- *Corresponding Author:
- I. Parmar
Department of Pharmacognosy, Faculty of Pharmacy, Sana’a University, India
E-mail: ijparmar2266@gmail.com
| Date of Received | 29 May 2020 |
| Date of Revision | 11 February 2021 |
| Date of Acceptance | 05 May 2021 |
| Indian J Pharm Sci 2021;;83(3):402-415 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Impurity is an unwanted substance present in the active pharmaceutical ingredients that form during the synthesis process of active pharmaceutical ingredients or any unwanted constituent that is produced besides the active ingredient during the formulation or the aging of active pharmaceutical ingredients. Even the insignificant quantity of impurity existing in the medicinal product may harm the patient life and compromises the purity and superiority of the medicinal product. According to International council for harmonisation guidelines, analytical monitoring of impurity is a prerequisite and mandatory requirement for approval of market authorization of the new drug substance. Any pharmaceutical product would be capable to serve their intended therapeutic activity when they are free from impurity. Thus, an impurity existing in an active pharmaceutical ingredient needs to be identified, and quantify with the help of modern analytical approaches. This review explores the basic information concerning impurity profiling, highlights the advantages of an analytical technique and also focuses on the limitation of different analytical methods for impurity profiling with possible ways to overcome the limitation.
Keywords
Impurity profiling, active pharmaceutical ingredients, drug substance, hyphenated technique, chromatographic separation techniques
The therapeutically active product comprising of active pharmaceutical ingredients (API) and excipients, the API is responsible for producing pharmacological effects after absorption in systemic flow in the living body. But in some circumstances, the active constituent or excipients could not be 100 % pure and may contain some other component that may arise in the medicinal product from different sources, i.e., from synthesis, an excipient, residual solvent, degradation product. These unwanted components other than API and excipients are known as impurities. Many definitions for impurity and impurity profiling have been well-defined in different reviews and guidelines. Impurity is the product or substance form in the synthesis includes intermediate or the side product of intermediate that forms during the side reaction or unwanted chemical reaction [1,2].
In any pharmaceutical product or drug substance, if the impurities are expected to be present, then they need to be identified and characterized by using appropriate analytical methods and this procedure is known as impurity profiling. Impurity profiling is a systemic process to identify the unknown impurity and to isolate the impurity to elucidate the structure. It is an important approach designed for identifying and quantifying the impurities existing in the medicinal substance [3]. Impurities are needed to be recognized and characterize, should be there in acceptable limits which do not produce any toxicological effect in living body thus, to synthesis the medicinal product of best quality and efficacy demands for impurity reporting in upcoming days. Impurity profiling requires highly sensitive, selective and efficient analytical techniques to regulate the trace quantity of impurity. The highly accurate, precise and sensitive technique is demanded in the current era for impurity profiling because the impurity may exist in a very trace amount in the drug substance that is very difficult to isolate and identify with lower sensitivity and accuracy techniques and in most of the cases the structure of impurities are derivatives or degradants of the parent drug molecule thus, hyphenated analytical techniques is a vital requirement for impurity identification. Various current approaches in investigative techniques are now available for identification, characterization and the structure elucidation of impurity [4].
Impurity profiling significance:
In assuring the high standard quality of drug products reached into the market, it’s important in screening impurities existing in the medicinal product throughout manufacturing. As compared to the online spectrum obtained during the earlier impurity profiling study that is required for registration of the drug master file, the standard impurity spectrum is of better purity and superiority. Synthesized impurity is used for toxicological studies and also use as the standard for determining impurity [5]. The impurity existing in the pharmaceutical product may vary the dissolution and solubility of the drug constituent and may affect the systemic circulation thus, it not only affects patient safety but also alter the biopharmaceutical behaviour of the drug substance. Thus, impurity profiling is essential for assuring the quality, efficacy and safety of pharmaceutical products [6,7].
