- *Corresponding Author:
- Dipanwita banik
Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh 201002, India
E-mail: dipanwitabanik@neist.res.in
| Date of Submission | 16 March 2021 |
| Date of Revision | 20 September 2021 |
| Date of Acceptance | 05 August 2022 |
| Indian J Pharm Sci 2022;84(4):1051-1062 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Antimicrobial resistance against Mycobacterium and Candida are vital problems in immunocompromised patients. The current study was aimed to investigate the antimicrobial efficacy of fifteen folk medicinal plants found in Assam. Ethanolic plant extracts by well diffusion assay showed antimicrobial activity of 7 species against Mycobacterium smegmatis (ATCC®607TM) and 9 species against Candida albicans (ATCC®90028TM). Nepenthes khasiana exhibited highest zone of inhibition 21±0.5 mm against Mycobacterium smegmatis and this is the first report of Nepenthes khasiana showing anti-Mycobacterium activity from North East India. Mesua ferrea leaf extract first time exhibited good anti-candidal activity than the other studied species with zone of inhibition 11.83±0.76 mm. The impact of morphological characters on mechanistic convergence of anti-Mycobacterium and anti-Candida activities among the studied species was also assessed. Approximately 27 discrete morphological characters were used to prepare data matrix. Majority rule consensus tree of morphological data matrix with branch support ≥50 % using Mesquite 3.61 found that nearly 66.67 % species exhibited mechanistic convergence of anti-Mycobacterium and anti-Candida activity in combination congruent to morphological clustering. However, the clades viz., rosids, Murraya, Crinum and Alpinia showed partial congruence with angiosperm phylogeny group IV classification. A larger dataset including more than one representative species of each genus of the studied family and use of extensive morphological characters may exhibit a better mechanistic convergence.
Keywords
Medicinal plants, morphological data matrix, mechanistic convergence, antimicrobial, Mycobacterium, Candida
Assam is covered with dense forests and catchment areas along the river Brahmaputra which nurture and sustain natural wealth of the state. The region is gifted with rich floral diversity due to the presence of favorable climatic condition, diversified physiography and absolute geographical location. Among the floral community medicinally and economically important flora are found in wild and home gardens. Plants with medicinal properties are utilized by various indigenous community for their primary health related problems[1-3].
Antimicrobial resistance has been playing a major concern all over the world. Diseases caused by drug resistant microbes failed to recover as the standard drugs did not work on respective pathogens. This increases the risk of patients to survive, sometimes lead to prolonged illness followed by excessive healthcare cost. The genus Mycobacterium has got prioritization as drug resistant bacteriumcausing Tuberculosis (TB) all over the world for which new treatments are urgently needed[4]. Recently, Non TB Mycobacteria (NTM) has been gaining importance as they are found to cause some respiratory problems in immunocompromised patients[5]. Moreover, Human Immunodeficiency Virus (HIV) victims are more vulnerable to the infections caused by NTM. Fast growingNTM are responsible for causing infections of joint, skin, soft tissue and lymph node etc.[6]. Further, Candida albicans (C. albicans) is an opportunistic pathogen commonly found in genitourinary and gastrointestinal tract[7]. Generally causes infections on skin, vagina, mouth etc. Hospitalized patients with weak immune system are prone to resistant Candida infections. In immunocompetent patients (undergoing anticancer and HIV treatments) Candida can invade into bloodstream and infects internal organ system. About 7 % bloodstream infections are severe because they are resistant to available drugs[8].
On the basis of traditional knowledge and medicinal properties we have selected fifteen plants of Assam commonly growing in home gardens including cultivated one for anti- Mycobacterium and anti-candidal activity study. Further, morphological characters of each species were studied to prepare morphological character-based data matrix to analyses the mechanistic convergence of anti-Mycobacterium and anti-candidal activity of the studied plants.
Materials and Methods
Collection of plant material:
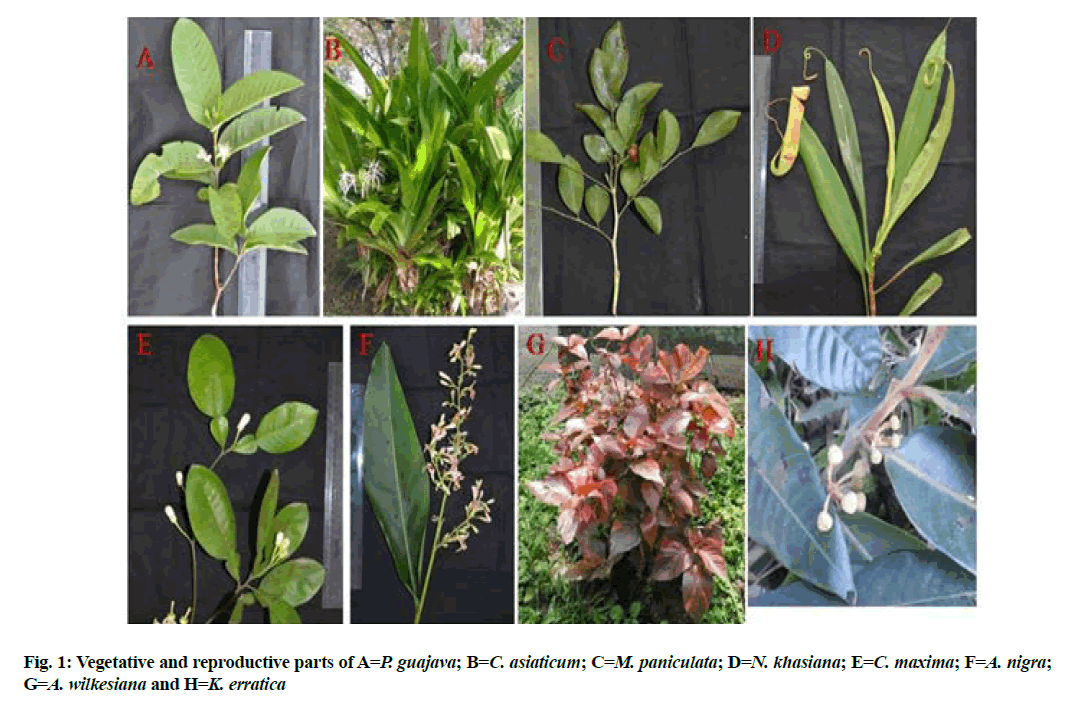
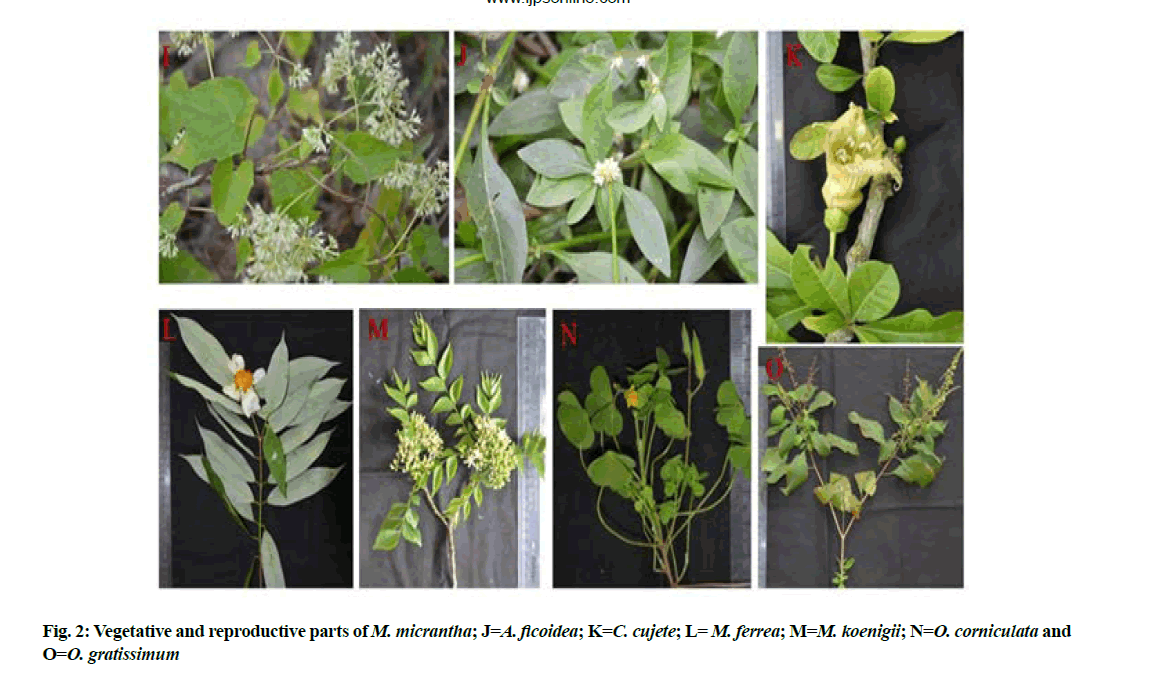
15 species were selected based on folk medicinal use with prospective antimicrobial activity vide published literature (Table 1)[9-40]. All the species were collected from adjoining areas of Council of Scientific and Industrial Research (CSIR)-North East Institute of Science and Technology (NEIST), Jorhat and Golaghat (Assam) with vegetative and reproductive parts (fig. 1 and fig. 2) and the field collection notes viz., name of the species, family, location, date of collection, collector’s name and number were recorded (Table 2). Herbarium sheets were deposited at the herbarium of CSIR-NEIST, Jorhat.
