile with an improved cytotoxic effect on cell lines of breast cancer[81].
MSNPs in lung cancer:
Lung cancer is classified into 2 types Non-Small Cell Lung Cancer (NSCLC) and Small cell Lung Cancer (SCLC). The NSCLC was found to be less sensitive to chemotherapy and radiation therapy. This type of lungs cancer arises on epithelial cells from bronchi to terminal alveoli. Hence, the challenges of the treatment lie here. MSNPs showed a promising effect on delivering the drugs by the inhalation route. Using MSNPs, the anticancer drugs cisplatin and DOX conjugated with BCL2 and MRP1 small interfering (si)RNAs are localized to lungs for local therapeutics effect. In vivo experimentation with murine resulted 73 % localization of MSNPs in the lungs and a small amount in the spleen, heart, kidney, and liver[82]. Hence it can lead to the development of successful treatment for delivering anticancer drugs in the lungs and for suppression of tumor.
MSNPs in brain cancer:
There are various ways developed to detect cancer in the body but particularly brain cancer is still associated with many difficulties. Malignant glioma, Glioblastoma Multiforme (GBM) is a dangerous form of cancer that is characterized by the fast injury of the brain parenchyma shows lesser resistance to chemotherapy, and a higher frequency of relapse, and a lesser survival rate. The MSNPs have been studied for the delivery of the GBM. Drug GBM was loaded in the MSNPs with protein grafted. The MSNPs were conjugated with the Transferrin (Tf), a glycoprotein on the external surface of the MSNPs with poly (d, l-lactic-co-glycolic acid) NPs of DOX. The purpose of the study was to increase the efficacy of the nanoparticles of DOX with Tf as a gatekeeper and a targeting agent to the malignant cell. This showed increased efficiency in delivering DOX compared to the conventional DOX. MSNPs containing Tf stood out to be a strong system to hinder tumor spread and reduce systemic toxicity[79,83].
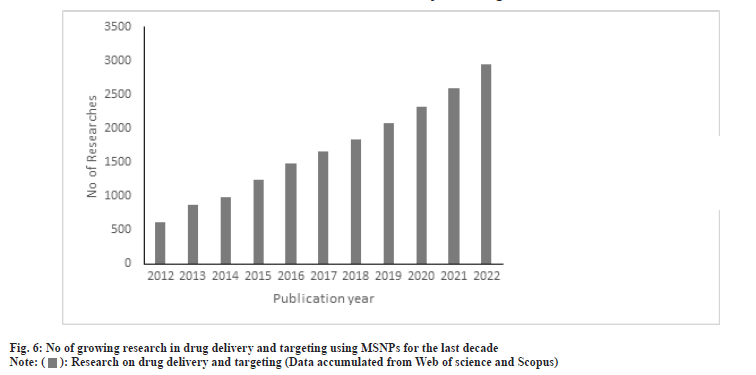
The application of MSNPs in the biomedical field has been studied widely over the last decades. The conclusion derived from the reports suggests that the toxicities depend on the characteristics of the carriers but were found to vary to derive a conclusion. Fu et al.[84] and other collaborators reported that the oral administration of MSNPs was safe in vivo, when it was administered parenterally they reported that it was deposited in the liver and spleen which was cleared off in a month when studied on mice[83-85]. Though the carrier shows tremendous potential for biocompatibility and efficacy, a lack of in-depth understanding of the interactions in human physiology needs to be addressed. In the last decade, the MSNPs showed safety in clinical trials[86]. Orally delivered ibuprofen and simvastatin showed a 1.95 and 3.5 times respectively improvement in bioavailability[87]. The oral bioavailability of fenofibrate was improved by 54 % over the conventional dosage form[88]. These data reveal that the safety of MSNPs is established for oral delivery of drugs[89-95]. The number of growing research in this field is an indication of its application in the drug delivery and targeting as analysed in fig. 6.