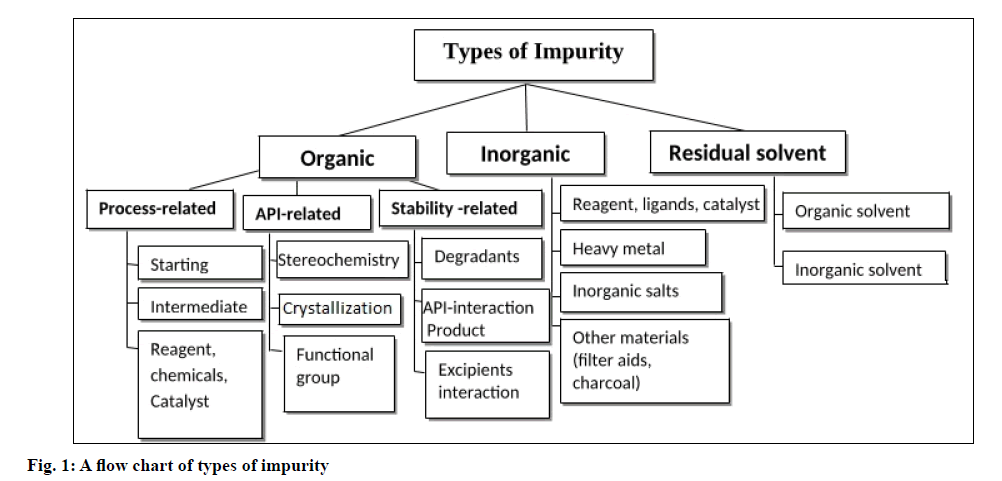
Types of impurities:
Impurity may be divided into three types such as an organic, inorganic and residual solvent. The further classification of impurity is shown in the fig. 1 [1].
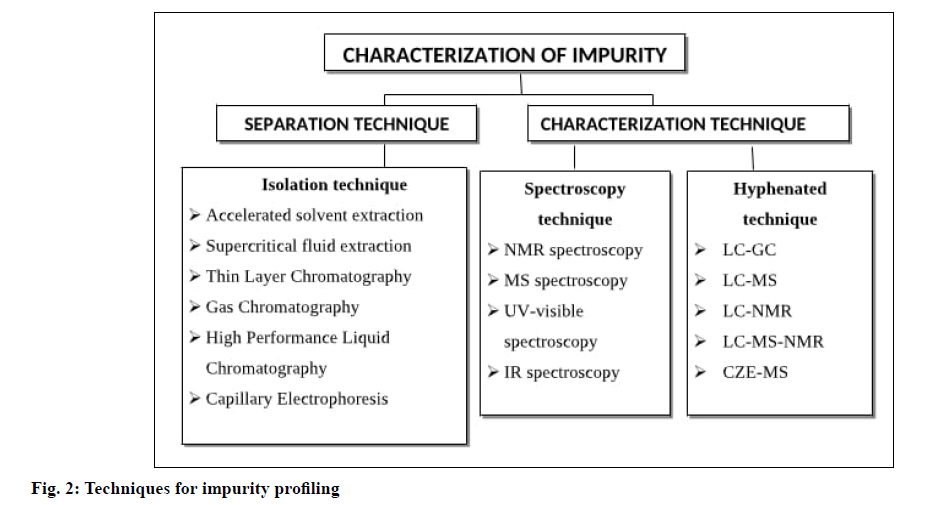
Characterization of Impurity
The detected impurity is further characterized by diverse spectroscopic techniques, before the characterization sufficient amount of impurity essential be isolated by suitable separation techniques. As stated in the International Council for Harmonisation (ICH) standard that when the level of impurity is greater than 0.1 % then it should be recognized and characterized appropriately. The various techniques for impurity profiling are given in fig. 2 and the examples of analytical techniques use in structure elucidation of impurity in different API are shown in Table 1.
| Sr No | Drug | Impurities | Method to detect impurities | Method to identify impurities | References |
|---|---|---|---|---|---|
| 1 | Ezetimibe | A. 2-(4-hydroxybenzyl)-N,5-bis(4-fluorophenyl)
Pentanamide |
HPLC (Waters Alliance 2695 separation module) | MS,NMR,FT-IR,TLC | 64 |
| 2 | Halobetasol propionate | A. Diflorasone diacetate |
UPLC (ACQUITYUPLC System) | LC–MS/MS | 65 |
| 3 | Icatibant | 4,4(5,5(1E,1E)-3,3(4,4methylenebis(thiophene-4,2-diyl))bis(2-carboxyprop-1-ene-3,1-diyl)bis(2-butyl-1H-imidazole-5,1-diyl))bis(methylene)dibenzoic acid | Agilent 1260 HPLC system (Agilent Technologies, Waldbronn, Germany) equipped | NMR,MS-MS | 66 |
| 4 | IIIM-290 (preclinical candidate) | A. Rohitukine, |
HPLC (LC-6AD HPLC system) | NMR, FTIR, and ESI–MS | 67 |
| 5 | Tolterodine tartrate | A. N-(3-(2-hydroxy-5-methylphenyl)-3-phenylpropyl)-N,N-diisopropyl hydroxylammonium trifluoro acetate salt.
