- *Corresponding Author:
- J. L. Jiang
Rehabilitation Medicine Center, West China Hospital of Sichuan University, Chengdu, Sichuan 611135, China
E-mail: zjy2011zjh@126.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “182-190” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The analysis yielded significant revelations regarding the effectiveness and safety of utilizing diclofenac sodium as a treatment choice for knee osteoarthritis in the elderly population. The keywords "diclofenac sodium" and "knee osteoarthritis" were used to search the Chinese National Knowledge Infrastructure and Wanfang databases, while the terms "diclofenac" and "knee osteoarthritis" were employed to search Web of Science, Embase, Cochrane Library and PubMed. Two assessors independently evaluated the quality of the included articles using the Cochrane scoring system. Extraction of data from the selected studies was performed, and the effect of diclofenac sodium was quantified through meta-analysis with RevMan 5.3. Following the process of literature search, selection, and quality assessment, a total of 8 studies were identified as suitable for incorporation in the meta-analysis. The meta-analysis results indicated that participants in the diclofenac sodium experimental group experienced significant enhancements in Western Ontario and McMaster universities osteoarthritis index pain, physical function, and stiffness scores, surpassing those of the control group. Furthermore, the experimental group displayed a superior treatment efficacy. According to the meta-analysis results, the diclofenac sodium experimental group demonstrated a higher occurrence of gastrointestinal adverse events than the control group. In opposition, no significant discrepancy was detected in the occurrence of skin and other adverse events between the two groups. This meta-analysis conclusively established that diclofenac sodium exerts statistically significant effects on pain, physical function, and stiffness improvement in elderly patients suffering from knee osteoarthritis. Taking into account the results of this meta-analysis and systematic review, diclofenac sodium can be recommended as a primary non-surgical treatment option for knee osteoarthritis in elderly individuals. Nonetheless, healthcare professionals should closely monitor and manage the occurrence of gastrointestinal adverse events.
Keywords
Diclofenac sodium, knee osteoarthritis, nonsteroidal anti-inflammatory drugs, meta-analysis
Chronic pain, inflammation, and reduced overall joint function define Osteoarthritis (OA), the most prevalent joint disease. The aging population is more susceptible to this condition[1]. Factors like decreased gastric mucosal defense, diminished renal function, and concurrent medication usage make the elderly population particularly vulnerable to gastrointestinal adverse events caused by Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). Topical NSAIDs are highly recommended as a grade 1A non-surgical treatment option for knee OA, according to the guidelines provided by the International Osteoarthritis Research Society (OARSI)[2-4]. Diclofenac sodium is a frequently prescribed NSAID for managing conditions such as rheumatoid OA, ankylosing spondylitis and arthritis[5]. Meng et al.[6] review on NSAIDs, capsaicin, and salicylates for knee OA mentioned diclofenac sodium as a first-line treatment option but did not describe the safety profiles among the treatments. Derry et al.[7] review found that diclofenac sodium effectively relieves pain caused by OA but did not specifically target knee OA. Presently, there is a range of studies investigating the efficacy of diclofenac sodium in the management of knee OA, but there are inconsistencies in the research findings. To establish evidence-based and reliable clinical recommendations, conducting a meta-analysis of placebo-controlled studies is essential to thoroughly examine the efficacy and safety of diclofenac sodium in the management of knee OA.
Materials and Methods
Study selection:
This study followed the 2010 PRISMA guidelines and also incorporated the use of the AMSTAR guidelines for evaluating the methodological quality of systematic reviews[8]. A comprehensive systematic literature search was operated across multiple databases, called Cochrane Library, Web of Science, Embase, PubMed, Wanfang and China National Knowledge Infrastructure, to gather relevant information. The search spanned studies published until June 30, 2023. The search terms used were "diclofenac sodium" and "knee arthritis". Moreover, a comprehensive review of the reference lists of relevant articles was undertaken to uncover any potential additional literature of relevance to the study. Two assessors independently screened and selected studies based on eligibility criteria. Trial data related to predefined endpoints were subsequently extracted.
