- *Corresponding Author:
- Biqian Dong
Department of Anesthesiology,
Affiliated Hospital of North Sichuan Medical College,
Nanchong 637000, China
E-mail: dongbiqian@nsmc.edu.cn
| This article was originally published in a special issue, “Diagnostic and Therapeutic Advances in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:83(5) spl issue “185-194” |
Abstract
Adopt meta-analysis method to study the relationship between troponin I and prognosis of sepsis patients and to explore the predictive value of troponin I on prognosis of sepsis patients. In PubMed, EMBASE and Ovid MEDLINE, the clinical studies published as of September 2021 were comprehensively searched and screened through inclusion and exclusion criteria. The qualified clinical studies were incorporated to conduct quality evaluation and data extraction. Adopt RevMan 5.3 software for meta-analysis and evaluate the relationship between the troponin I level and indicators such as the mortality rate, length of hospital stay in patients with sepsis. The statistical results are represented by forest plots and the publication bias is tested by funnel plots. This research contains 14 independent studies and 3104 patients participated in the study. Meta-analysis results showed that compared with patients with sepsis in the troponin I-negative group; The mortality of patients in the troponin I positive group was significantly higher [odds ratio=2.21, 95 % confidence interval (1.88, 2.61), p<0.001]; No significant difference was found in incidence of septic shock in troponin I-positive group [odds ratio=1.32, 95 % confidence interval (0.65, 2.70), p=0.44]; It possessed no significant difference in length of hospital stay in troponin I-positive group [standardized mean difference=0.08, 95 % confidence interval] (-0.10, 0.25), p=0.39]. Compared with troponin I-negative sepsis patients, troponin I-positive sepsis patients have a higher fatality rate; however, the incidence of septic shock and the length of hospitalization between the two groups were not significantly different. Elevated troponin I has certain predictive value for prognosis of sepsis patients.
Keywords
Troponin I, sepsis, prognosis, meta-analysis
Sepsis is a common critical illness in Intensive Care Unit (ICU). It is often induced by severe trauma, burns, major surgery, infection, etc., which can lead to septic shock and multiple organ dysfunction syndrome; its fatality rate is high and it seriously affects the quality of life of patients[1]. In 2017, an estimated 489 million sepsis events were recorded globally and 11 million sepsis-related deaths were reported, accounted for 19.7 % of global deaths[2]. About 20 % of these surviving patients have cognitive dysfunction[3]. Sepsis patient’s conditions develop rapidly. Diagnosis and treatment technology and monitoring measures continue to progress, but the incidence and fatality rate of sepsis are still high, which is a prominent problem facing the global medical community[4]. Early recognition, evaluation and appropriate treatment are the keys to reducing deaths and improving prognosis in sepsis patients. The pathogenesis of sepsis is complex, involving changes in the function of multiple organs in the body[5]. Previous studies have shown that the pathogenesis of sepsis includes inflammatory response imbalance, immune dysfunction, abnormal blood coagulation, neuroendocrine-immune network, mitochondrial damage, endoplasmic reticulum stress, autophagy, gene polymorphism, etc.[6-13].
Cardiac Troponin I (cTnI) is an important regulator of myocardial muscle contraction. It is one of the most sensitive and specific markers of cardiomyocyte injury. It can detect slight myocardial injury and is the main biochemical index for rapid diagnosis of acute myocardial infarction and acute coronary syndrome[14,15]. Cardiac dysfunction is an extremely common complication among severe sepsis and septic shock patients. Studies have shown that about 40- 50 % of patients with sepsis will have myocardial inhibition[16]. Inflammatory factors produced by the body of patients with sepsis, such as tumor necrosis factor and interleukin, these myocardial inhibitors mediate the apoptosis of cardiomyocytes and the lysis of cTnI through the activation of enzymes, leading to cTn I in the blood increased. The superoxide released by white blood cells during the progress of sepsis can also cause the destruction of myocardial cells and increase cTnI. In septic shock, the ischemia and hypoxia of the myocardium caused by changes in microcirculation can also cause the destruction of cardiomyocytes. Elevated cTnI is an important indicator of cardiac dysfunction in patients with sepsis and multiple studies have shown that elevated cTnI can independently predict the prognosis of patients with sepsis, but there are also studies that hold the opposite argument[17], which may have different conclusions due to different sample sizes and regions. Therefore, this study adopted meta-analysis methods, based on the retrieval of relevant literature on cTnI and the prognosis of sepsis patients, comprehensively and meticulously explored the relationship between cTnI and the prognosis of sepsis patients.