B. 3-(2-hydroxy-5-methyl phenyl)-N-isopropyl-3-phenyl propane-1-amine oxide |
HPLC (Waters ACQUITYT UPLC system) | MS, NMR | 68 |
| 6 | Olanzapine | A. 2-methyl-4-(4-methylpiperazin-1-yl)-10-((methylthio)methyl)-thieno[2,3-b][1,5] benzodiazepine B. 10-(3-(1H-benzo[d]imidazol-2-yl)-5-methylthiophen-2-yl)-2-methyl-4-(4-methylpiperazin-1-yl)-thieno[2,3-b][1,5]benzodiazepine |
HPLC(Shimadu LC-20AD) | UV,FT-IR, LCMS/TOF, NMR and X-ray diffraction analysis | 69 |
| 7 | Isoproterenol hydrochloride | A. isoproterenone or (1-(3,4-dihydroxyphenyl)-2(isopropylamino)ethanone hydrochloride) B. 4-[2-(propan-2- ylamino)ethyl]benzene-1,2-diol |
UHPLC(Nexera-X2) | NMR, IR,LCMS/ESI | 70 |
| 8 | Meprobamate | Carbamic acid 2-carbamoyloxymethyl-2-methyl-pent-3-enyl ester | SFC(Waters Acquity UPC) | LC–MS | 71 |
| 9 | Metoprolol tartrate | C27H45NO13-adduct of lactose and Metoprolol formed by Maillard reaction | HPLC (Waters Model Alliance 2695 Separation Module) | NMR, IR | 72 |
| 10 | Dapoxetine | A. 1-(2E)-cinnamyloxynaphthalene B. 1-(2Z)-cinnamyloxynaphthalene |
TLC | IR, NMR, and MS | 73 |
| 11 | Rosuvastatin | A. Anti-isomer impurity: (3R,5R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl) amino]pyrimidin-5-yl]-3,5-dihydroxy-6(E)-heptenoic acid. B. Lactone impurity: N-{4-(4-fluoro-phenyl)-5- [2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)- vinyl]-6-isopropyl-pyrimidin-2-yl}-N-methylmethanesulfonamide. |
UPLC (Waters Acquity system) | UPLC-MS | 74 |
| 12 | Simvastatin | A. 7-[7-(2,2-dimethyl-butyryloxy)-2,6- dimethyl-1,2,6,7,8,8a-hexahydro-naphthalen-1- yl]-3-hydroxy-5-hydroxymethyl-heptanoic acid | HPLC(Waters Acquity system) | MS/MS | 75 |
| 13 | Amlodipine Maleate | A. 5-ethyl-7-methyl-6-(2-chlorophenyl)-8-methyl- 3,4,6,7-tetrahydro-2H-1,4-benzoxazine-5,7-dicarboxylate | HPLC(Agilent 1100 series) | LC-MS/MS NMR, IR |
76 |
| 14 | Ofloxacin | A. des carboxy ofloxacin B. ofloxacin-N-oxide C. N-des methyl ofloxacin D. 9-methyl piperizine E. difluoro pyrido benzoxazine carboxylic acid |
HPLC(Water Breeze) | - | 77 |
Table 1: Analytical Method Used to Detect and Identify the Structure of Impurity in Active Pharmaceutical Ingredients
Separation Techniques
Accelerated solvent extraction:
The accelerated solvent extraction technique is unique and extensively uses a technique that involves the extraction of a chemically active constituent with the help of solvent which penetrates within the pores of the solid matrix for extracting the desire chemical constituent. It has found its application in various pharmaceutical fields. Most widely applied for extraction of a natural chemical constituent from herbal plant materials. The application is not only restricted for the extraction of natural constituents but has also found its application in the impurity profiling of drug substances. The technique has been extensively employed in screening microorganism, dietary supplements, insecticide residue, an examination of environmental samples and organic contaminants. This technique has several benefits over the traditional method (soxhlet extraction, maceration, purification, turbo-extraction and sonication).