Inclusion criteria and exclusion criteria:
Inclusion criteria: The studies had to focus on the clinical treatment of knee OA using diclofenac sodium. Secondly, they needed to provide data suitable for statistical analysis or include at least one of the specified clinical outcomes, such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, stiffness score, physical function score, treatment efficacy, or adverse events. Lastly, studies from the same author or research center that reported multiple studies were considered, with a preference for recent, larger-scale, or high-quality publications. Even if the studies involved entirely different patient populations from the same center, their data were still included and analyzed.
Excluded criteria: Letters, commentaries, conference reports, reviews, case reports, animal experimental studies, clinical trial registrations, etc. and articles that lacked the necessary data for statistical analysis.
Quality assessment of methods:
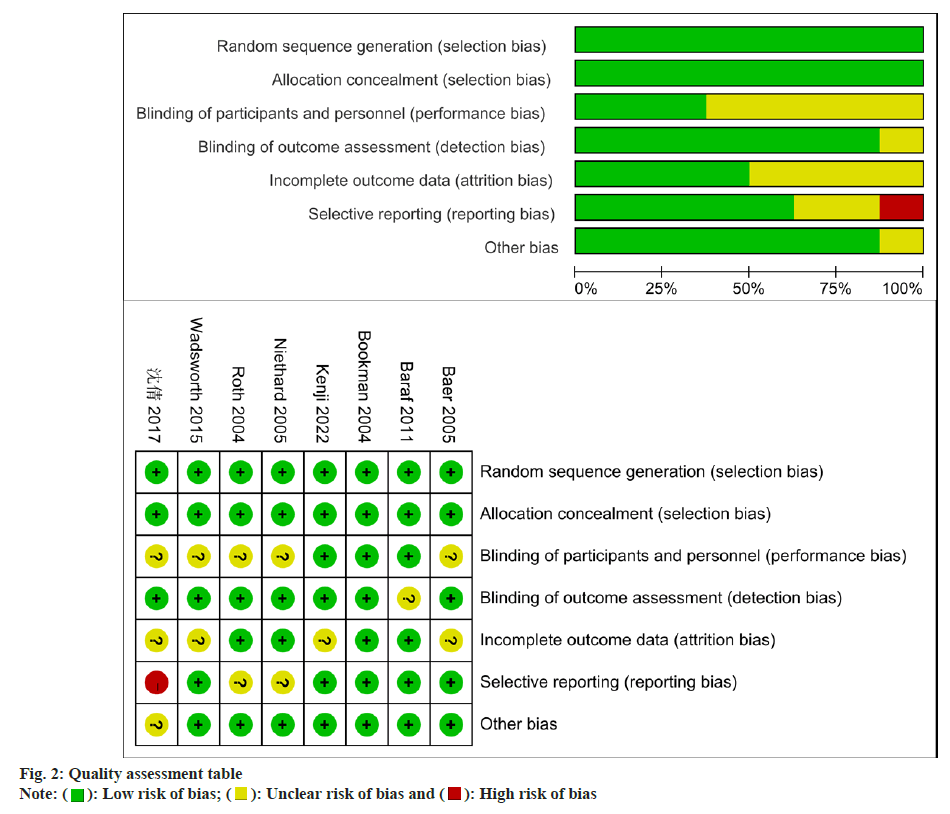
The quality assessment of Randomized Controlled Trials (RCTs) in this study was performed by two assessors who applied the Cochrane risk of bias assessment tool. The tool considers various criteria, such as random sequence generation, incomplete outcome data, blinding, allocation concealment, selective reporting, and other potential biases. Based on established guidelines, the assessors systematically evaluated each criterion and assessed the potential risk of bias in the studies that were included in the assessment. Through the examination of these criteria, a comprehensive evaluation of the methodological quality of each study is achieved, thereby minimizing bias and establishing the credibility and validity of the results.
Data extraction:
The extracted data include study characteristics (authors, publication year, country, study design, study duration, and sample size), patient characteristics (age, gender, Body Mass Index (BMI), baseline WOMAC pain, physical function, and stiffness scores), and treatment outcomes after medication (WOMAC pain score, physical function score, stiffness score, treatment efficacy, and adverse events). For the purpose of comparability, the WOMAC pain, stiffness, and physical function scores were normalized to a 20-point scale in all studies. If a study presented median and range data, the mean and Standard Deviations (SDs) were approximated using the approach outlined by Hozo et al.,[9].