Conclusion
MSNPs have been profoundly studied for drug delivery and created a huge impact in the field of diagnosis and treatment of various diseases. It has shown its potential in fabrication, multifunctional nanocarrier for site-specific delivery, theranostic purpose and multiple drug loading. The unique physicochemical properties and functionalization of the surface for a triggered response make it a novel carrier to overcome the difficulties associated with the temporal and spatial placement of the drug. Compare to other inorganic nanoparticles it has shown its greater effectivity against cell penetration and is also found to be biocompatible. The invention of Cornell Dots or C Dots to trace the cancer cells in the body proves its acceptability for clinical translation. Though the remarkable outcomes of silica nanoparticles are promising, still the question lies in the reproducibility of its production on an industrial scale and the development of sensitive analytical procedures for its characterization. The non-clinical development of long-term tissue compatibility, genotoxicity, and teratogenic effect should be explored more for clinical translation. The in vivo degradation mechanism should be established to support the absence of chronic toxicity. Therefore, once the bridging between preclinical and clinical study is established, the use of MSNPs for drug delivery and pharmaceutical development will support an optimistic future in patient care.
Conflict of interest:
Authors declare that there is no conflict of interest.
References
- Krukemeyer MG, Krenn V, Huebner F, Wagner W, Resch R. History and possible uses of nanomedicine based on nanoparticles and nanotechnological progress. J Nanomed Nanotechnol 2015;6(6):336. [ Google Scholar]
- Martínez-Carmona M, Colilla M, Vallet-Regí M. Smart mesoporous nanomaterials for antitumor therapy. Nanomaterials 2015;5(4):1906-37.
[Crossref] [Google Scholar] [PubMed]
- Kwon S, Singh RK, Perez RA, Abou Neel EA, Kim HW, Chrzanowski W. Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng 2013;4:2041731413503357.
[Crossref] [Google Scholar] [PubMed]
- Qiao ZA, Zhang L, Guo M, Liu Y, Huo Q. Synthesis of mesoporous silica nanoparticles via controlled hydrolysis and condensation of silicon alkoxide. Chem Mater 2009;21(16):3823-9.
- Sun B, Zhou G, Zhang H. Synthesis, functionalization, and applications of morphology-controllable silica-based nanostructures: A review. Prog Solid State Chem 2016;44(1):1-9.
- Deodhar GV, Adams ML, Trewyn BG. Controlled release and intracellular protein delivery from mesoporous silica nanoparticles. Biotechnol J 2017;12(1):1600408.
[Crossref] [Google Scholar] [PubMed]
- Cha W, Fan R, Miao Y, Zhou Y, Qin C, Shan X, et al. Mesoporous silica nanoparticles as carriers for intracellular delivery of nucleic acids and subsequent therapeutic applications. Molecules 2017;22(5):782.
[Crossref] [Google Scholar] [PubMed]
- Haussecker D. Current issues of RNAi therapeutics delivery and development. J Control Release 2014;195:49-54.
[Crossref] [Google Scholar] [PubMed]
- Wierzbicki AS, Viljoen A. Anti-sense oligonucleotide therapies for the treatment of hyperlipidaemia. Expert Opin Biol Ther 2016;16(9):1125-34.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Wang P, Tang X, Elzatahry AA, Wang S, Al-Dahyan D, et al. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent Sci 2017;3(8):839-46.
- Taleghani AS, Nakhjiri AT, Khakzad MJ, Rezayat SM, Ebrahimnejad P, Heydarinasab A, et al. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: A review. J Mol Liquid 2021;328:115417.
- Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 1992;114(27):10834-43.
- Dilebo J. A mini review on factors affecting particle size, sphericity and pore size of mesoporous silica nanoparticles. Int J Pharm Sci Res 2020;11(2):15-20.
- Desai D, Åkerfelt M, Prabhakar N, Toriseva M, Näreoja T, Zhang J, et al. Factors affecting intracellular delivery and release of hydrophilic versus hydrophobic cargo from mesoporous silica nanoparticles on 2d and 3d cell cultures. Pharmaceutics 2018;10(4):237.
[Crossref] [Google Scholar] [PubMed]
- Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Targeted Ther 2018;3(1):7-19.
[Crossref] [Google Scholar] [PubMed]
- Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater 2014;26(1):435-51.
- Kresge AC, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992;359(6397):710-2.
- Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018;10(3):118-49.