| Scientific names | Name of the user tribe/country | Plant parts used | Health ailments/Indications of use | References |
|---|---|---|---|---|
| A. wilkesiana Mull. Arg. | Nigeria, Mauritius | Le | Asthma, skin infections, antibacterial activity against E. coli, S. aureus, Klebsiella pneumoniae, antifungal activity and others | [25-27] |
| A. nigra (Gaertn.) Burtt | Assam, Manipur, Tripura | Le, shoo, see, rhi | Infections and others. Alpiniasp. is reported with anti-bronchitis, antibacterial and antifungal activity. | [1], [28-30] |
| A. ficoidea (L.) P. Beauv. | Lodha tribe in West Bengal, Pakistan | w. pl. | Herbal tribal medicine for asthma, cough, diarrhea and others. | [31,32] |
| C. maxima (Burm.) Merr. | Traditional Medicine, Asia, SE Asia, America | Fr, le | Cold, inflammation, leprosy, respiratory ailments, antifungal and antibacterial activity against Salmonella typhimurium and E. coliand others. | [33,34] |
| C. cujete L. | Mayan healers, Himalayan, peninsular India | Ba, le, fr pulp, see, Fr decoction | Cold, respiratory trouble, bronchitis, cough, asthma, activity against M. smegmatis, Mycobacterium tuberculosis (multi drug resistant isolate), Mycobacterium fortuitum and others. | [35-38] |
| C. asiaticum L. | Traditional Medicine, South Pacific islands, Asia | Le, bulbs | Infections, antimicrobial activity against E. coli, Klebsiella pneumoniae, P. aeruginosa, S. aureus and others. | [39-41] |
| K. erratica (Hook. F. & Thomson) J. Sinclair | India | La | Oral problems. | [13] |
| M. ferrea L. | Chirang Reserve, Assam, Meetei, Manipur | Flw, le, see isolated coumarins | Respiratory, dermal infection, anti-Mycobacteriumactivity, anti-Candida albicans activity and others. | [24], [42-44] |
| M. micrantha Kunth | Chorei tribe, (Assam) Malaysia | Young le, W. pl. | Respiratory problems, ulcers, itches, rashes on skin, healing wounds, antimicrobial activity viz., against E. coli, S. aureus, P. aeruginosa, Salmonella typhi, Streptococcus pneumoniae, Mycobacterium sp. and others. | [1], [45,46] |
| M. koenigii (L.) Spreng. | Ayurvedic use, Apatani (Arunachal Pradesh) | Le, v. oil | Inflammation, fungal infection, anti-M. smegmatis mc2155 activity, anti-Candida activity | [12], [47-49] |
| M. paniculata (L.) Jack | Thai | Le | Anti-Mycobacterium activity, anti-AIDS activity, anti-C. albicans activity | [24], [48,50] |
| N. Khasiana Hook.f. | Khasi, Garo tribes (Meghalaya, NE India) | Pit., fluid of unopened pit. | Leprosy and other ailments. | [51] |
| O. gratissimum L. | India, Malaysia, Africa | Le v. oil | Antimicrobial activity against common bacterial and fungal pathogens causing respiratory, dermal infections and others. | [52] |
| O. corniculata L. | Apatani, North Tripura, Bangladesh | Shoo, le | Dermal infection, antimicrobial activity against S. aureus, Salmonella typhiand others. | [35], [47], [53] |
| P. guajava L. | Khasi, Jayantia, Garo tribes, Tropical America | Ba, le, fr | Infections, wounds, inflammation, antibacterial activity and others. Leaf nano particle has anti-mycobacterial activity. | [54-56] |
Note: Bark, Le: Leaves, R: Roots, Flw: Flowers, Shoo: Shoot, Fr: Fruit, See: Seeds, Rhi: Rhizome, W. pl.: Whole plant, v. oil: Volatile oil, La: Latex, Pit.: Pitcher
Table 1: Folk Medicinal uses of 15 Studied Species
| S. no. | Names of the species | Collection localities | Voucher no. |
|---|---|---|---|
| 1 | A. wilkesiana Mull.Arg. | CSIR-NEIST, Jorhat campus | S. Saikia 1608 |
| 2 | A. nigra (Gaertn.) Burtt | CSIR-NEIST, Jorhat campus | S. Saikia 1610 |
| 3 | Alternanthera ficoidea (L.) P. Beauv. | CSIR-NEIST, Jorhat campus | S. Saikia 1838 |
| 4 | C. maxima (Burm.) Merr. | CSIR-NEIST, Jorhat campus | S. Saikia 1612 |
| 5 | C. cujete L. | Lohpohia, Jorhat | S. Saikia & D. Banik 1846 |
| 6 | C. asiaticum L. | CSIR-NEIST, Jorhat campus | S. Saikia 1609 |
| 7 | K. erratica (Hook.f. and Thomson) J. Sinclair | Deoparbat, Golaghat, Assam | S. Saikia and J. Saikia 1320 |
| 8 | M. ferrea L. | CSIR-NEIST, Jorhat campus | S. Saikia 1611 |
| 9 | M. micrantha Kunth | Tezpur | S. Saikia1831 |
| 10 | M. koenigii (L.) Spreng. | CSIR-NEIST, Jorhat campus | S. Saikia 1837 |
| 11 | M. paniculata (L.) Jack | CSIR-NEIST, Jorhat campus | S. Saikia 1830 |
| 12 | N. khasiana Hook.f. | CSIR-NEIST, Jorhat campus | S. Saikia 1834 |
| 13 | O. gratissimum L. | CSIR-NEIST, Jorhat campus | S. Saikia 1836 |
| 14 | O. corniculata L. | CSIR-NEIST, Jorhat campus | S. Saikia 1833 |
| 15 | P. guajava L. | CSIR-NEIST, Jorhat campus | S. Saikia 1832 |
Table 2: Collected Plant Specimens
Study of macro and microscopic characters:
The morphological characters of the species were critically examined from live specimens and measurement was recorded in metric scale. Magnus-TZ trinocular stereo zoom microscope was used to study micro-morphological characters. Identification of all the specimens was determined by consulting taxonomic literature like national and regional flora, protologue, revisionary work[41-45] and by consulting authentic specimens deposited in the herbarium of Eastern Circle Botanical Survey of India, Shillong (Assam) and several other herbaria available online viz., royal botanic gardens, the natural history museum, royal botanic garden Edinburgh, the New York botanical garden, Conservatoire et Jardin botaniques de la Ville de Geneve. The acronyms of the herbaria were used vide Index Herbariorum[46,47]. The species names were verified vide viz., The Plant List (www.theplantlist.org), International Plant Name Index (www.ipni.org), Plants of the World Online, Kew Science (http://www.plantsoftheworldonline.org/) and JSTOR (www.plants.jstor.org).