Statistical analysis:
In this research, the software (Cochrane Collaboration's Review Manager 5.3), headquartered in Oxford, United Kingdom (UK), will be utilized. In the meta-analysis, the assessment of continuous variables will involve calculating Weighted Mean Difference (WMD) along with a 95 % Confidence Interval (CI), while for categorical variables, Odds Ratio (OR) with a 95 % CI will be computed. Heterogeneity will be assessed using the I2 statistic. Heterogeneity in meta-analysis can be categorized as high, moderate, or low based on the I2 value; >50 % corresponds to high heterogeneity, 25 % to 50 % suggests moderate heterogeneity, and <25 % indicates low heterogeneity. The decision of whether to use a fixed-effects model or a random-effects model in the study is contingent upon the outcomes of the heterogeneity test. Statistical significance for differences will be assessed using a cutoff of p<0.05.
Results and Discussion
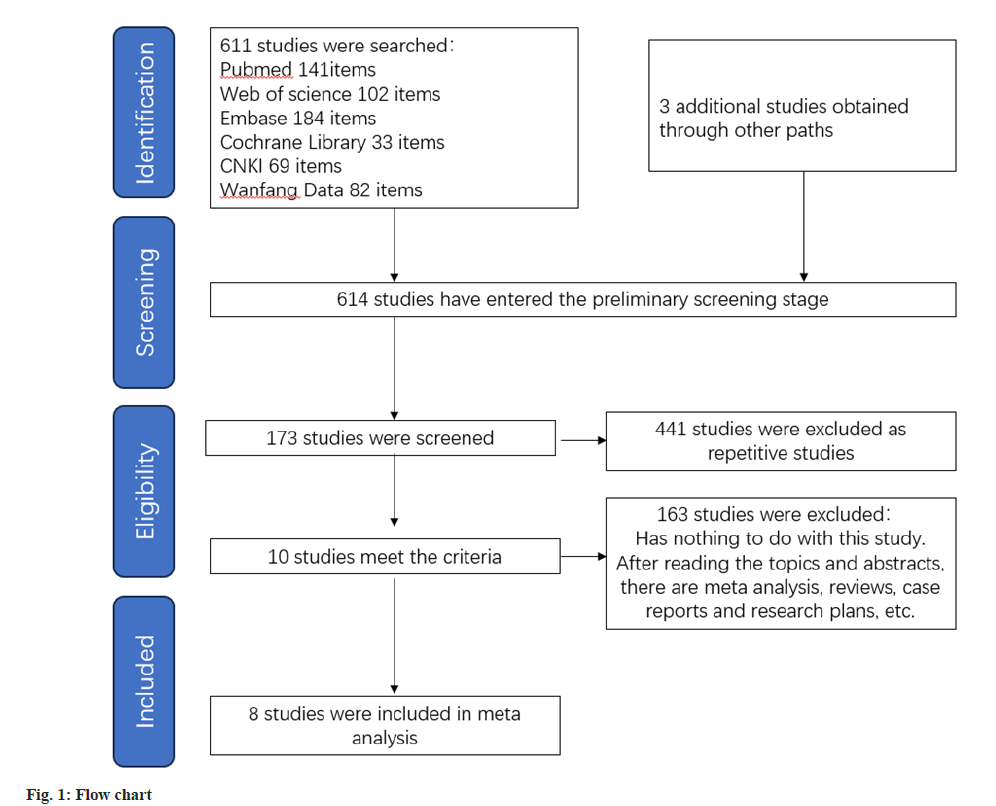
Our literature search and stringent application of inclusion criteria yielded 8 studies that fulfill the criteria for meta-analysis. The selection process is visually represented in fig. 1, while the quality assessment outcomes for the included studies are displayed (fig. 2). For easy reference, the key characteristics were shown in Table 1, which can be identified by their corresponding references[10-17]. A total of 2229 patients were included in the analysis conducted. Specifically, 1122 elderly patients with knee OA formed the observation group, receiving treatment with diclofenac sodium. The control group consisted of 1107 patients who were treated without the use of diclofenac sodium. These studies included 3 from Canada, 2 from the United States and 1 each from China, Germany, and Japan.