[Crossref] [Google Scholar] [PubMed]
- Danks AE, Hall SR, Schnepp ZJ. The evolution of ‘sol–gel’chemistry as a technique for materials synthesis. Mater Horizon 2016;3(2):91-112.
- Mahoney L, Koodali RT. Versatility of evaporation-induced self-assembly (EISA) method for preparation of mesoporous TiO2 for energy and environmental applications. Materials 2014;7(4):2697-746.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Deng Y, Liu J, Wu H, Zhao D. Homopolymer induced phase evolution in mesoporous silica from evaporation induced self-assembly process. Microporous Mesoporous Mater 2008;116(1-3):633-40.
- Hung CT, Bai H. Adsorption behaviors of organic vapors using mesoporous silica particles made by evaporation induced self-assembly method. Chem Eng Sci 2008;63(7):1997-2005.
- Kumar S, Malik MM, Purohit R. Synthesis methods of mesoporous silica materials. Mater Today Proc 2017;4(2):350-7.
- Wu CG, Bein T. Microwave synthesis of molecular sieve MCM-41. Chem Commun 1996;1(8):925-6.
- Run M, Wu S, Wu G. Ultrasonic synthesis of mesoporous molecular sieve. Microporous Mesoporous Mater 2004;74(1-3):37-47.
- Huang R, Shen YW, Guan YY, Jiang YX, Wu Y, Rahman K, et al. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater 2020;116:1-5.
[Crossref] [Google Scholar] [PubMed]
- Amgoth C, Joshi S. Thermosensitive block copolymer [(PNIPAM)-b-(Glycine)] thin film as protective layer for drug loaded mesoporous silica nanoparticles. Mater Res Express 2017;4(10):105306.
- Thi TT, Nguyen TN, Hoang DT, Nguyen DH. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater Sci Eng 2019;99:631-56.
- Teng Z, Wang R, Zhou Y, Kolios M, Wang Y, Zhang N, et al. A magnetic droplet vaporization approach using perfluorohexane-encapsulated magnetic mesoporous particles for ultrasound imaging and tumor ablation. Biomaterials 2017;134:43-50.
[Crossref] [Google Scholar] [PubMed]
- Tian Z, Yu X, Ruan Z, Zhu M, Zhu Y, Hanagata N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo-and magnetic hyperthermia therapy. Microporous Mesoporous Mater 2018;256:1-9.
- Chang JH, Kim J, Lee H. PNIPAm grafted amino-functionalized mesoporous silica for thermo-responsive chromium elimination. Appl Surface Sci 2017;424:115-21.
- Le Thi TN, Nguyen TH, Hoang DQ, Tran TV, Nguyen NT, Nguyen DH. Development of new magnetic nanoparticles: Oligochitosan obtained by γ-rays and–coated Fe3O4 nanoparticles. Appl Surface Sci 2017;422:863-8.
- Chen WH, Luo GF, Lei Q, Cao FY, Fan JX, Qiu WX, et al. Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials 2016;76:87-101.
[Crossref] [Google Scholar] [PubMed]
- Shao D, Lu MM, Zhao YW, Zhang F, Tan YF, Zheng X, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater 2017;49:531-40.
[Crossref] [Google Scholar] [PubMed]
- Hegazy M, Zhou P, Wu G, Wang L, Rahoui N, Taloub N, et al. Construction of polymer coated core–shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polymer Chem 2017;8(38):5852-64.
- Azevedo RC, Sousa RG, Macedo WA, Sousa EM. Combining mesoporous silica–magnetite and thermally-sensitive polymers for applications in hyperthermia. J Sol-Gel Sci Technol 2014;72:208-18.
- Freitas FS, Gonçalves AS, de Morais A, Benedetti JE, Nogueira AF. Graphene-like MoS2 as a low-cost counter electrode material for dye-sensitized solar cells. Energy Environ Sci 2012;1:11002-3.
- Avedian N, Zaaeri F, Daryasari MP, Javar HA, Khoobi M. pH-sensitive biocompatible mesoporous magnetic nanoparticles labeled with folic acid as an efficient carrier for controlled anticancer drug delivery. J Drug Deliv Sci Technol 2018;44:323-32.