Materials and Methods
Retrieval strategy:
We conducted a review of systems and meta-analysis. On PubMed, EMBASE and Ovid MEDLINE we conducted a systematic literature search to determine all studies involving humans published as of September 2021, comparing the outcomes of sepsis patients with elevated and non-elevated cTn. The search criteria combine the medical subject terms "troponin" and "sepsis". Search has no language limit. Identify and review articles related to this systematic review based on the title. The reviewed articles are obtained from the electronic search and the relevant articles in the reference list are also searched. Preliminarily screen the obtained articles and read the abstracts. According to the pre-determined literature inclusion and exclusion criteria, articles and full texts that meet the inclusion criteria are obtained. Two researchers will conduct specific evaluations of the obtained articles. If there are objections to the same research evaluation, the third evaluator will evaluate whether to include the article and obtain the final included article. And two researchers independently extracted relevant data for each study. When the included data are inconsistent, the third researcher decides whether to include it. When the data is missing or incomplete, contact the first author or corresponding author to obtain the relevant data and the research that fails to obtain the relevant data will be excluded.
Inclusion and exclusion criteria:
Inclusion criteria: All published articles; the diagnosis and inclusion criteria of sepsis in the literature should meet the diagnostic standard of American College of Chest Physicians/Society of Critical Care Medicine; a unified standard cTnI determination was performed on the included subjects; complete data can be extracted.
Exclusion criteria: Review, meta-analysis, conference abstracts, case reports, animal studies and other nonclinical research literature; duplicate literature, research reports on the same cohort of people in different databases or different languages; literature without full text; literature for which complete data cannot be obtained.
Literature quality evaluation:
As the included literatures are cohort studies or case-control studies, a literature quality evaluation scale, Newcastle-Ottawa Scale (NOS) was used for evaluation. NOS is evaluated in three dimensions, namely selection, comparability and exposure. The score ranges from 0 (lowest) to 9 (highest). A score ≥7 indicates that the quality of the literature is high. This study included literature with a score of ≥7.
Data extraction:
Use Microsoft Excel 2003 software to create the following table, extract and record the data. Basic information table includes basic information such as first author, age, country, research design type, sample size, etc. Data table which uses the literature author’s years as a label, record the number of cTnI elevated and normal groups, the number of deaths, the length of hospital stay and the number of patients requiring vasoactive drugs, mechanical ventilation and continuous renal replacement therapy during hospitalization period, literature quality evaluation scale.
Statistical analysis:
Use RevMan 5.3 software for meta-analysis. Dichotomous variables adopt Odds Ratio (OR) or Relative Risk (RR) as the effect size. Continuous variables use standard mean difference or weighted mean difference as the effect size. Use the Q test to test and analyze the heterogeneity among the studies. When the p>0.1, the heterogeneity among the independent studies is small. At this time, we adopted fixed effect model to calculate the combined comprehensive effect; if the p≤0.1, it is considered that the test results are significantly different and the heterogeneity among the studies is relatively large. If there is heterogeneity among the research objects, the I2 index can be used to quantitatively analyze the heterogeneity. When I2≤50 %, it can be considered that there is no heterogeneity among the independent research results (I2=0) and the fixed effect model can be selected; When I2>50 %, it means that the heterogeneity among groups is large, it is unacceptable. At this time, the reasons for the heterogeneity should be analyzed, mainly as follows.
Clinical heterogeneity is the difference in object characteristics, diagnosis, intervention, evaluation outcomes and other factors. Methodological heterogeneity is the difference in research design and research quality. Statistical heterogeneity refers to the effect observed in each independent study, whose variability exceeds the variability caused by the opportunity itself. When the heterogeneity is obvious, according to the different sources of heterogeneity, methods such as subgroup analysis or sensitivity analysis can be selected to explain the reasons for the heterogeneity. After the analysis of heterogeneity, the study believes that the combined data still has important clinical significance and the random effects model can be selected to calculate the combined effect. Meta-analysis results are represented by forest plot and funnel plot analysis is used to test publication bias.