To improve the extraction process; this technique utilizes high temperatures and pressure. The elevated temperature will enhance the extraction kinetics thus, it leads to a decrease in the viscosity of the sample medium, which enhances the diffusion of the liquid into the sample medium. High pressure will force the solvent inside the sample medium pore and hence ease the extraction process [8,9].
Benefits of accelerated solvent extraction technique over other techniques [10]:
The accelerated solvent extraction technique is a quick and competent technique for extracting chemical constituents from the solid sample. The extraction technique is faster. A smaller volume of solvent consumption, thereby reduce environmental pollution.
Extraction yield increases, by decreasing the inclusive extraction cost. It is a reproducible technique.
Limitations: Thermo labile compound might be susceptible when exposed at the higher temperature, thus when they are exposed to high temperature it undergoes degradation.
Supercritical fluid extraction:
A substance over its critical temperature and pressure is known as a supercritical fluid (SCF). It has a property of both fluid and gas, it has gas like mass transmission and fluid-like solvating power hence provide an additional advantage over other methods. The supercritical fluid has wide applicability over the extraction of the natural component from plant materials besides also remained active in impurity profiling [11].
Advantages of supercritical fluid extraction over other techniques [12,13]:
The supercritical fluid extraction technique has a property of both liquid and gas thus can diffuse inside the sample medium identical to gas and dissolve the solid similar to a liquid and lead to accelerating the mass transfer process. Increase the efficiency of extraction. A chiefly employed supercritical fluid is carbon dioxide as it’s cheaper and easily available. Supercritical fluid CO2 is eco-friendly and identifies as safe by the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA). The critical condition of CO2 is at 30.9º temperature and 73.8 bar pressure. Similarly, used for thermo labile samples or easily oxidizable compounds as it remains operated at low temperatures.
Limitations: Supercritical fluid CO2 has low polarity; this can be overcome with the addition of co-solvent (methanol). By adding co-solvent, it will be alternating the polarity of SCF and enhance the solvating power.
Thin-layer chromatography (TLC):
TLC is a unique and most reliable analytical tool for testing the purity and identity of any compound. Thinlayer chromatography would need shorter development time, visualization of the separated compound is easy, separation procedure is quicker and TLC is the cheaper technique and minor sample size is required and reduced the quantity of solvent consumption. TLC can test a wider polarity range, so all the contamination can be detected. By using the newly hyphenated Liquid chromatography-mass spectrometry (LC-MS) technique identification of unknown impurity can be achieved, avoiding time-consuming isolation through simply scrapping off followed through mass spectrometry (MS) determination for clarifying the structure of unknown impurity. Thin-layer chromatography (TLC) /Highperformance thin-layer chromatography (HPTLC) and other planar chromatography techniques used for various applications in the pharmaceutical field in the analysis of numbers of drugs [14,15].
Limitations: Results of TLC are not completely reproducible and reliable as it’s an open structure. Critical factors like environmental changes and analyst variation may influence the result. It provides only qualitative data, quantification is not possible. It’s a non-automated technique, the manual error may also influence the correctness and exactness of the result. To resolve the weakness of TLC recent advances have been made that permit quantitative assessment through high precision densitometer and automatic multiple developments (AMD) is a distinct developing chamber that improves the selectivity of the method.
Advantages of HPTLC over TLC [16]:
The limitation of TLC can be overcome with the advance and automated High performance thin layer chromatography technique. It provides reproducible results and optimizes the analysis condition. HPTLC has a high resolution, sensitivity and speed; as compared to TLC. Quantification can be accomplished automatically by scanning the plate with the densitometer. Simultaneously, two analysts can complete the analysis at a time. Less solvent consumption in the analysis.
Gas chromatography (GC):
Gas chromatography is a unique investigative tool, most applicable for impurity profiling. The chief benefit of GC is that it is capable of isolating the volatiles from non-volatile medium over the specially employed sample pre-treatment. A residual solvent can be determined by GC since no other method can be employed for residual solvent determination. GC has high separation power, good selectivity and a wide choice of flame ionization detector (FID). GC renders the highest separation potential and for detection of the sample; linearity can be achieved up to 107 units. The dynamic headspace mode of sample pre-treatment provides the highest sensitivity [17].