- Pourjavadi A, Tehrani ZM, Jokar S. Chitosan based supramolecular polypseudorotaxane as a pH-responsive polymer and their hybridization with mesoporous silica-coated magnetic graphene oxide for triggered anticancer drug delivery. Polymer 2015;76:52-61.
- Chen PJ, Hu SH, Hsiao CS, Chen YY, Liu DM, Chen SY. Multifunctional magnetically removable nanogated lids of Fe3O4–capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J Mater Chem 2011;21(8):2535-43.
- Manzano M, Vallet-Regí M. Ultrasound responsive mesoporous silica nanoparticles for biomedical applications. Chem Commun 2019;55(19):2731-40.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Wu J, Chen J, Jin Y, Hu T, Walters KB, Ding S. Fabrication of p H‐sensitive poly (2‐(diethylamino) ethyl methacrylate)/palygorskite composite microspheres via pickering emulsion polymerization and their release behavior. J Appl Polymer Sci 2015;132(26):1-7.
- Niedermayer S, Weiss V, Herrmann A, Schmidt A, Datz S, Müller K, et al. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale 2015;7(17):7953-64.
- Wong RC, Chow SY, Zhao S, Fong WP, Ng DK, Lo PC. pH-responsive dimeric zinc (II) phthalocyanine in mesoporous silica nanoparticles as an activatable nanophotosensitizing system for photodynamic therapy. ACS Appl Mater Interfaces 2017;9(28):23487-96.
[Crossref] [Google Scholar] [PubMed]
- Liu R, Zhang Y, Zhao X, Agarwal A, Mueller LJ, Feng P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J Am Chem Soc 2010;132(5):1500-1.
[Crossref] [Google Scholar] [PubMed]
- Paquin F, Rivnay J, Salleo A, Stingelin N, Silva C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J Mater Chem C 2015;3(207890):10715-22.
- Zhao R, Li T, Zheng G, Jiang K, Fan L, Shao J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017;143:1-6.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Luo Z, Zhang J, Luo T, Zhou J, Zhao X, et al. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016;83:51-65.
[Crossref] [Google Scholar] [PubMed]
- Tian Z, Xu Y, Zhu Y. Aldehyde-functionalized dendritic mesoporous silica nanoparticles as potential nanocarriers for pH-responsive protein drug delivery. Mater Sci Eng C Mater Biol Appl 2017;71:452-9.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Chen X, Zhang X, Gooding JJ, Zhou Y. Carbon‐quantum‐dots‐loaded mesoporous silica nanocarriers with pH‐switchable zwitterionic surface and enzyme‐responsive pore‐cap for targeted imaging and drug delivery to tumor. Adv Healthc Mater 2016;5(12):1401-7.
[Crossref] [Google Scholar] [PubMed]
- Qian R, Ding L, Ju H. Switchable fluorescent imaging of intracellular telomerase activity using telomerase-responsive mesoporous silica nanoparticle. J Am Chem Soc 2013;135(36):13282-5.
[Crossref] [Google Scholar] [PubMed]
- Srivastava P, Hira SK, Sharma A, Kashif M, Srivastava P, Srivastava DN, et al. Telomerase responsive delivery of doxorubicin from mesoporous silica nanoparticles in multiple malignancies: therapeutic efficacies against experimental aggressive murine lymphoma. Bioconjug Chem 2018;29(6):2107-19.
[Crossref] [Google Scholar] [PubMed]
- Geng J, Li M, Wu L, Chen C, Qu X. Mesoporous silica nanoparticle‐based H2O2 responsive controlled‐release system used for Alzheimer's disease treatment. Adv Healthc Mater 2012;1(3):332-6.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Yin T, Liu Y, Sun J, Zhou Y, Liu J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater 2016;46:177-90.
[Crossref] [Google Scholar] [PubMed]
- Šoltys M, Kovačík P, Dammer O, Beránek J, Štěpánek F. Effect of solvent selection on drug loading and amorphisation in mesoporous silica particles. Int J Pharm 2019;555:19-27.
- Pagar OB, Nagare HS, Chine YM, Autade RR, Narode PR, Sanklecha VM. Mesoporous silica: A Review. Int J Pharm Drug Anal 2018;6(2):1-12.