Literature search results:
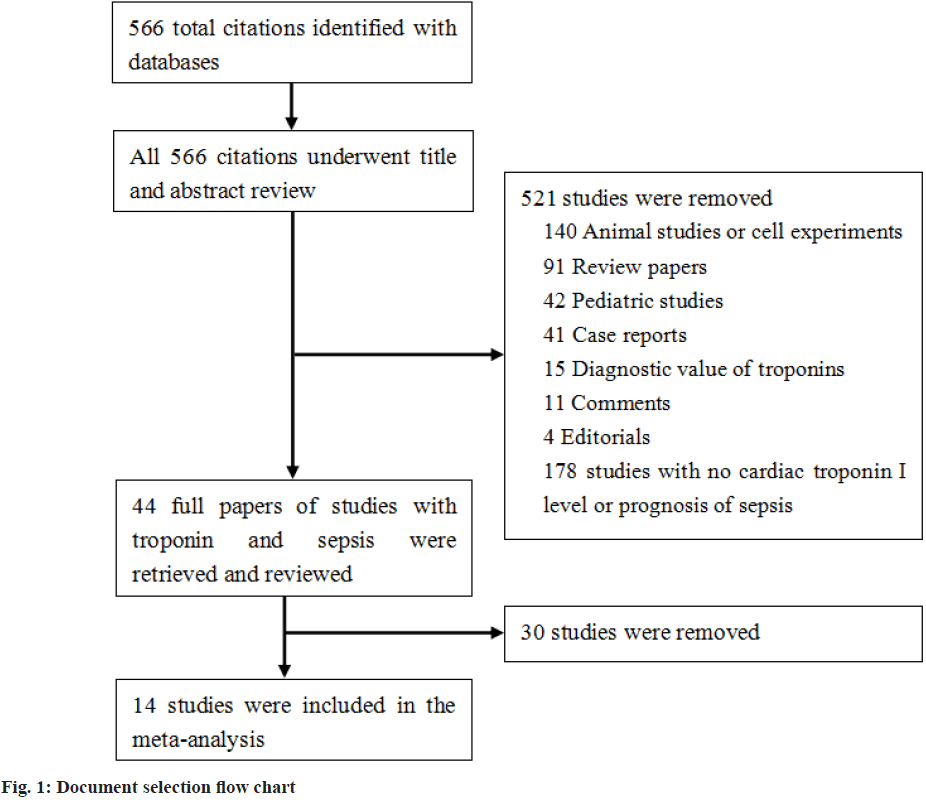
A total of 566 literatures were retrieved in PubMed, EMBASE, Ovid and MEDLINE. 44 literatures that met the inclusion criteria were selected by excluding duplicate literature and reading the titles and abstracts of literature. After reading the full text, the documents that did not meet the requirements were eliminated and 14 literatures were finally adopted as shown in literature screening process in fig. 1.
Baseline characteristics of included studies:
14 studies were included[18-31], whose publication year was between 2001-2021; the sample size ranged from 20-1124, with a total of 3104 patients; the average age range was 55-76 y old; males were 43.80-77.45 %. 10 studies were prospective studies and 4 studies were retrospective analysis (Table 1).