Limitations: It cannot be practice for the study of the non-volatile and thermally unstable compound. GC is a unique separation technique where ultraviolet (UV) spectra cannot be taken. A direct injection mode of sample pre-treatment decreases the sensitivity.
The significance of derivatization technique [18,19]:
Derivatization technique is required for analysis of the compound which undergoes degradation when exposing to higher temperatures. The derivatization technique replaces the polar functional group, to increase its volatility and detection ability. Trimethylsilyl is most widely used as a derivatizing agent for derivatization in GC, which results in the silylation of -OH and -COOH group and enhances the thermal stability and volatility.
Derivatization also uses to furnish the information of the functional group present in the compound, through making a comparison of retention time of the derivative compound and un-derivative substance and additionally mass spectra acquire by GC/MS.
High-performance liquid chromatography (HPLC):
The HPLC is an automated, separation technique that provides high sensitivity, selectivity and highresolution power. The technique is fast and efficient, for testing purity and the separation of impurities existing in the drug substance. Determination of impurities in biological material, the reverse phase HPLC technique is extensively used. When HPLC is employed as a separation technique, UV-detector can produce good quality UV spectra. Sample preparation is easy in this system, even the error is also minimized [20].
Limitations: To detect the compound in HPLC, the compound should possess, following structural features such as UV chromophore and the fluorescence element or electrochemical activity, to attain the appropriate detection power. The drug substance that does not contain any structural elements like UV chromophore and a fluorescence element or electrochemical activity is analyzed by derivatization of the drug substance [21].
Capillary electrophoresis (CE):
Capillary electrophoresis is a very valuable technique in impurity profiling. Impurity determination is an issue, since the impurity may be relatively complex and usually, impurities may be structurally analogous to the core drug substance. High peak efficiency is the benefit and strength of CE over other separation techniques. CE can be operated in diverse modes, thus there is an increase in the separation capability [22].
Advantages of capillary electrophoresis over liquid chromatography (LC) [23]:
The analyte ranging from smaller ions to larger protein molecules can be analyzed using the identical capillary and provides higher separation efficiency. The instrument operated under a wide range of pH of the solution using aqueous or non-aqueous conditions deprived of resetting of the instrumental setup. Most extraction techniques make use of an organic solvent for the extraction, but CE is the only technique that mainly utilizes the aqueous buffer. Thus, the global scarcity of organic solvent mainly acetonitrile can be overcome. CE is an eco-friendly technique and reduces waste production. A pseudo-stationary phase is used in the micelle electrokinetic chromatography (MEKC) mode, which is employed for the extraction of neutral molecules. The pseudo-stationary phase is a design by accumulating a desirable surfactant in the background electrolyte. For derivatization reactions of the analyte, sample preparations need to be performing within the capillary.
Limitations: Due to the low optical path length of the UV detector, capillary electrophoresis has a low sensitivity. There is overheating of the sample, therefore limited to the use of low voltage. In terms of instrumentation, a few options available. As compared to the liquid chromatography technique, CE is more complicated.
Charaterization Technique
UV- Visible Spectroscopy:
To regulate and determine the purity of the drug material, unique and easiest methods are UV-VIS spectroscopy. The spectrophotometric active drug constituent can be controlled using UV-VIS spectroscopy. It’s also used for the impurity detection within the drug substance. The gradient HPLC technique is most widely used for impurity profiling, where the peaks are tracked using the retention time [24].
But the problem to track and differentiate between the impurity and drug substance peak is the retention time may shift or when the peaks are not properly resolved. Thus, to resolve this issue UV detection using a diode array detector that provides the UV spectrum of the unknown peak and to perform the matching using some algorithm, to a spectrum in a spectral library. The spectral library is prepared by using the reference standard. The investigation is mainly focused on the major peaks and the minor peaks, generally at less than 0.1 UV area percent, have not been characterized [25].
Advantages of UV-Visible Spectroscopy [26]: This technique is cost-effective over other analytical techniques and sample preparation is easy and quick. Require lesser analysis time and provide higher precision and accuracy. Provide a wide range of alternatives for the selection of solvents and chemicals used in the analysis of the sample. Sample can be reuse and recover for further analysis, as it does not undergo any destructive effect during analysis.