Morphological data matrix and cluster analysis:
Morphological data matrix of 15 folk medicinal plant species was prepared using 27 discrete characters viz., 4 binary and 23 multistate characters where for quantitative characters the mean value of natural range was considered to assign the character state (Table 3). Michelia champaca (M. champaca)from Magnoliaceae was used as out group in the study. Altogether 432 data points were prepared including out group following standard procedure[48,49]. The morphological cluster analysis was conducted through reconstructing majority rule consensus tree where 174 most parsimonious trees were constructed using the software Mesquite 3.61 (build 927) to visualise the cladogram[50]. Mesquite’s heuristic search resulted 174 equally good trees based on tree length. For rearrangements of the trees, Sub-tree Pruning and Re-grafting (SPR) method was considered. The majority rule consensus tree was formed with branch support ≥50 % where the branches with frequency less than 0.5 were allowed to get collapsed. The Consistency Index and Retention Index (CI and RI) and parsimony tree length were obtained from tree analysis while characters were treated as un-weighted and unordered.
| S. No. | Character state number with names of diagnostic morphological characters |
|---|---|
| 1 | Habit: 0, Herb; 1, Rhizomatous herb, bulbous herb; 2, Climber, creeper; 3, Subshrub, undershrub; 4, Shrub; 5, tree. |
| 2 | Roots: 0, Tap root; 1, Adventitious root; 2, Tap and adventitious root. |
| 3 | Twig indumentums: 0, Glabrous; 1, Puberulous; 2, Velutinous; 3, Villous; 4, Strigose; 5, Stellate. |
| 4 | Leaf petiole: 0, Sessile; 1, Pseudo petiolate; 2, Petiolate; 3, Wing petiolate. |
| 5 | Petiole indumentum: 0, Glabrous; 1, Slightly pubescent; 2, Pubescent. |
| 6 | Petiole size: 0, 0-0.35 cm; 1, 0.7-1.65 cm; 2, 2.25 cm; 3, 4.55-9.25 cm; 4, 0.7 cm; 5, 5.3 cm. |
| 7 | Leaf lamina shape: 0, Ovate; 1, Cordate; 2, Oblong; 3, Elliptic; 4, Lanceolate; 5, Obcordate; 6, Spathulate. |
| 8 | Leaf arrangement: 0, Alternate; 1, Alternately fascicled with tubercle; 2, Opposite; 3, Opposite decussate; 4, Radical. |
| 9 | Leaf apex: 0, Acute; 1, Acuminate; 2, Acute to acuminate; 3, Cuneate; 4, Cuspidate; 5, Caudate; 6, Obtuse; 7, Emerginate; 8, Acuminate with pitcher. |
| 10 | Leaf lamina margin: 0, Entire; 1, Crenulate; 2, Serrate; 3, Dentate. |
| 11 | Leaf lamina size (Length): 0, 0.85 cm; 1, 2.25-7.45 cm; 2, 12.0-13.2 cm; 3, 14.5-15.75 cm; 4, 23.5 cm; 5, 35.0 cm; 6, 49.5 cm; 7, 80.0 cm. |
| 12 | Leaf lamina size (Width): 0, 0.85 cm; 1, 2.15-2.45 cm; 2, 3.0-3.75 cm; 3, 4-5.0 cm; 4, 6-7.0 cm; 5, 9.6 cm; 6, 12.0-12.5 cm. |
| 13 | Inflorescence: 0,Solitary; 1, Solitary cauline; 2, Raceme; 3, Panicle; 4, Spike; 5, Corymb, corymbose panicle; 6, Umbel, cymose umbel; 7, Head; 8, Verticillaster; 9, Tubercle. |
| 14 | Colour of flower: 0, White; 1, Greenish white; 2, Pink; 3, Reddish; 4, Greenish red; 5, Yellow; 6, Brown. |
| 15 | Fragrance of flower: 0, Absent; 1, Present. |
| 16 | Flower sex:0, Bisexual; 1, Unisexual. |
| 17 | Filament no.:0, 5; 1, 10; 2, 13-13.5; 3, 20, 7.5; 4, 4; 5, 6; 6, 1; Many, 7. |
| 18 | Anther shape: 0, Linear; 1, Ellipsoid; 2, Reniform; 3, Vermiform; 4, Saggitate; 5, Appendiculate; 6, Linear curved; 7, Rotundus; 8, Ecrestate. |
| 19 | Stigma shape: 0, Capitate; 1, Truncate; 2, Discoid; 3, Peltate; 4, Laciniate; 5, Bifid; 6, Bilipped each ellipsoid; 7, Lobed and lobulate. |
| 20 | Style length: 0, 0-0.5 mm; 1, 3.5-5 mm; 2, 6-6.5 mm; 3, 8-13 mm; 4, 32 mm; 5, 46 mm; 6, 95 mm. |
| 21 | Ovary position: 0, Hypogynous; 1, Epigynous. |
| 22 | Fruit type: 0, Drupe; 1, Capsule; 2, Berry; 3, Hesperidium; 4, Utricle; 5, Carcerulus; 6, Achenes. |
| 23 | Fruit stalk length: 0, Sessile; 1, 1.5 mm; 2, 5.5-7.5 mm; 3, 9 mm; 4, 12 mm; 5, 15 mm; 6, 60 mm. |
| 24 | Fruit indumentums: 0, Glabrous; 1, Puberulous; 2, Sparsely pubescent; 3, Valutinous; 4, Hispid. |
| 25 | Seed shape: 0, Globose, rotundus, subglobose; 1, Triangular transversely globose; 2, Ovoid; 3, Obovoid; 4, Linear obovoid; 5, 3-4 tri- tetragonal; 6, Spindle shaped, winged; 7, Irregularly angular; 8, Unequally pyriform. |
| 26 | Leaf type: 0, Simple; 1, Unifoliate compound; 2, Trifoliate compound; 3, Pinnately compound. |
| 27 | Androecium character: 0, Ployandrous (stamens many & free); 1, Stamens in 2 whorls; 2, Stamens united at base; 3, Stamens epiphyllous in 2 whorls, stamens in 2 whorls united at base; 4, Monadelphous; 5, Polyadelphous; 6, Didynamous, epipetalous; 7, One stamen fertile rest modified into petaloid staminodes; 8, Syngenesious; 9, Filaments modified as columnar disc, stamens connate. |
Table 3: Character States of diagnostic morphological characters of studied plants
Solvent extraction of plant material and study of antimicrobial activity:
The collected species and plant parts as listed in Table 2 were shade dried, powdered in a Willy Mill and macerated in ethanol (95 %) for 48 h. Filtrate was taken through Whatman no. 1 filter paper and was evaporated at 40° under reduced pressure using rotary evaporator (Buchi, Switzerland). The concentrated extracts were completely dried in lyophilizer (Delvec pumps Pvt. Ltd., India) at -50°. Strains of Mycobacterium smegmatis (M. smegmatis)(ATCC®607TM) and C. albicans (ATCC®90028TM) were procured from HiMedia. M. smegmatis was cultured on sterile brain heart infusion agar and broth media and C. albicans was cultured on yeast malt agar and potato dextrose broth media. Sterile Mueller Hinton Agar (MHA) media was poured on petriplates to solidify and inoculum (ca.1×108 Colony Forming Unit (CFU)/ml) was spread on it. CFU concentration was determined comparing with McFarland solution (absorbance 0.12±0.003 at 625 nm)[51]. Well diffusion assay was used to evaluate antimicrobial activity[52]. Dimethyl Sulfoxide (DMSO) was used to prepare stock solution of the ethanol extract and was used as negative control. Isoniazid (1.5 mg/ml) was used as positive control for M. smegmatis and fluconazole (60 µg/ml) for
C. albicans. Petriplates were incubated at 37° for M. smegmatis and at 30° for C. albicans. Experiment was carried out in triplicates (n=3), Zone of Inhibition (ZOI) was measured using zone scale (HIMEDIA), data were presented as mean±Standard Deviation (SD) and statistical analysis was carried out in Microsoft Excel 2007.