| Author and year | Research design type | Total number of cases | Study group and number of cases | Age | Gender (male) | Observation index | study area | setting | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Abdalla et al.[18] | Retrospective analysis | 125 | Troponin-I positive (n=45)/Troponin-I negative (n=80) | 74.4/70.3 | 29/50 | Septic shock=28/35; Hospital mortality=11/12; Duration of mechanical ventilation in hours=55.6±24.8/49.6±33; ICU length of stay in days=4.6±3.1/4.3±3; Hospital length of stay in days=9.7±6/8.4±4.4 | USA | ICU | 8 |
| Ammann et al.[19] | Prospective study | 20 | Troponin-I positive (n=17)/Troponin-I negative (n=3) | 66.15±8.16 | 13/2 | mortality=5/1 | Switzerland | ICU | 7 |
| Ammann et al.[20] | Prospective study | 58 | Troponin-I positive (n=28)/Troponin-I negative (n=30) | 55±21 | 28 | D 30 mortality=13/3 | Switzerland | ICU | 7 |
| Bouhemad et al.[21] | Prospective study | 54 | Troponin-I positive (n=22)/Troponin-I negative (n=32) | 56±17 | 17/24 | D 10 mortality=6/3 | France | ICU | 7 |
| Brivet et al.[22] | Prospective study | 145 | Troponin-I positive (n=69)/Troponin-I negative (n=76) | - | - | Hospital mortality=34/20 | France | ICU | 7 |
| Frencken et al.[23] | Prospective study | 1124 | Elevated High-Sensitive cTn T (hs-cTnT) on Admission (n=673)/Normal hs-cTnI on Admission (n=451) | 65 (53-73)/60 (49-68) | 397/287 | ICU mortality=153/54; Hospital mortality=238/99; 1-year mortality=342/167 | Netherlands | ICU | 7 |
| Innocenti et al.[24] | Prospective study | 256 | Troponin-I levels increased (n=114)/normal Troponin-I level (n=142) | 76±11/70±14 | 64/82 | septic shock=49/44; D 7 mortality=20/13; D 28 mortality=39/29 | Italy | Emergency Department High-Dependency Unit | 8 |
| John et al.[25] | Retrospective analysis | 105 | Troponin-I positive (n=48)/Troponin negative (n=57) | 57.3±15/56.7±14.3 | 31/31 | ICU mortality=25/17; D 28 mortality=28/22 | USA | ICU | 8 |
| John et al.[26] | Prospective study | 598 | Troponin-I positive (n=451)/Troponin negative (n=147) | 61.5±17.0/56.3±18.4 | 252/81 | D 28 mortality=145/20 | 164 centers in 11 countries | ICU | 8 |
| Kang et al.[27] | Prospective study | 121 | elevated cTnI group (n=50)/lower cTnI group (n=71) | 67±12/66±12 | 22/31 | Septic shock=6/24; within 90 d mortality=30/25 | Korea | Internal medicine | 7 |
| Mehta et al.[28] | Prospective study | 37 | cTnI positive (n=16)/cTnI negative (n=21) | 69.3±14.5/67.9±14 | 08/12 | Mortality=10/5; Length of stay in ICU (days)=9.8±6.5/4.71±2.2; Mechanical ventilation=11/10 | USA | ICU | 8 |