Limitations: The drug structure must have a UV chromophore in its structure, to absorb the UV radiation. The UV cut-off is considered as an essential parameter for the selection of the solvent for the preparation of a stock solution. Thus, the absorption maxima of the drug substance and solvent used for the preparation of the stock solution should not be identical [27].
Infrared (IR) spectroscopy:
The drug material will absorb a specific wavelength when exposed to electromagnetic energy, a specific bond present in the structure will absorb at a characteristic’s wavelength. Thus, this technique can be active to detect the sample structure by identifying the functional group prevailing in the sample. A lot of time is consumed in the sample preparation in the chromatographic technique, even though it has many advantages such as the better resolution of the impurities even in the multi-component sample. A new advance in the impurity profiling method is the use of a spectroscopy method coupled with chemometric as a replacement for the chromatographic method. The Fourier transform infrared spectroscopy (FT-IR) technique is a fast, less expensive technique, but for separation and determination of impurity, this technique cannot be applied directly. Hence chemometric technique is requiring chiefly multivariate regression for impurity profiling. The chemometric technique seems to be crucial in the extraction of information from complex data sets. The combination of IR with chemometrics will simplify and improve the quality control method of the drug substance in the manufacturing process [28].
The method has been reported that has applied the FTIR method along with the chemometric method for the identification of impurity in the simvastatin. Two methods were applied such as cluster analysis and principal component analysis for the data obtained from FT-IR spectroscopy. The partial least square model was built to predict the relative content of the lovastatin, the main impurity of simvastatin and the sum of statin like impurities. The method was able to predict the impurities content in the drug product containing simvastatin with good prediction ability (R2>0.95). Each molecule has a unique fingerprint spectrum thus use to analyze the drug and its impurities [29].
Limitations: Sample preparation is very time-intense. It can’t give detailed information as nuclear magnetic resonance (NMR) spectrometry. The method is destructive; the sample cannot be reused for further analysis. For detection in the IR range, require IR active sample.
Mass spectrometry (MS):
Mass spectrometry offers high reproducibility, specificity and it’s a highly sensitive technique in the analysis of a trace compound and for elucidating the structure. The identification of biomolecules or protein molecules present in a biological sample can be completed using mass spectroscopy and the study of high molecular mass, non-volatile and thermally susceptible compound can be possible by the introduction of a soft ionization technique. The parent molecule undergoes ionization to produce ions or fragments and they travel to the analyzer compartment of the instrument where ions are resolved according to their mass to charge ratio. The mass spectrum will provide data concerning the molecular composition of the parent drug compound. MS is coupled with different chromatographic techniques. This hyphenated technique has wide application in the determination of impurity structure [30].
One of the drawbacks in mass spectrometry techniques that the excipients present in the formulation may also contribute to the mass spectrum that resulting in a complex mass spectrum since it also suppresses the analyte signal. Whereas, in the UV spectrometry technique, excipients do not interfere in the UV chromatogram. This problem in mass spectrometry analysis can be overcome by using tandem mass spectrometry which reduces solvent interference [31].
Mass spectrometry without chromatographic separation:
Tandem mass spectrometry (MS-MS) is an advanced analytical method that utilizes a broad range of mass analyzers in a sequential arrangement. Each of these analyzer configurations has certain characteristic features that offer a particular advantage over the other configuration. Tandem mass spectrometry can have a detection limit up to ppm-level. Thus, due to the high sensitivity of this technique, it does not demand prior tedious chromatographic extraction of the compound on a preparative scale. The tandem mass spectrometry can provide the online spectra; therefore, minimize the time for extraction and also reduce the solvent interference in the mass spectrum [32,33]. An example of the enormous specificity and sensitivity attain by tandem mass analysis is the characterization of clindamycin in which six impurities were detected with the online mode of analysis [34].
Limitations of Mass spectrometry without chromatographic separation:
The method is restricted to the differentiation of the isomers with the same molecular weight. The differentiation between the stereoisomer and positional isomers can be made possible by the prior separation of the sample by chromatographic technique followed by mass spectrometry analysis.
Mass spectrometry with chromatographic separation:
Different chromatographic methods are employed in the isolation of impurities and the fraction isolated can be analyzed in mass spectrometry. The differentiation between the stereoisomer and positional isomers can be made possible. An example of differentiation of stereoisomer, described in discrimination of eight chloramphenicol isomers by LC-MS/MS sequentially to examine the natural occurrence of chloramphenicol. In mass spectrometry with chromatographic separation, the analyte of interest can be isolated and pre-concentrated for further detailed analysis and the structure elucidation has been possible using this offline approach [35].