Results and Discussion
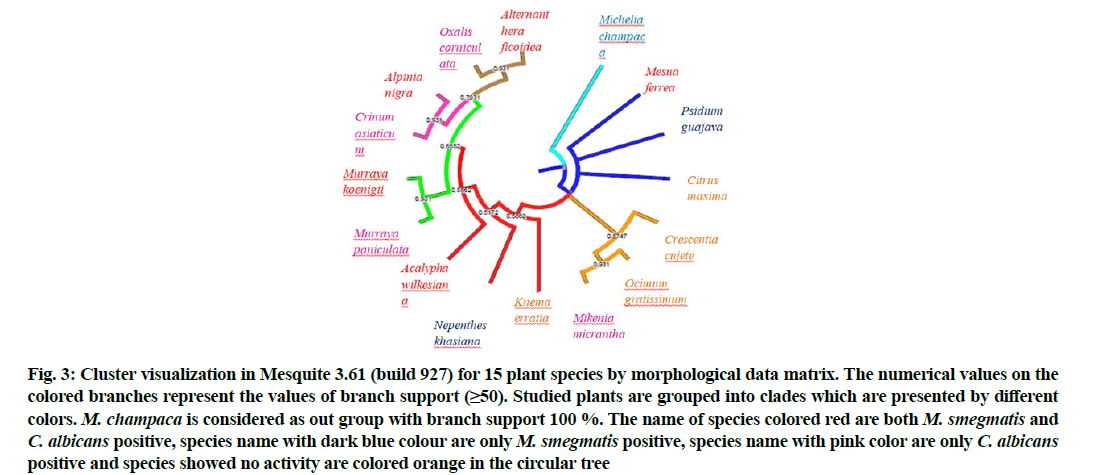
The reconstructed consensus tree obtained with Mesquite 3.61 (Build 927) exhibited the tree length 199, CI 0.73869347 and RI 0.43478261. The tree showed monophyletic and paraphyletic group in two distinct clades including all the 15 species (fig. 3). Psidium guajava (P. guajava), Mesua ferrea (M. ferrea) and Citrus maxima (C. maxima) showed polytomy. According to recent classification of Angiosperm Phylogeny Group IV (APG IV), Mesua, Psidium and Citrus belong to the orders Malpighiales, Myrtales and Sapindales respectively and are found in the same clade rosids[53] which is congruent in the morphological matrix-based cladogram. M. ferrea, P. guajava and C. maxima share certain common characters viz., tree with tap root system, white flowers, fragrant and bisexual, leaf lamina entire and fruit glabrous. The subclade Mikania and Ocimum share 8 common morphological characters viz., simple leaf, lamina dentate, flower white, fragrant, bisexual, stigma bifid, average style length 6-6.5 mm and glabrous fruit indumentum and the subclade is supported with frequency 0.93. Crescentia clade is supported with frequency 0.57 and includes the subclade of Ocimum+Mikania. The other clade supported with a frequency 0.65 comprises 3 subclades viz., Alternanthera+Oxalis with subclade frequency 0.93, Alpinia+Crinum with subclade frequency 0.93 and Murraya subclade with frequency 0.93. Knema, Acalypha and Nepenthes with frequency 0.58, 0.51 and 0.58 exhibited paraphyly to the same. In APG IV classification, Oxalis and Alternanthera belong to two distinct orders viz., Oxalidales and Caryophyllales, but in the present morphological matrix-based cluster analysis they appeared within the same clade with frequency 0.93. The subclade with Oxalis corniculata (O. corniculata) and Alternanthera ficoidea (A. ficoidea) shares the common characters viz., herbaceous habit with adventitious roots, velutinous twig and entire lamina, bisexual flower with 10 filaments, hypogenous ovary and ovoid seeds. However, phylogenetically, the taxa Crinum and Alpinia belong to Asparagales and Zingiberales respectively and are very close to each other[53] which is congruent to morphological cluster analysis. Similarly, the genus Murraya with two species Murraya paniculata (M. paniculata)and M. koenigii appeared within the same subclade. M. champaca used in the study as out group was supported with frequency 1.00 in the majority rule consensus tree. Thus, the morphological cluster analysis could discriminate the identity of the genus Murraya, clustering of Crinum and Alpinia through morphological characters. However, a larger morphological matrix with more numbers of taxa i.e., including more than one species from each genus of the studied plant families as well as extensive use of morphological characters may help to better understand the congruence with APG IV system of classification[53].