- Seljak KB, Kocbek P, Gašperlin M. Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J Drug Deliv Sci Technol 2020;59:101906.
- Ambrogi V, Perioli L, Pagano C, Marmottini F, Ricci M, Sagnella A, et al. Use of SBA-15 for furosemide oral delivery enhancement. Eur J Pharm Sci 2012;46(1-2):43-8.
[Crossref] [Google Scholar] [PubMed]
- Trzeciak K, Kaźmierski S, Wielgus E, Potrzebowski MJ. DiSupLo-New extremely easy and efficient method for loading of active pharmaceutical ingredients into the pores of MCM-41 mesoporous silica particles. Microporous Mesoporous Mater 2020;308:110506.
- Niu X, Wan L, Hou Z, Wang T, Sun C, Sun J, et al. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int J Pharm 2013;452(1-2):382-9.
[Crossref] [Google Scholar] [PubMed]
- Waters LJ, Hussain T, Parkes G, Hanrahan JP, Tobin JM. Inclusion of fenofibrate in a series of mesoporous silicas using microwave irradiation. Eur J Pharm Biopharm 2013;85(3):936-41.
[Crossref] [Google Scholar] [PubMed]
- Abu-Zied BM, Schwieger W, Asiri AM. Effect of ball milling on the structural and textural features of MCM-41 mesoporous material. Microporous Mesoporous Mater 2015;218:153-9.
- Castillo RR, Lozano D, González B, Manzano M, Izquierdo-Barba I, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin Drug Deliv 2019;16(4):415-39.
[Crossref] [Google Scholar] [PubMed]
- He Q, Zhang J, Shi J, Zhu Z, Zhang L, Bu W, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials 2010;31(6):1085-92.
[Crossref] [Google Scholar] [PubMed]
- Encinas N, Angulo M, Astorga C, Colilla M, Izquierdo-Barba I, Vallet-Regí M. Mixed-charge pseudo-zwitterionic mesoporous silica nanoparticles with low-fouling and reduced cell uptake properties. Acta Biomater 2019;84:317-27.
[Crossref] [Google Scholar] [PubMed]
- Ngamcherdtrakul W, Sangvanich T, Reda M, Gu S, Bejan D, Yantasee W. Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int J Nanomed 2018:4015-27.
[Crossref] [Google Scholar] [PubMed]
- Montalvo-Quiros S, Aragoneses-Cazorla G, Garcia-Alcalde L, Vallet-Regí M, González B, Luque-Garcia JL. Cancer cell targeting and therapeutic delivery of silver nanoparticles by mesoporous silica nanocarriers: Insights into the action mechanisms using quantitative proteomics. Nanoscale 2019;11(10):4531-45.
- Lee J, Oh ET, Choi MH, Kim HG, Park HJ, Kim C. Dual-functional cyclic peptide switch on mesoporous nanocontainers for selective CD44 targeting and on–off gatekeeping triggered by conformational transformation. New J Chem 2018;42(15):12938-44.
- Wang K, Yao H, Meng Y, Wang Y, Yan X, Huang R. Specific aptamer-conjugated mesoporous silica–carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater 2015;16:196-205.
[Crossref] [Google Scholar] [PubMed]
- Shahin SA, Wang R, Simargi SI, Contreras A, Echavarria LP, Qu L, et al. Hyaluronic acid conjugated nanoparticle delivery of siRNA against TWIST reduces tumor burden and enhances sensitivity to cisplatin in ovarian cancer. Nanomedicine 2018;14(4):1381-94.
[Crossref] [Google Scholar] [PubMed]
- Horcajada P, Ramila A, Pérez-Pariente J, Vallet-Regı M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater 2004;68(1-3):105-9.
- Le TT, Elzhry Elyafi AK, Mohammed AR, Al-Khattawi A. Delivery of poorly soluble drugs via mesoporous silica: Impact of drug overloading on release and thermal profiles. Pharmaceutics 2019;11(6):269.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Sun J, Zhang L, Wang J, Ren B. Influence of different structured channels of mesoporous silicate on the controlled ibuprofen delivery. Mater Chem Phys 2012;135(2-3):786-97.