| Scott et al.[29] | Prospective study | 66 | Troponin-I Elevated (n=42)/Troponin-I Normal (n=24) | 67.2±18/59.9±16 | 26/13 | Mortality=12/5; Hospital days=33.0±28/37.3±28; Ventilator days=13.2±14/15.2±15 | USA | ICU | 8 |
| Tiruvoipati et al.[30] | Retrospective analysis | 293 | Elevated troponin-I group (n=165)/Low troponin-I group (n=128) | 66.5(57-76)/73(63-79) | 80/67 | Septic shock=92/47; ICU mortality=42/14; Hospital mortality=60/19; ICU length of stay (days)=4 (1-7)/3 (2-6); Hospital length of stay (days)=10(5-23)/11(6-23) | Australia | ICU | 8 |
| Wu et al.[31] | Retrospective analysis | 102 | TnI high expression group (n=46)/low expression group (n=56) | 74.4/70.3 | 29/50 | 1 y survival rate: 87.12 %/95.60 % (Mortality=4/3); 2 y survival rate: 71.08 %/89.81 % (Mortality=14/6); 3 y survival rate: 44.66 %/66.19 % (Mortality=26/19) | China | ICU | 7 |
Table 1: Baseline Characteristics That Was Included in Studies
Meta-analysis:
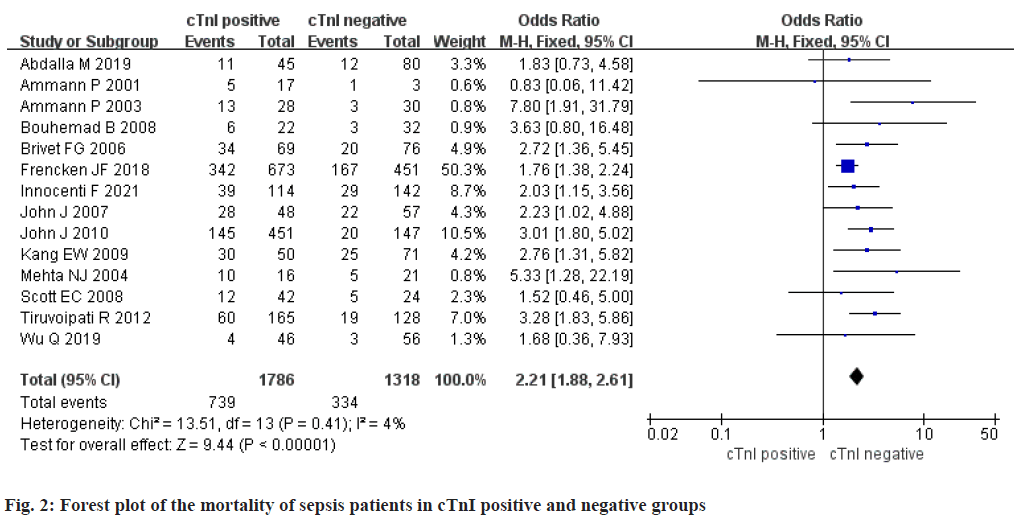
Mortality of sepsis patients in cTnI positive group and negative group: A total of 14 studies were included. There were 1786 cTnI positive patients and 1318 negative patients. Study data had no statistical heterogeneity (p>0.1 and I2<50 %), so we adopted fixed effect model. Meta-analysis result in the mortality rate of cTnI positive group was higher than negative group, they were remarkably different [OR=2.21, Confidence Interval (CI) (1.88, 2.61), Z=9.44, p< 0.001] (fig. 2).
Incidence of Septic Shock in Patients with Sepsis in cTnI positive group and negative group:
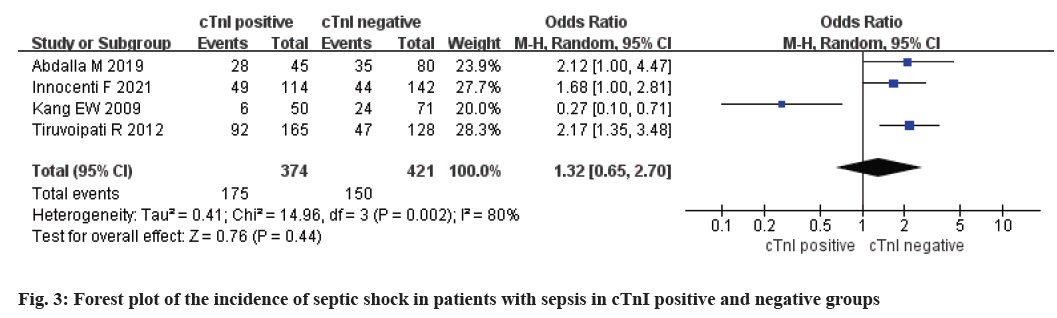
A total of 4 studies were included. There were 374 patients with positive cTnI and 421 patients with negative cTnI. The study data was statistically heterogeneous (p<0.1 and I2>50 %), so we adopted random effect model. Meta-analysis results in neither cTnI positive group nor cTnI negative group possessed no significant difference in septic shock incidence [OR=1.32, 95 % CI (0.65, 2.70), Z=0.74, p=0.44] (fig. 3).
Lengths of hospital stay of sepsis patients in cTnI positive group and negative group:
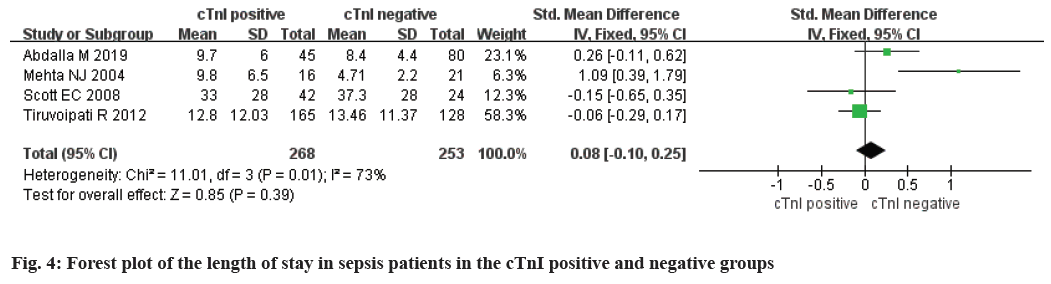
A total of 4 studies were included. There were 268 cTnI positive patients and 253 negative patients. The study data was statistically heterogeneous (p<0.1 and I2>50 %), so we adopted random effect model. Metaanalysis results in neither cTnI positive group nor cTnI negative group possessed no significant difference in hospitalization length [Standardized Mean Difference (SMD)=0.08, 95 % CI (-0.10, 0.25), Z=0.85, p=0.39] (fig. 4).