Fig. 3: Cluster visualization in Mesquite 3.61 (build 927) for 15 plant species by morphological data matrix. The numerical values on the colored branches represent the values of branch support (≥50). Studied plants are grouped into clades which are presented by different colors. M. champaca is considered as out group with branch support 100 %. The name of species colored red are both M. smegmatis and C. albicans positive, species name with dark blue colour are only M. smegmatis positive, species name with pink color are only C. albicans positive and species showed no activity are colored orange in the circular tree
The genera studied in the experiment have been reported with folk medicinal use. Ethanolic extracts of lesser explored plant parts of 15 species were tested against M. smegmatis and C. albicans. Among the 15 species viz., M. koenigii, M. ferrea, Alpinia nigra, (A. nigra) A. ficoidea and Acalypha wilkesiana (A. wilkesiana) have showed activity against both the pathogens. On the other hand, Ocimum gratissimum (O. gratissimum), Knema erratica (K. erratica), C. maxima and Crescentia cujete (C. cujete) didn’t show any activity. The study confirmed the anti-Mycobacterium activity of 7 species and anti-Candida activity of 9 species out of 15 studied species (Table 4). Among the tested plant species tender shoot with leaves of Nepenthes khasiana (N. khasiana) exhibited highest ZOI against M. smegmatis and extracts of M. koenigii, M. ferrea and Crinum asiaticum (C. asiaticum) exhibited good antifungal activity against C. albicans. Earlier reports showed that Acalypha indica and Alpinia galanga possess anti-TB activity[54,55] and seed extracts of M. ferrea was inactive against C. albicans[56]. In this experiment, the plants N. khasiana, A. nigra, A. ficoidea and A. wilkesiana are reported first time with anti-M. smegmatis activity and M. ferrea leaf extract with anti-C. albicans activity. ZOI study exhibited that the anti-Mycobacterium activity was stronger than anti-Candida activity in the studied species (Table 4). As antimicrobial activity is commonly studied against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) the antimicrobial activity of these 15 species were also reviewed against E. coli and S. aureus where A. wilkesiana, C. maxima, A. nigra and P. guajavashowed promising activity i.e., inhibition zone 17.00±0.00-25.10±0.2 mm against E. coli and 22.00±0.23-30.0±0.1 mm against S. aureus (Table 5)[57-71]. Similarly, the bioactive molecules and chemical constituents of these species were also reviewed where Saikia et al., 2020 showed abundance of (-)-Isoledene, (±)-Debromofliformin, δ-Cadinene, t-Caryophyllene in the floral essential oil of Mikania micrantha (M. micrantha) which exhibited cytotoxicity with IC50 <6 µg/ml against HeLa and <11 µg/ml against PA1 cell lines and antimicrobial activity with ZOI approximately 10-18 mm against Pseudomonas aeruginosa(P. aeruginosa), C. albicans, S. aureus and M. smegmatis (Table 6)[72-85]. Therefore, after the experimental validation of traditional use of these 15 folk medicinal species, further extensive studies are required to investigate the efficacy of these medicinal plants along with the bioactive molecules for the translational use and development of antibacterial and antifungal treatments to replace the resistant drugs.
| S. no. | Name of plants | Plant parts used for ethanol extraction | M. smegmatis | C. albicans |
|---|---|---|---|---|
| (ZOI in mm) | (ZOI in mm) | |||
| 1 |
|
Leaves | 13.66±0.57 | 9.66±0.57 |
| 2 | A. nigra | Leaves | 15.83±0.28 | 9.83± 0.28 |
| 3 | A. ficoidea | Whole plant with flower | 10.33±0.57 | 10±0 |
| 4 | C. maxima | Fruit pulp | -- | -- |
| 5 | C. cujete | Fruit pulp | -- | -- |
| 6 | C. asiaticum | Pseudo shoot | -- | 11.16±0.28 |
| 7 | K. erratica | Leaves | -- | -- |
| 8 | M. ferrea | Leaves | 10.83±0.28 | 11.83±0.76 |
| 9 | M.micrantha | Leaves and stem | -- | 10±0 |
| 10 | M. koenigii | Leaves | 14.16± 0.28 | 11.83±0.28 |
| 11 | M. paniculata | Leaves | -- | 10.33±0.57 |
| 12 | N. khasiana | Tendershoot with leaves | 21±0.5 | -- |
| 13 | O. gratissimum | Leaves andshoot | -- | -- |
| 14 | O. corniculata | Whole plant | -- | 10.33±0.28 |
| 15 | P. guajava | Leaves | 11.33±0.57 | -- |
| Fluconazole | NA | 15.83±0.28 | ||
| Isoniazid | 21.76±0.25 | NA |
Note: *ZOI=Zone of inhibition in mm (means of triplicate±SD), ‘--’ means no activity, NA=not applicable
Table 4: antimicrobial activity of the 15 plant species against m. Smegmatis and c. Albicans
| S. no. | Name of plants | Plant parts used for solvent extraction | E. coli | S. aureus | References |
|---|---|---|---|---|---|
| (ZOI in mm) | (ZOI in mm) | ||||
| 1 | A. wilkesiana | Leaves (EtOH) | 25.10±0.2 | 30.0±0.1 | [57] |
| 2 | A. nigra | Leaves (MeOH) | 18.25±0.68 | 22.00±0.23 | [58] |
| 3 | A. ficoidea | Whole plant (Aqueous) | -- | n/r | [59] |
| 4 | C. maxima | Fruit pulp and seed (EtOH) | 22 | 30 | [60] |
| 5 | C. cujete | Fruit pulp (EtOH) | -- | 3.75 | [61] |
| 6 | C. asiaticum | Bulb (EtOH) | 11.8 | 7.1 | [62] |
| 7 | K. erratica | Leaves | n/r | n/r | |
| 8 | M. ferrea | Leaves (EtOH) | 17.5±0.5 | 17.0±0.5 | [63] |
| 9 | M.micrantha | Leaves (EtOH) | 8.01±0.01 | 9.33±0.01 | [64] |
| 10 | M. koenigii | Leaves (EtOH) | 22.3 | 10.5 | [65] |
| 11 | M. paniculata | Leaves (EtOH) | -- | -- | [66] |
| 12 | N. khasiana | AuNP Leaves (Aqueous) | 8 | n/r | [67] |
| 13 | O. gratissimum | Leaves (EtOH) | 5.00±0.09 | 8.00±0.11 | [68] |
| 14 | O. corniculata | Whole plant (EtOH) | 14±1.64 | n/r | [69] |
| 15 | O. corniculata | Whole plant (MeOH) | 11±1.31 | 8.07±0.49 | [70] |
| 16 | P. guajava | Leaves | 17.00±0.00 | 26.00±0.00 | [71] |
Note: *ZOI=Zone of inhibition in mm (means of triplicate±SD), ‘--’ means no activity, n/r=not reported
Table 5: antimicrobial activity of the 15 plant species reported against e. Coli and s. Aureus
| S. no. | Name of plants | Marker chemical compounds | References |
|---|---|---|---|
| 1 | A. wilkesiana | Ethyl gallate, pyrogallol | [72] |
| 2 | A. nigra | β-caryophyllene, α-pinene, β-pinene | [73] |
| 3 | A. ficoidea | 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 3-ethoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris, 9,12,15-octadecatrienoic acid, methyl ester | [74] |
| 4 | C. maxima | β-sitosterol, marmin, naringenin, naringin | [75] |
| 5 | C. cujete | Naphthoquinones | [76] |
| 6 | C. asiaticum | Lycoriside, pahnilycorine, | [77] |
| 7 | K. erratica | n/r | |
| 8 | M. ferrea | α-Humulene, β-caryophyllene oxide, t-caryophyllene | [78] |
| 9 | M.micrantha | (-)-Isoledene, (±)-Debromofliformin, δ-Cadinene, t-caryophyllene | [79] |
| 10 | M. koenigii | 1-Methyl-pyrrolidine-2-carboxylic acid, Ethyl à-d-glucopyranoside | [80] |
| 11 | M. paniculata | β-Caryophyllene, (E,E)-Geranyl linalool, isospathulenol, methyl palmitate | [81] |
| 12 | N. khasiana | 5-O-methyl droserone, droserone, naphthoquinones, plumbagin | [82] |
| 13 | O. gratissimum | Eugenol, germacrene D, terpinolene | [83] |
| 14 | O. corniculata | 5-Hydroxy-6,7,8,4’-tetramethoxyflavone, 5,7,4’ -Trihydroxy-6,8-dimethoxyflavone | [84] |
| 15 | P. guajava | β-sitosterol, guajanoic acid, oleanolic acid, ursolic acid, uvaol | [85] |
Table 6: Some Reported Marker Chemical Compounds of the 15 Plant Species