- Zhu YF, Shi JL, Li YS, Chen HR, Shen WH, Dong XP. Hollow mesoporous spheres with cubic pore network as a potential carrier for drug storage and its in vitro release kinetics. J Mater Res 2005;20(1):54-61.
- Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release 2010;145(3):257-63.
[Crossref] [Google Scholar] [PubMed]
- Fang IJ, Trewyn BG. Application of mesoporous silica nanoparticles in intracellular delivery of molecules and proteins. Methods Enzymol 2012;508:41-59.
[Crossref] [Google Scholar] [PubMed]
- Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, et al. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf B Biointerface 2017;154:287-96.
- Kumar B, Kulanthaivel S, Mondal A, Mishra S, Banerjee B, Bhaumik A, et al. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf B Biointerface 2017;150:352-61.
- Alyassin Y, Sayed EG, Mehta P, Ruparelia K, Arshad MS, Rasekh M, et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov Today 2020;25(8):1513-20.
[Crossref] [Google Scholar] [PubMed]
- Lin J, Cai Q, Tang Y, Xu Y, Wang Q, Li T, et al. PEGylated Lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: Design, characterization and its cytotoxic effect. Int J Pharm 2018;536(1):272-82.
[Crossref] [Google Scholar] [PubMed]
- Bhavsar D, Gajjar J, Sawant K. Formulation and development of smart pH responsive mesoporous silica nanoparticles for breast cancer targeted delivery of anastrozole: In vitro and in vivo characterizations. Microporous Mesoporous Mater 2019;279:107-16.
- Taratula O, Garbuzenko OB, Chen AM, Minko T. Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target 2011;19(10):900–14. [Crossref] [Google Scholar] [PubMed]
- Chen Y, Chen H, Shi J. In vivo bio‐safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater 2013;25(23):3144-76.
[Crossref] [Google Scholar] [PubMed]
- Fu C, Liu T, Li L, Liu H, Chen D, Tang F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013;34(10):2565-75.
[Crossref] [Google Scholar] [PubMed]
- Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol 2012;25(11):2265-84.
- Janjua TI, Cao Y, Yu C, Popat A. Clinical translation of silica nanoparticles. Nat Rev Mater 2021;6(12):1072-4.
- Tan A, Eskandar NG, Rao S, Prestidge CA. First in man bioavailability and tolerability studies of a silica–lipid hybrid (Lipoceramic) formulation: A Phase I study with ibuprofen. Drug Deliv Transl Res 2014;4:212-21.
[Crossref] [Google Scholar] [PubMed]
- Bukara K, Schueller L, Rosier J, Martens MA, Daems T, Verheyden L, et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur J Pharm Biopharm 2016;108:220-5.
[Crossref] [Google Scholar] [PubMed]
- Pham SH, Choi Y, Choi J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 2020;12(7):630.
[Crossref] [Google Scholar] [PubMed]
- Knežević NŽ, Trewyn BG, Lin VS. Light‐and pH‐responsive release of doxorubicin from a mesoporous silica‐based nanocarrier. Chemistry 2011;17(12):3338-42.
[Crossref] [Google Scholar] [PubMed]
- Li T, Shi S, Goel S, Shen X, Xie X, Chen Z, et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater 2019;89:1-3.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Peng J, Liu Z, Zou H, Wu RA. In situ and timed extraction of cellular peptides from live HeLa cells by photo-switchable mesoporous silica nanocarriers. Anal Chem 2016;88(17):8380-4.
[Crossref] [Google Scholar] [PubMed]
- Beňová E, Zeleňák V, Halamová D, Almáši M, Petrul'Ová V, Psotka M, et al. A drug delivery system based on switchable photo-controlled p-coumaric acid derivatives anchored on mesoporous silica. J Mater Chem 2017;5(4):817-25.
- Wang F, Xu W, Ouyang Y, Zhang L, Liu H. Reversible crosslinking terpolymer shell-based mesoporous silica nanoparticles as on-off nanocarriers for pyrene-releasing application. J Taiwan Inst Chem Eng 2018;91:578-87.
- Zeleňák V, Beňová E, Almáši M, Halamová D, Hornebecq V, Hronský V. Photo-switchable nanoporous silica supports for controlled drug delivery. New J Chem 2018;42(16):13263-71.