Risk assessment of bias:
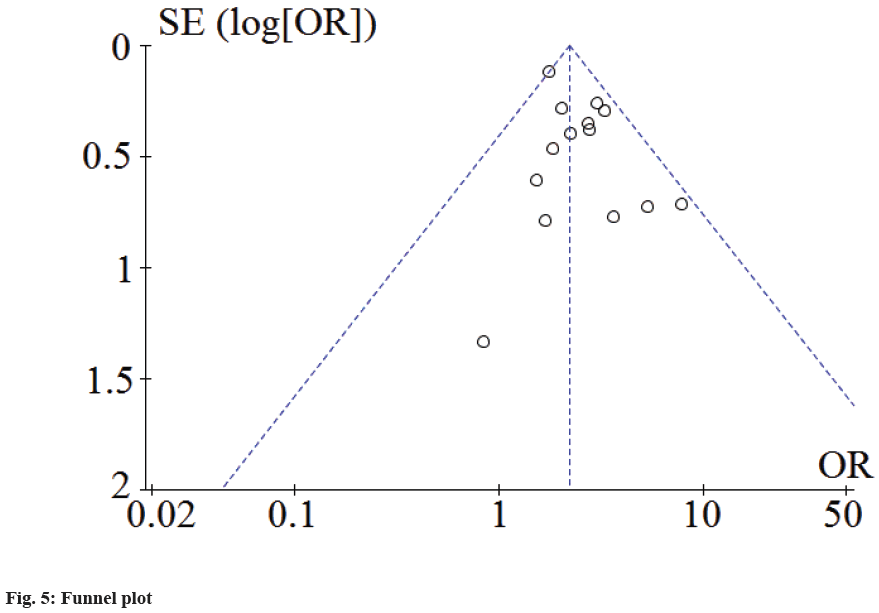
The prognosis of sepsis patients in cTnI positive group and negative group were included in the study for funnel plot analysis. The results showed that all studies fell within or above the virtual 95 % CI line, indicating that the study had a low risk of bias, as shown in fig. 5.
cTnI is one of the biomarkers for predicting myocardial damage and the increase in serum cTnI level indicates the higher the degree of myocardial damage in patients[32]. In addition to evaluating the prognosis of cardiovascular diseases, cTnI is also used for the evaluation of other diseases. For example, cTnI is not only used for the early diagnosis of acute myocardial infarction[33], but also can predict the 180 d mortality risk of cancer patients in the emergency department[34]. Among patients with sepsis, the proportion of patients with elevated cTnI is also very high[35] and its value in judging the prognosis of sepsis is also controversial. This meta-analysis is to collate the results of previous studies and use evidence-based medicine to systematically evaluate the relationship between positive (elevated) cTnI and the prognosis of sepsis.