| Characterstics | Study details | |||||||
|---|---|---|---|---|---|---|---|---|
| Roth et al.[15] | Bookman et al.[12] | Baer et al.[10] | Niethard et al.[13] | Baraf et al.[11] | Wadsworth et al.[16] | Shen et al.[17] | Tomatsu et al.[14] | |
| Sample size | ||||||||
| Observation group | 164 | 84 | 107 | 117 | 274 | 130 | 100 | 146 |
| Control group | 162 | 80 | 109 | 120 | 264 | 129 | 100 | 143 |
| Age | ||||||||
| Observation group | 63.4±10.5 | 62.5±11.7 | 65.0±11.0 | 66±9 | 71.8±5.4 | 60.2±9.2 | 66.51±2.12 | 54.60±8.36 |
| Control group | 64.9±10.6 | 62.1±11.4 | 64.4±10.9 | 66±9 | 72.1±5.3 | 61.9±9.1 | 66.12±2. 09 | 55.92±8.36 |
| Gender(male/female) | ||||||||
| Observation group | 51/113 | 32/52 | 51/56 | 73/44 | 99/175 | 46/84 | 51/49 | 21/125 |
| Control group | 54/108 | 26/54 | 43/66 | 78/42 | 89/175 | 39/90 | 54/46 | 16/127 |
| BMI | ||||||||
| Observation group | NA | NA | NA | 29±6 | 29.8±5.9 | 32.5±7.7 | NA | 29.77±4.65 |
| Control group | NA | NA | NA | 28±5 | 30.5±5.7 | 31.8±6.8 | NA | 29.19±4.23 |
| Body weight | ||||||||
| Observation group | 92.8±21.8 | 81.3±16.4 | 89.9±18.1 | NA | NA | 93.3±22.8 | NA | 70.72±12.77 |
| Control group | 89.1±20.3 | 83.9±21.6 | 86.5±17.3 | NA | NA | 89.8±21.1 | NA | 68.98±10.72 |
| WOMAC pain | ||||||||
| Observation group | 13.0±3.3 | 9.1±3.5 | 13.0±3.2 | 9.6±3.2 | 11.9±2.4 | 12.4±3.1 | 14. 98±6.51 | NA |
| Control group | 13.0±3.4 | 9.3±3.5 | 12.8±3.1 | 9.4±3.2 | 11.7±2.4 | 12.6±3.4 | 15.11±5.92 | NA |
| WOMAC Body function | ||||||||
| Observation group | 42.0±11.7 | 29.5±13.7 | 40.7±11.9 | 53±15 | 39.4±10.2 | 42.9±10.4 | 31. 23±15. 17 | NA |
| Control group | 41.3±11.5 | 30.5±11.9 | 40.4±11.2 | 51±15 | 38.7±10.8 | 43.3±10.3 | 30.98±16.11 | NA |
| WOMAC stiffness | ||||||||
| Observation group | 5.2±1.5 | 3.7±1.7 | 5.2±1.5 | 9.6±4.2 | NA | 5.3±1.5 | 3.67±2.12 | NA |
| Control group | 5.2±1.5 | 3.5±1.7 | 5.2±1.5 | 9.2±3.4 | NA | 5.4±1.3 | 3.98±2.14 | NA |
Table 1: General Characteristics of the Inclusion Study
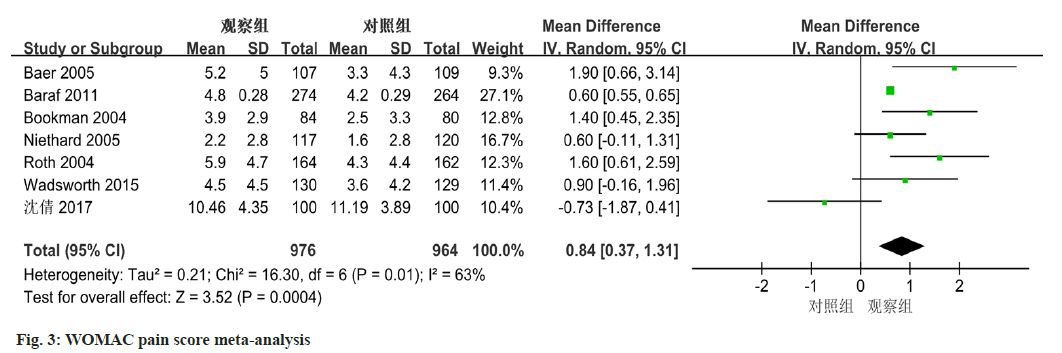
Among the 8 studies, 7 of them provided data on WOMAC pain scores. A level of heterogeneity was observed, with an I2 value of 63 % and a statistically significant p-value of 0.01. Utilizing a random-effects model, the meta-analysis displayed a significant difference in WOMAC pain scores between the diclofenac sodium group and the control group. The analysis revealed a mean difference of 0.84 (95 % CI 0.37-1.31, p=0.0004) (fig. 3).