The consensus tree generated by Mesquite 3.61 (Build 927) using morphological data matrix of 15 folk medicinal species was used to visualize the mechanistic convergence of antimicrobial activity where anti-Mycobacterium and anti-Candida activities were manually mapped on the consensus tree (fig. 3). The study showed that the clade I comprising 3 subclades viz., Alternanthera+Oxalis, Alpinia+Crinum and Murraya sub-clade exhibited mechanistic convergence of anti-Mycobacterium and anti-Candida activities where A. ficoidea, A. nigra and M. koenigii exhibited activity against both M. smegmatis and C. albicans and their adjacent members in the respective subclades exhibited activity against C. albicans. Further, immediate adjacent paraphyly exhibited to the clade I, by A. wilkesiana with activity against both the test organisms and N. khasiana with activity only against M. smegmatis. Thus, mechanistic convergence of antimicrobial activity was found congruent in clade I. In clade II, M. ferrea exhibited activity against both M. smegmatis and C. albicans whereas, P. guajavaonly against M. smegmatis, though the other clade member C. maxima did not show any activity. The clade III comprising C. cujete, O. gratissimum and M. micrantha did not exhibit any activity against M. smegmatis and only M. micrantha exhibited anti-Candida activity. Thus, nearly 66.67 % (out of 15 species nearly 10) species exhibited mechanistic convergence of antimicrobial activity against both M. smegmatis and C. albicans in combination as well as individually which are in congruence with their morphological clustering in above 3 clades. Absence of activity in C. cujete and O. gratissimum was also observed in the clade III. Thus, none of the clades reflected mechanistic convergence of individual antimicrobial activity of M. smegmatis and C. albicans but mechanistic convergence of activity was exhibited in combination against both M. smegmatis and C. albicans in clade I, partially in clade II and also through paraphyly by A. wilkesiana and N. khasiana to clade I irrespective of their status as per APG IV. However, a larger dataset with more than one representative species from each genus of studied plant families using extensive morphological characters may have reflected better mechanistic convergence.
The cladogram reconstructed from morphological matrix did not show congruence in clade support in respect to their individual antimicrobial activity. Phylogenetic analysis was conducted with morphological data matrix of more than 35 taxa including more than 115 characters to assign the generic circumscription of the genus Bromelia of the subfamily Bromelioideae and showed critical insights on paraphyly of the genus and the evolution among the subfamily[48]. Likewise, the current study exhibited the impact of morphological characters of 15 folk medicinal species in morphological data matrix-based phylogeny. Morphological clustering through reconstructing majority rule consensus tree using Mesquite 3.61 (build 927) with branch support ≥50 % showed congruence with APG IV for rosids, the clade comprising Mesua, Psidium and Citrus of Malpighiales, Myrtales and Sapindales, clustering of Murraya, Crinum and Alpinia.
Among the studied 15 folk medicinal plants 7 species exhibited activity against M. smegmatis and 9 species against C. albicans. Moreover, this is the first report of N. khasiana with prominent anti-Mycobacterium activity along with 3 more species. Among the studied plant species M. ferrea leaf extract showed best activity against C. albicans. The study validated the traditional uses of a few plants as antimicrobial agents and will initiate further translational research on anti-Mycobacterium and anti-Candida activity of folk medicinal plants used among cross cultural ethnic tribes. Further, the morphological matrix partly showed mechanistic convergence of anti-Mycobacterium and anti-candidal activity in combination among 66.67 % of the studied species. A larger dataset with more than one representative species from each genus of the studied plant families and use of extensive morphological characters may exhibit a better understanding of mechanistic convergence of anti-Mycobacterium and anti-Candida activity.
Acknowledgements:
We thank the Director, CSIR-North East Institute of Science and Technology, Jorhat, Assam 785006 (NMN 202142) for providing laboratory facilities to smoothly carry out the experiment.
Conflict of interests:
The authors declared no conflict of interests.
References
- Choudhury S, Sharma P, Choudhury MD, Sharma GD. Ethnomedicinal plants used by Chorei tribes of Southern Assam, North eastern India. Asian Pac J Trop Dis 2012;2(1):S141-7.
- Choudhury PR, Choudhury MD, Ningthoujam SS, Das D, Nath D, Talukdar AD. Ethnomedicinal plants used by traditional healers of North Tripura district, Tripura, North East India. J Ethnopharmacol 2015;166:135-48.
[Crossref] [Google Scholar] [PubMed]
- Rakotoarivelo NH, Rakotoarivony F, Ramarosandratana AV, Jeannoda VH, Kuhlman AR, Randrianasolo A, et al. Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J Ethnobiol Ethnomed 2015;11(1):1-6.
- Antimicrobial resistance. World Health Organization; 2021.
- Sousa S, Bandeira M, Carvalho PA, Duarte A, Jordao L. Nontuberculous mycobacteria pathogenesis and biofilm assembly. Int J Mycobacteriol 2015;4(1):36-43.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Lu D, Zhang X, Li H, Meng S, Pan YS, et al. Mycobacterium smegmatis in skin biopsy specimens from patients with suppurative granulomatous inflammation. J Clin Microbiol 2013;51(3):1028-30.
[Crossref] [Google Scholar] [PubMed]
- Spampinato C, Leonardi D. Candida infections, causes, targets and resistance mechanisms: Traditional and alternative antifungal agents. Biomed Res Int 2013;2013:204237.
[Crossref] [Google Scholar] [PubMed]
- Drug-resistant Candida species. CDC; 2019.
- Anokwuru CP, Sinisi A, Samie A, Taglialatela-Scafati O. Antibacterial and antioxidant constituents of Acalypha wilkesiana. Nat Prod Res 2015;29(12):1180-3.
[Crossref] [Google Scholar] [PubMed]
- Kingsley O, Marshall AA. Medicinal potential of Acalypha wilkesiana leaves. Adv Res 2014;2(11):655-65.
- Quds T, Ahmed S, Ali MS, Onocha PA, Azhar I. Antiemetic activity of Acalypha fimbriata Schumach. and Thonn., Acalypha ornata Hochst., and Acalypha wilkesiana cv. Godseffiana Muell Arg. Phytopharmacol 2012;3(2):335-40.
- Ahmed AA, Sharmen F, Mannan A, Rahman MA. Phytochemical, analgesic, antibacterial and cytotoxic effects of Alpinia nigra (Gaertn.) Burtt leaf extract. J Tradit Complement Med 2015;5(4):248-52.
[Crossref] [Google Scholar] [PubMed]
- Roy B, Swargiary A, Giri BR. Alpinia nigra (Family Zingiberaceae): An anthelmintic medicinal plant of north-east India. Adv Life Sci 2012;2(3):39-51.
- Basak S, Sarma GC, Rangan L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol 2010;132(1):286-96.
[Crossref] [Google Scholar] [PubMed]
- Pal DC, Jain SK. Notes on Lodha medicine in Midnapur district, West Bengal, India. Econ Bot 1989;43(4):464-70.
- Saqib F, Janbaz KH. Rationalizing ethnopharmacological uses of Alternanthera sessilis: A folk medicinal plant of Pakistan to manage diarrhea, asthma and hypertension. J Ethnopharmacol 2016;182:110-21.