Discussion
This research contained 14 studies, 3104 samples in total. The meta-analysis results proved that the fatality rate of sepsis patients with cTnI positive was significantly increased (OR=2.21, 95 % CI: 1.88- 2.61, Z=9.44, p<0.001), elevated cTnI indicated the possibility of myocardial injury and cardiac dysfunction in patients. The study of Scott et al.[29] showed that sepsis patients with elevated cTnI had lower cardiac index. Mehta et al.[28] also found that the left ventricular ejection fraction of sepsis patients in the cTnI elevated group was remarkably lower than cTnI normal group and the probability of abnormal ventricular wall motion was higher. Innocenti et al.[24] proved that the survival rate of sepsis patients with myocardial dysfunction was significantly lower than that of sepsis patients without myocardial dysfunction. It shows that cTnI has important predictive value for the cardiac function and prognosis of patients with sepsis. Early determination of cTnI has a suggestive significance for reducing heart dysfunction events in patients with sepsis and improving the prognosis of sepsis patients. In addition to suggesting the presence of myocardial injury, cTnI also predicts the possibility of severe complications such as septic myocardial depression, heart failure, acute coronary syndrome, acute pulmonary embolism and even multiple organ failure. These complications aggravate the severity of the patient's disease, leading to an increase in the mortality rate of the patient. The results of studies by Koszta et al.[36], Roch et al.[37], Cheng et al.[38] and Huang et al.[39] all showed that the cTnI level of patients who died of sepsis was remarkably higher than surviving patients; The study by John et al.[26] also showed that cTnI could be used as a predictor of mortality in severe sepsis patients, suggesting that sepsis patients with elevated cTnI had a higher hospital mortality rate and ICU mortality rate. Meta-analysis results also showed that elevated cTnI did not increase septic shock incidence and length of stay in sepsis patients, which may be caused by small number of included studies. The results of Abdalla et al.[18] proved that elevated cTnI increased the time of mechanical ventilation in sepsis patients, but Scott et al.[29] showed that the elevated cTnI did not increase the time of mechanical ventilation in sepsis patients. Sepsis is prone to myocardial damage and impaired cardiac function may also affect the function of the lungs through the heart-lung interaction, which can cause circulatory and respiratory system disorders, making patients more prone to pulmonary edema and lung infections, resulting in the extension of mechanical ventilation time. Elevated cTnI may not increase the possibility of respiratory failure in patients with sepsis, but it will affect the prognosis of sepsis patients.
Conclusion
In patients with sepsis, the elevated cTnI indicates a higher probability of a poor prognosis. Early detection of cTnI levels in patients with sepsis has important prompting value for the evaluation of their prognosis. It can detect myocardial dysfunction in patients with sepsis as soon as possible and then adjust treatment strategies in time and carry out effective interventions to reduce the mortality of patients in hospital.
Authors' contributions:
Biqian Dong and Wanqiu Fan have contributed equally to this work and share first authorship.
Conflict of interests:
The authors declared no conflicts of interest.
References
- van Wagenberg L, Witteveen E, Wieske L, Horn J. Causes of mortality in ICU-acquired weakness. J Intensive Care Med 2020;35(3):293-6.
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020;395(10219):200-11.
- Perner A, Cecconi M, Cronhjort M, Darmon M, Jakob SM, Pettilä V, et al. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med 2018;44(6):791-8.
- Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: A review of advances in management. Adv Ther 2017;34(11):2393-411.
- Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet 2018;392(10141):75-87.
- Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol 2013;34(3):129-36.
- Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis 2019;19(12):e422-36.
- Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med 2004;30(6):1032-40.
- Ren C, Yao RQ, Zhang H, Feng YW, Yao YM. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflammation 2020;17(1):1-14.
- Sun J, Zhang J, Tian J, Virzì GM, Digvijay K, Cueto L, et al. Mitochondria in sepsis-induced AKI. J Am Soc Nephrol 2019;30(7):1151-61.
- Chen X, Wang Y, Xie X, Chen H, Zhu Q, Ge Z, et al. Heme oxygenase-1 reduces sepsis-induced endoplasmic reticulum stress and acute lung injury. Mediators Inflamm 2018;2018:9413876.
- Choi ME. Autophagy in kidney disease. Annu Rev Physiol 2020;82:297-322.
- Hu P, Chen Y, Pang J, Chen X. Association between IL-6 polymorphisms and sepsis. Innate Immun 2019;25(8):465-72.
- ?irin G, Borlu F. Is Cardiac Troponin I Valuable to Detect Low-Level Myocardial Damage in Congestive Heart Failure? Sisli Etfal Hastan Tip Bul 2019;53(2):172-8.
- Xia C, Zhou D, Su Y, Zhou G, Yao L, Sun W, et al. A liquid-crystal-based immunosensor for the detection of cardiac troponin I. Analyst 2020;145(13):4569-75.
- Fernandes Jr CJ, Akamine N, Knobel E. Myocardial depression in sepsis. Shock 2008;30(7):14-7.