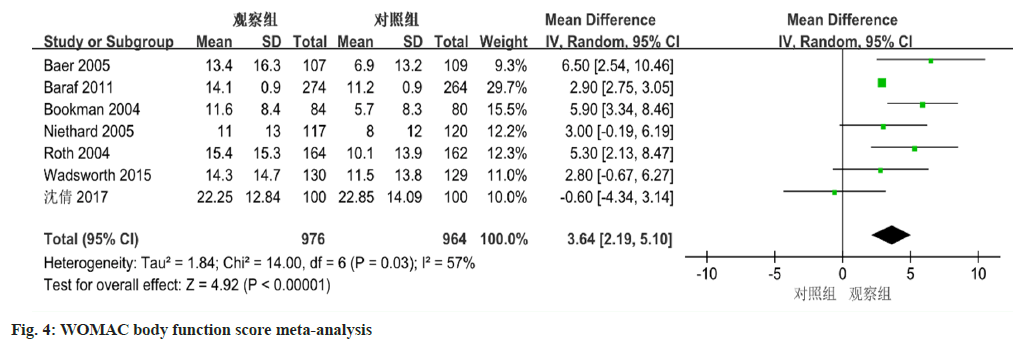
The meta-analysis, employing a random-effects model, yielded significant differences in WOMAC physical function scores between diclofenac sodium treatment group and the control group. The analysis revealed a mean difference of 3.64 (95 % CI 2.19-5.10, p<0.0001) (fig. 4).
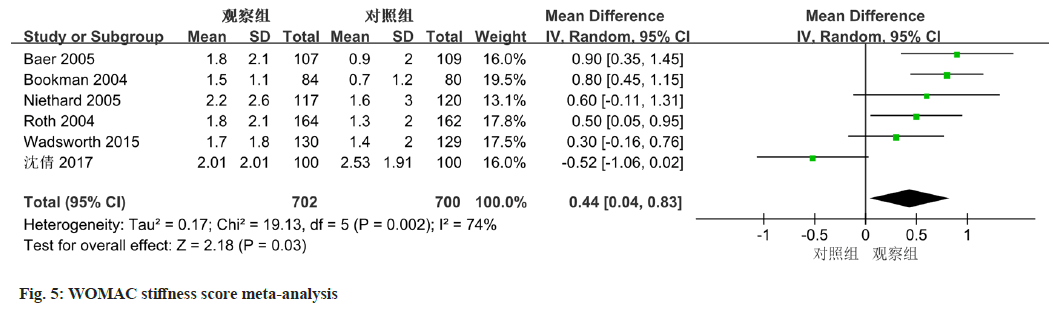
Among the 8 studies, six of them provided data on WOMAC stiffness scores. There was a notable amount of heterogeneity among the included studies, evidenced by an I2 value of 74 % and a statistically significant p-value of 0.002. Employing a random- effects model, the results revealed a notable improvement in WOMAC stiffness scores for the observation group (diclofenac sodium treatment) compared to the control. The mean difference was estimated to be 0.44 (95 % CI 0.04-0.83, p=0.03) (fig. 5).