[Crossref] [Google Scholar] [PubMed]
- Singh A, Navneet. Citrus maxima (Burm.) Merr. A traditional medicine: Its antimicrobial potential and pharmacological update for commercial exploitation in herbal drugs-A review. Int J ChemTech Res 2017;10(5):642-51.
- Vijaylakshmi P, Radha R. An overview: Citrus maxima. J Phytopharmacol 2015;4(5):263-7.
- Agarwal M, Chauhan S. Anti-mycobaterial potential of Crescentia cujete (bignoniaceae). Int J Adv Res Bot 2015;1(1):1-9.
- Islam MM, Shova NA, Rahman T, Bashar AA, Rahmatullah M. Crescentia genus of medicinal plants: A review. J Med Plants Stud 2019;7(3):112-6.
- Parvin M, Das N, Jahan N, Akhter M, Nahar L, Islam M. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res Notes 2015;8(1):412.
[Crossref] [Google Scholar] [PubMed]
- Mackenzie Theis MR, Bell K, de Golier T. Crescentia cujete (calabash tree) seed extract and fruit pulp juice contract isolated uterine smooth muscle tissues from Mus musculus. J Med Plants 2017;5(5):10-5.
- Haque M, Jahan S, Rahmatullah M. Ethnomedicinal uses of Crinum asiaticum: A review. World J Pharm Pharm Sci 2014;3(9):119-28.
- Nair JJ, van Staden J, Bonnet SL, Wilhelm A. Antibacterial properties of the family Amaryllidaceae: Evaluation of plant extracts in vitro. Nat Prod Commun 2017;12(7):1145-51.
- Surain P, Aneja KR. Anticandidal potential of Crinum asiaticum leaves extract against selected oral and vaginal Candida pathogens. J Innov Biol 2014;1(1):27-30.
- Ningombam DS, Devi SP, Singh PK, Pinokiyo A, Thongam B. Documentation and assessment on knowledge of ethno-medicinal practitioners: A case study on local Meetei healers of Manipur. Int J Pharm Biosci 2014;9:53-70.
- Panda SK, Das R, Leyssen P, Neyts J, Luyten W. Assessing medicinal plants traditionally used in the Chirang reserve forest, Northeast India for antimicrobial activity. J Ethnopharmacol 2018;225:220-33.
[Crossref] [Google Scholar] [PubMed]
- Roy B, Swargiary A, Giri BR. Alpinia nigra (Family Zingiberaceae): An anthelmintic medicinal plant of North-East India. Adv Life Sci 2012;2(3):39-51.
- Ishak AH, Shafie NH, Esa NM, Bahari H. Nutritional, phytochemical and pharmacological properties of Mikania micrantha Kunth. Pertanika J Scholarly Res Rev 2016;2(3):123-32.
- Matawali A, Chin LP, Eng HS, Gansau JA. Antibacterial and phytochemical investigations of Mikania micrantha HBK (Asteraceae) from Sabah, Malaysia. Trans Sci Tech 2016;3(1-2):244-50.
- Kala CP. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed 2005;1(1):11.
[Crossref] [Google Scholar] [PubMed]
- Naik SK, Mohanty S, Padhi A, Pati R, Sonawane A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement Altern Med 2014;14(1):87.
[Crossref] [Google Scholar] [PubMed]
- Vats M, Singh H, Sardana S. Phytochemical screening and antimicrobial activity of roots of Murraya koenigii (Linn.) Spreng.(Rutaceae). Braz J Microbiol 2011;42(4):1569-73.
[Google Scholar] [PubMed]
- Kusuma SA, Irma EH, Yuliasih N. In vitro antifungal activity of the orange jasmine (Murraya paniculata [L.] Jack.) leaves ethanol extract from Indonesia against Candida albicans. Int J Sci Eng Appl Sci 2017;3(5):273-8.
- Sanusi SB, Bakar MA, Mohamed M, Sabran SF, Mainasara MM. Ethnobotanical, phytochemical, and pharmacological properties of Nepenthes species: A review. Asian J Pharm Clin Res 2017;10(11):16-9.
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid Based Complement Altern Med 2016;2016:3012462.
- Rahman MS, Khan MM, Jamal MA. Anti-bacterial evaluation and minimum inhibitory concentration analysis of Oxalis corniculata and Ocimum santum against bacterial pathogens. Biotechnology 2010;9(4):533-6.
- Kote JR, Mulani RM, Kadam AS, Solankar BM. Anti-mycobacterial activity of nanoparticles from Psidium guajava L. J Microbiol Biotechnol Res 2014;4(5):14-7.
- Laloo D, Hemalatha S. Ethnomedicinal plants used for diarrhea by tribals of Meghalaya, Northeast India. Pharmacogn Rev 2011;5(10):147-54.
[Crossref] [Google Scholar] [PubMed]
- Sanches NR, Garcia Cortez DA, Schiavini MS, Nakamura CV, Dias Filho BP. An evaluation of antibacterial activities of Psidium guajava (L.). Braz Arch Biol Technol 2005;48(3):429-36.
- Hooker JD. The Flora of British India, 1st ed. London: L. Reeve and Company Limited Ashford, Kent UK; 1885. p. 608.
- Kanjilal UN, Kanjilal PC, Das A. Flora of Assam Vol I Part 1, Ranunculaceae to Elaeocarpaceae. Allied Book Centre, Dehradun; 1934.
- Haridasan K, Rao RR. Forest flora of Meghalaya, Vol 1, Bishen Singh Mahendra Pal Singh 23-A, Connaught Place, Dehradun; 1985.
- Balakrishnan R, Vijayraja D, Jo SH, Ganesan P, Su-Kim I, Choi DK. Medicinal profile, phytochemistry and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants 2020;9(2):101.
[Crossref] [Google Scholar] [PubMed]
- Banik D, Bora PP. A taxonomic study on the diversity of Indian Knema Lour.(Myristicaceae). Taiwania 2016;61(2):141-58.
- Holmgren PK, Holmgren NH, Barnett LC. Index Herbariorum-Part 1: The Herbaria of the world. New York Botanic Garden, New York; 1990.
- Index herbariorum. New York Botanical Garden (NYBG), Steere Herbarium.
- Monteiro RF, Mantovani A, Forzza RC. Morphological phylogenetic analysis of two early-diverging genera of Bromelioideae (Bromeliaceae). Rodriguésia 2015;66:505-21.
- Bora PK, Kemprai P, Barman R, Das D, Nazir A, Saikia SP, et al. A sensitive 1H NMR spectroscopic method for the quantification of capsaicin and capsaicinoid: Morpho-chemical characterisation of chili land races from Northeast India. Phytochem Anal 2021;32(1):91-103.
[Crossref] [Google Scholar] [PubMed]
- Maddison WP. Mesquite: A modular system for evolutionary analysis. Evolution 2008;62:1103-18.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protocols 2008;3(2):163-75.
- Methods for dilution antimicrobial susceptibility tests for bacteria that grows aerobically. Approved Standard, 9th ed, MO7-A9, Clinical and Laboratory Standards Institute (CLSI), Wayne; 2012.
- Angiosperm Phylogeny Group, Chase MW, Christenhusz MJ, Fay MF, Byng JW, Judd WS, Soltis DE, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 2016;181(1):1-20.
- Gupta R, Thakur B, Singh P, Singh HB, Sharma VD, Katoch VM, et al. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacteriumtuberculosis isolates. Indian J Med Res 2010;131(6):809.