- Tiruvoipati R, Sultana N, Lewis D. Cardiac troponin I does not independently predict mortality in critically ill patients with severe sepsis. Emerg Med Australas 2012;24(2):151-8.
- Abdalla M, Sohal S, Baha’a Al-Azzam WM. Effect of troponin I elevation on duration of mechanical ventilation and length of intensive care unit stay in patients with Sepsis. J Clin Med Res 2019;11(2):127-32.
- Ammann P, Fehr T, Minder E, Günter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med 2001;27(6):965-9.
- Ammann P, Maggiorini M, Bertel O, Haenseler E, Joller-Jemelka HI, Oechslin E, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003;41(11):2004-9.
- Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby JJ. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med 2008;36(3):766-74.
- Brivet FG, Jacobs FM, Colin P, Prat D, Grigoriu B. Cardiac troponin level is not an independent predictor of mortality in septic patients requiring medical intensive care unit admission. Crit Care 2006;10(1):1-404.
- Frencken JF, Donker DW, Spitoni C, Koster-Brouwer ME, Soliman IW, Ong DS, et al. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes 2018;11(2):e004040.
- Innocenti F, Palmieri V, Stefanone VT, D’Argenzio F, Cigana M, Montuori M, et al. Comparison of Troponin I levels versus myocardial dysfunction on prognosis in sepsis. Intern Emerg Med 2021:1-9.
- John J, Awab A, Norman D, Dernaika T, Kinasewitz GT. Activated protein C improves survival in severe sepsis patients with elevated troponin. Intensive Care Med 2007;33(12):2122-8.
- John J, Woodward DB, Wang Y, Yan SB, Fisher D, Kinasewitz GT, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care 2010;25(2):270-5.
- Kang EW, Na HJ, Hong SM, Shin SK, Kang SW, Choi KH, et al. Prognostic value of elevated cardiac troponin I in ESRD patients with sepsis. Nephrol Dial Transplant 2009;24(5):1568-73.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004;95(1):13-7.
- Scott EC, Ho HC, Yu M, Chapital AD, Koss W, Takanishi Jr DM. Pre-existing cardiac disease, troponin I elevation and mortality in patients with severe sepsis and septic shock. Anaesth Intensive Care 2008;36(1):51-9.
- Tiruvoipati R, Sultana N, Lewis D. Cardiac troponin I does not independently predict mortality in critically ill patients with severe sepsis. Emerg Med Australas 2012;24(2):151-8.
- Wu Q, Xiao Z, Pu Y, Zhou J, Wang D, Huang Z, et al. TnI and IL-18 levels are associated with prognosis of sepsis. Postgrad Med J 2019;95(1123):240-4.
- Adamcova M, Šterba M, Šimunek T, Potacova A, Popelova O, Mazurova Y, et al. Troponin as a marker of myocardiac damage in drug-induced cardiotoxicity. Expert Opin Drug Saf 2005;4(3):457-72.
- Boeddinghaus J, Twerenbold R, Nestelberger T, Badertscher P, Wildi K, Puelacher C, et al. Clinical validation of a novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem 2018;64(9):1347-60.
- Park SH, Kim T, Cha WC, Yoon H, Hwang SY, Shin TG, et al. Cardiac troponin I predicts clinical outcome of patients with cancer at emergency department. Clin Cardiol 2020;43(12):1585-91.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000;46(5):650-7.
- Koszta G, Kacska Z, Szatmári K, Szerafin T, Fülesdi B. Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac surgery. J Anesth 2012;26(6):812-21.
- Roch A, Allardet-Servent J, Michelet P, Oddoze C, Forel JM, Barrau K, et al. NH2 terminal pro-brain natriuretic peptide plasma level as an early marker of prognosis and cardiac dysfunction in septic shock patients. Crit Care Med 2005;33(5):1001-7.
- Cheng H, Fan WZ, Wang SC, Liu ZH, Zang HL, Wang LZ, et al. N-terminal pro-brain natriuretic peptide and cardiac troponin I for the prognostic utility in elderly patients with severe sepsis or septic shock in intensive care unit: A retrospective study. J Crit Care 2015;30(3):654-e9.
- Huang J, Zhou Y, Liu H. Construction of death early-warning model for patients with septic myocardial depression: a retrospective analysis of 129 patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018;30(5):461-5.