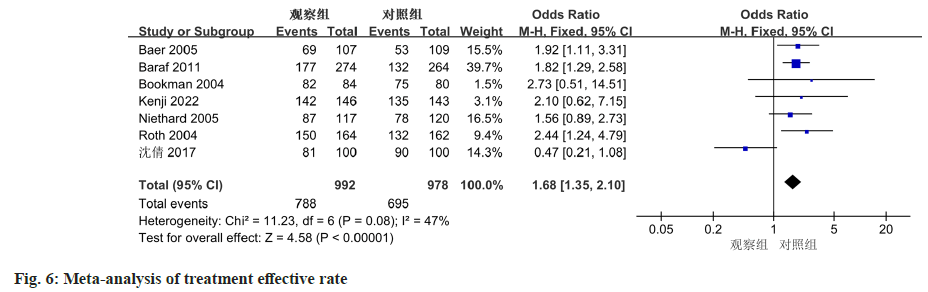
The meta-analysis included a total of 7 studies, with each of them reporting treatment efficacy outcomes. The included studies did not exhibit any significant heterogeneity, indicated by an I2 value of 47 % and a non-significant p-value of 0.08. The meta-analysis revealed a notable difference in overall treatment efficacy between diclofenac sodium treatment group and the control group. The OR was estimated to be 1.68 (95 % CI 1.35-2.10, p<0.0001) (fig. 6).
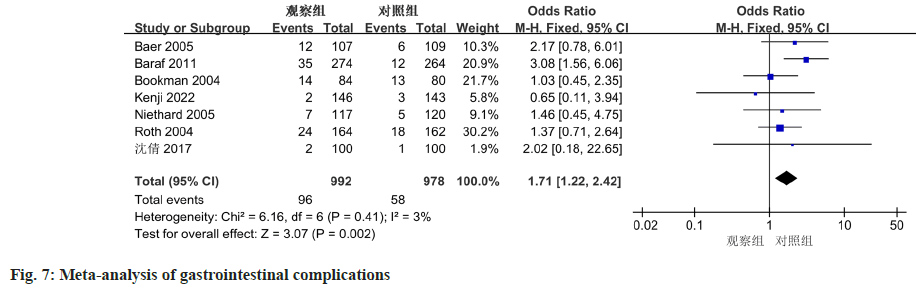
The analysis of adverse events related to drug treatment across the seven included studies revealed a minimal degree of heterogeneity (I2=3 %, p=0.41), indicating good agreement in the results. The results demonstrated a notable difference in the occurrence of gastrointestinal adverse events between the diclofenac sodium treatment group and the control group (OR=1.71, 95 % CI 1.22-2.42, p=0.002) (fig. 7).
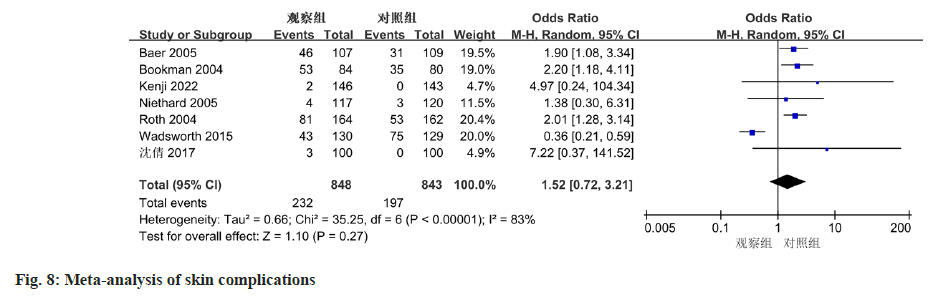
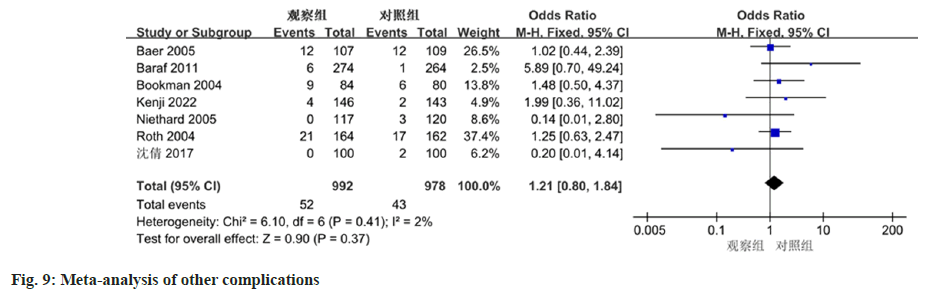
The meta-analysis, which included data from 7 studies, revealed no significant discrepancy in the occurrence of skin-related adverse events between diclofenac sodium treatment group and the control group. (OR=1.52, 95 % CI 0.72-3.21, p=0.27), as displayed in fig. 8. For other adverse events, excluding gastrointestinal and skin-related complications, the heterogeneity of the 7 included studies was low (I2=2 %, p=0.41). Based on the meta-analysis results shown in fig. 9, no notable difference was observed between the two groups (OR=1.21, 95 % CI 0.80-1.84, p=0.37).