[Google Scholar] [PubMed]
- Phongpaichit S, Vuddhakul V, Subhadhirasakul S, Wattanapiromsakul CJ. Evaluation of the antimycobacterial activity of extracts from plants used as self-medication by AIDS patients in Thailand. Pharm Biol 2006;44(1):71-5.
- Chanda S, Rakholiya K, Parekh J. Indian medicinal herb: Antimicrobial efficacy of Mesua ferrea L. seed extracted in different solvents against infection causing pathogenic strains. J Acute Dis 2013;2(4):277-81.
- Majekodunmi SO, Nubani SE. Formulation of Acalypha wilkesiana Muell. Arg. ethanol leaf extract into creams for the treatment of microbial skin infections. Int J Pharm Sci Invent 2014;3(10):45-53.
- Sahoo S, Kar B, Sahoo A, Nayak S. Phytoconstituents analysis and bioactivity study of Alpinia nigra (Gaertn.) Burtt. J Essent Oil Bear Plants 2017;20(6):1461-71.
- Baishya MK, Baishya D, Kalita MC. Biogenesis of silver nanoparticles using Alternanthera ficoidea leaf extract and its antibacterial potential. Int J Pharma Bio Sci 2013;4(3):104-10.
- Sahlan M, Damayanti V, Tristantini D, Hermansyah H, Wijanarko A, Olivia Y. Antimicrobial activities of pomelo (Citrus maxima) seed and pulp ethanolic extract. AIP Conf Proceed 2018;1933(1):030002.
- Hasanah U, Widhiastuti HT. Potency of ethanol extract from Berenuk (Crescentia cujete L.) fruit rind and flesh as antibacterial agents. IOP Conf Ser Earth Environ Sci 2018;187(1):012001.
- Riris ID, Simorangkir M, Silalahi A. Antioxidant, toxicity and antibacterial of ompu-ompu (Crinum asiaticum-L) ethanol extract. Rasayan J Chem 2018;11(3):1229-35.
- Adewale AI, Mirghani ME, Muyibi SA, Daoud JI, Abimbola MM. Antibacterial and cytotoxicity properties of the leaves extract of nahar (Mesua ferrea) plant. Adv Nat Appl Sci 2012;6(5):583-7.
- Nasution MY, Restuati M, Syahputra RA, Pulungan AS. Antibacterial activity of mandailing traditional plant leaves ethanol extract of Mikania micrantha. J Biosci Res 2019;16(1):793-7.
- Rastina R, Sudarwanto M, Wientarsih I. Aktivitas antibakteri ekstrak etanol daun kari (Murraya koenigii) terhadap Staphylococcus aureus, Escherichia coli dan Pseudomonas sp. J Kedokt Hewa 2015;9(2):185-8.
- Menezes IR, Santana TI, Varela VJ, Saraiva RA, Matias EF, Boligon AA, et al. Chemical composition and evaluation of acute toxicological, antimicrobial and modulatory resistance of the extract of Murraya paniculata. Pharm Biol 2015;53(2):185-91.
[Crossref] [Google Scholar] [PubMed]
- Bhau BS, Ghosh S, Puri S, Borah B, Sarmah DK, Khan R. Green synthesis of gold nanoparticles from the leaf extract of Nepenthes khasiana and antimicrobial assay. Adv Mater Lett 2015;6(1):55-8.
- Obinna C, Nwodo S, Olayinka O, Ikpo CO, Kehinde O. Antibacterial effects of extracts of Ocimum gratissimum and piper guineense on Escherichia coli and Staphylococcus aureus. Afr J Food Sci 2009;3(3):77-81.
- Golbarg H, Mehdipour Moghaddam MJ. Antibacterial potency of medicinal plants including Artemisia annua and Oxalis corniculata against multi-drug resistance E. coil. Biomed Res Int 2021;2021:9981915.
[Crossref] [Google Scholar] [PubMed]
- Misrahanum M, Almunawwarah SD, Helwati H, Maysarah H, Sadli S. Antimicrobial activity Jangjingki (Oxalis corniculata L.) against the growth of Staphylococcus aureus, Escherichia coli and Candida albicans. J Pharm Sci 2021;4(1):1-1.
- Metwally AM, Omar AA, Harraz FM, El Sohafy SM. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn Mag 2010;6(23):212-8.
[Crossref] [Google Scholar] [PubMed]
- Oladimeji OH, Tom EU, Attih EE. Ethyl gallate and pyrogallol from Acalypha wilkesiana var. Lace-acalypha (Muell &arg.). Eur Chem Bull 2014;3(8):788-91.
- Ghosh S, Ozek T, Tabanca N, Ali A, ur Rehman J, Khan IA, et al. Chemical composition and bioactivity studies of Alpinia nigra essential oils. Ind Crops Prod 2014;53:111-9.
- Patil RB, Kore BA. Phytoconstituents, pigments, gas chromatography mass spectrometry analysis, and allelopathy effect of Alternanthera ficoidea (L.) P. Beauv. Asian J Pharm Clin Res 2017;10(2):103-8.
- Xu YR, Zhang KF, Xie QJ, Lin JX, Huan KX, Liao Y. Chemical constituents from young fruits of Citrus maxima cv. Shatian. Zhong yao cai 2015;38(9):1879-81.
- Heltzel CE, Gunatilaka AL, Glass TE, Kingston DG. Furofuranonaphthoquinones: Bioactive compounds with a novel fused ring system from Crescentia cujete. Tetrahedron 1993;49(31):6757-62.
- Ghosal S, Shanthy A, Kumar A, Kumar Y. Palmilycorine and lycoriside: Acyloxy and acylglucosyloxy alkaloids from Crinum asiaticum. Phytochemistry 1985;24(11):2703-6.
- Keawsa-ard S, Kongtaweelert S. Antioxidant, antibacterial, anticancer activities and chemical constituents of the essential oil from Mesua ferrea leaves. Chiang Mai J Sci 2012;39(3):455-63.
- Saikia S, Tamuli KJ, Narzary B, Banik D, Bordoloi M. Chemical characterization, antimicrobial activity, and cytotoxic activity of Mikania micrantha Kunth flower essential oil from North East India. Chem Pap 2020;74(8):2515-28.
- Hema R, Kumaravel S, Alagusundaram K. GC/MS determination of bioactive components of Murraya koenigii. J Am Sci 2011;7(1):80-3.
- Dosoky NS, Satyal P, Gautam TP, Setzer WN. Composition and biological activities of Murraya paniculata (L.) Jack essential oil from Nepal. Medicines 2016;3(1):7.
[Crossref] [Google Scholar] [PubMed]
- Raj G, Kurup R, Hussain AA, Baby S. Distribution of naphthoquinones, plumbagin, droserone and 5-O-methyl droserone in chitin-induced and uninduced Nepenthes khasiana: Molecular events in prey capture. J Exp Bot 2011;62(15):5429-36.
- Joshi RK. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J Pharm Sci 2013;75(4):457.
[Crossref] [Google Scholar] [PubMed]
- Rehman A, Rehman A, Ahmad I. Antibacterial, antifungal and insecticidal potentials of Oxalis corniculata and its isolated compounds. Int J Anal Chem 2015;2015:842468.
[Crossref] [Google Scholar] [PubMed]
- Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava. Nat Prod Res 2004;18(2):135-40.
[Crossref] [Google Scholar] [PubMed]