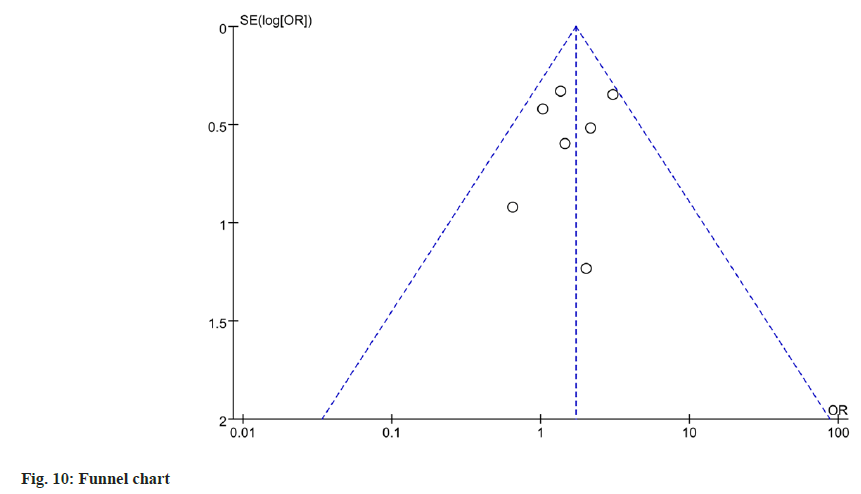
To evaluate publication bias for gastrointestinal adverse events, a funnel plot analysis was performed. Based on the funnel plot analysis, there was no significant evidence of publication bias as shown in (fig. 10).
This meta-analysis included 8 studies to look into the clinical effectiveness of diclofenac sodium in managing elderly knee OA. Diclofenac sodium demonstrated treatment advantages, significantly improving patients' pain and stiffness symptoms, and significantly improving physical function. The observation group receiving diclofenac sodium treatment exhibited a significant improvement in treatment efficacy compared to the control group. However, as an NSAID, diclofenac sodium has potential adverse effects that need to be considered. Literature review has consistently demonstrated a higher prevalence of gastrointestinal and skin-related adverse events in previous studies. Consistent with the findings of this study, the analysis revealed a significantly higher occurrence of gastrointestinal adverse events in diclofenac sodium group compared to the control. Nevertheless, no notable differences were observed between groups in terms of skin-related and other adverse events.
Studies have emphasized that the treatment of lower limb OA should focus on symptom relief rather than disease progression prevention, with arthroscopic surgery being the ultimate treatment option. Disease management should prioritize non-pharmacological approaches such as and exercise, weight loss, alignment correction, followed by medication management (topical and oral medications), with surgery being considered as a last resort. The findings provide strong evidence supporting the effectiveness of medication management in improving symptoms, alleviating pain and stiffness, and enhancing physical function. The outcomes of this study are consistent with the recommendation that healthcare providers should try NSAIDs, like diclofenac sodium, as a preliminary treatment approach prior to considering surgery for knee OA.
The results of a retrospective study including 105 patients with knee OA revealed that <20 % of the patients were either recommended or prescribed topical NSAIDs, while 31.4 % of the patients were referred for osteotomy surgery. These findings suggest that there may be a discrepancy between clinical practice in treating knee OA and the conservative recommendation in guidelines. The results of this study support the guideline recommendations, suggesting that healthcare providers should initiate a trial prescription of NSAID, such as diclofenac sodium, before resorting to surgical treatment for knee OA.
Age is a crucial factor contributing to the elevated frequency of adverse events related to oral NSAIDs, as the incidence of OA tends to escalate with age. These two factors present a contradiction. The results provide evidence that diclofenac sodium does indeed lead to a higher occurrence of gastrointestinal adverse events. However, NSAIDs have various routes of administration. A study suggested that combining topical diclofenac with a lower dosage of diclofenac sodium ester may reduce drug interactions and systemic adverse reactions[18-20]. In light of the lower risk profile of topical NSAIDs relative to oral administration, they should be recommended as the initial treatment option for non-surgical management of knee OA, with special attention to their suitability for elderly patients. Topical diclofenac sodium has significant advantages, reducing costs compared to other treatment modalities and minimizing barriers for patients to access the medication. Based on these factors, healthcare providers may consider topical diclofenac sodium as the preferred choice for initial management of OA in knee.
It may be necessary to take into account the limitations of the meta-analysis, even though the results are promising. Firstly, there is heterogeneity among the studies, which poses challenges in interpreting the significance of effect size differences. Therefore, it is important to approach the interpretation of the results regarding the efficacy and non-inferiority of the medication with caution. Besides, due to insufficient data, a meta-analysis on long-term outcomes and other indicators were not able to be conducted. It is important not to ignore these limitations when interpreting the results of the analysis.
To conclude, diclofenac sodium demonstrates significant benefits in managing pain, stiffness, and physical function in elderly patients who are suffering knee OA, justifying its consideration as the primary non-surgical treatment option. However, attention should be given to the occurrence of gastrointestinal adverse events.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26(3):355-69.
- Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 2019;27(11):1578-89.
[Crossref] [Google Scholar] [PubMed]
- Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72(2):220-33.
[Crossref] [Google Scholar] [PubMed]
- American Academy of orthopaedic surgeons. Management of Osteoarthritis of the Knee (Non-Arthroplasty). Evidence-Based Clinical Practice Guideline American Academy of Orthopaedic Surgeons 2021.
- Zhang Q, Huang J, Chen M. Effects of diclofenac sodium combined with sodium hyaluronate on knee joint function and serum inflammation in patients with knee osteoarthritis. Chin Med J 2023;8(9):3.
- Meng Z, Huang R. Topical treatment of degenerative knee osteoarthritis. Am J Med Sci 2018;355(1):6-12.
[Crossref] [Google Scholar] [PubMed]
- Derry S, Conaghan P, da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2016;4(4):CD007400.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160.
[Crossref] [Google Scholar] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5(1):1-10.
- Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: A randomised controlled, 6-week trial [ISRCTN53366886]. BMC Musculoskelet Disord 2005;6:1-9.
[Crossref] [Google Scholar] [PubMed]
- Baraf HS, Gloth FM, Barthel HR, Gold MS, Altman RD. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: Pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging 2011;28(1):27-40.
[Crossref] [Google Scholar] [PubMed]
- Bookman AA, Williams KS, Shainhouse JZ. Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: A randomized controlled trial. CMAJ 2004;171(4):333-8.
[Crossref] [Google Scholar] [PubMed]
- Niethard FU, Gold MS, Solomon GS, Liu JM, Unkauf M, Albrecht HH, et al. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J Rheumatol 2005;32(12):2384-92.
[Google Scholar] [PubMed]
- Tomatsu K, Yasuda S, Fuady A, Matsumoto H, Sumariyono. Efficacy and safety of S-flurbiprofen plaster in knee osteoarthritis patients: A 2-week randomized controlled Phase III clinical trial compared to diclofenac gel. Int J Rheum Dis 2022;25(5):563-70.
[Crossref] [Google Scholar] [PubMed]
- Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: A randomized, double-blind, vehicle-controlled clinical trial. Arch Int Med 2004;164(18):2017-23.
[Crossref] [Google Scholar] [PubMed]
- Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2 % topical solution for osteoarthritis of the knee: A randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin 2016;32(2):241-50.
[Crossref] [Google Scholar] [PubMed]
- Shen Q. Comparative study of acupuncture and diclofenac sodium emulsion in the treatment of senile knee osteoarthritis. J Clin Acupunct Moxibustion 2017;33(7):22-5.
- Hunter DJ. Lower extremity osteoarthritis management needs a paradigm shift. Br J Sports Med 2011;45(4):283-8.
[Crossref] [Google Scholar] [PubMed]
- DeHaan MN, Guzman J, Bayley MT, Bell MJ. Knee osteoarthritis clinical practice guidelines-how are we doing? J Rheumatol 2007;34(10):2099-105.
[Google Scholar] [PubMed]
- Gor A, Kothari N, Patel PK. Study of the efficacy and safety of oral diclofenac and decreased dose of oral diclofenac plus topical diclofenac in treatment of knee osteoarthritis. Int J Pharm Sci Res 2016;7(5):2083-9.
 High risk of bias
High risk of bias