Limitations: The drawback of this approach is that the solvent contamination may interfere in the chemical examination of the analyte of attention. The solvent interference or contamination may suppress the analysis of the compound of interest; this can be observed in the fast atom bombardment (FAB) ionization where the compound is more easily ionized. The resolution in the above situation, attain by using a continuous flow of fast atom bombardment ionization, electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) techniques. MS does not use for samples comprising of inorganic salts along with buffers.
Nuclear magnetic resonance (NMR) spectrometry:
The field of NMR has proved to be unique to the prosperous and rewarding sources of hypothetical and theoretical research in spin physics; now with new advances, NMR spectroscopy has been developed into a powerful tool and multidisciplinary in its own right. NMR spectrometry provides the magnetic property of the atomic nuclei, hence a useful tool in structure elucidation of unknown molecules. 1H and 13C NMR spectroscopy are mainly employed for structure elucidation. Other additional methods are twodimensional experiments such as heteronuclear single quantum coherence (HSQC) and double quantum filtered correlation spectroscopy (DFC-COSY) which are widely employed for structure elucidation [36]. The final and conclusive method for structure elucidation is NMR spectroscopy. The innovation in this technique involves modification of pulse-field gradient, solvent suppression, advancement in probe tools and high magnetic field configuration which will provide the driving energy for the structure elucidation of the unknown impurity [37].
NMR plays an important task in detecting insignificant impurities devoid of or following chromatographic separation. It is a great technique in determining the configuration and structure elucidation of synthetic and organic molecules, providing that they must be accessible in acceptable purity and amount including molecular mass, not more than 50 kDa [38,39].
Limitations: The sensitivity is a critical problem in the effective application of NMR spectroscopy. While the unequivocal determination of trace quantity of analyte or impurities is of key significance concerning a figure of essential industrialized welfare, such as in-market approval application, quality control and quality assurance of the formulations, regulatory features and protection of the patent right. After the separation of impurity by using the regular TLC plate and analytical HPLC column, it is impossible to acquire the NMR spectra. The preparative scale separation requires isolating enough quantity of impurity for NMR spectroscopy. Another disadvantage of NMR spectroscopy is that it requires a long time to understand the spectra and the NMR instrument is very expensive
Hyphenated Techniques
The hyphenated technique is a recent approach in the field of analytical science, where the two or more technique is a couple with the help of an interface. The standard of impurity is often unavailable for the characterization of impurity, thus; to generate the fingerprint of that unknown impurity, the hyphenated technique plays an important role.
The key idea behind the coupling of MS with the other chromatography method is that there is an increase in the signal-to-noise ratio, hence the noise decrease even faster which increases the selectivity of the analyte, due to an increase in the number of coupling.
Now due to lacking funds and less often use of this technique, some research laboratories cannot afford the installation of hyphenated techniques in their premises, as this technique is very expensive. In this laboratory the structure elucidation of the chromatographically separated fraction followed by the mass spectroscopy analysis for structure identification of the eluted sample fraction; which is very tedious and time-consuming [40].
Liquid chromatography-gas chromatography (LC-GC):
LC-GC is an extremely powerful practice; it combines the wide separation mechanism of LC and the high efficiency of GC. It is especially appropriate for a pure sample where high sensitivity and selectivity is required. LC-GC can exploit high sample capacity. Extensively used in offline mode. But there is some drawback in operating in an offline mode such as timeconsuming analysis, exhaustive in operation and low reproducibility [41].
Advantage of LC-GC in online mode:
An analysis of the small quantity of a sample, it is difficult to perform the analysis in offline mode, therefore analysis in online mode is a quick, reliable and efficient technique for providing higher sensitivity and analysis of the various sample. The technique is faster, completely automated, additional sensitive and extremely reproducible. Less solvent consumption and also minimize the problems related to the sample manipulation or artifact formation due to atmospheric air interference. The different applications of the LCGC technique, specified in the reference.
Limitations: LC-GC technique requires a complex instrument that used at different interface. Require trained users for careful optimization of various parameters.
Gas chromatography-Mass spectrometry (GC-MS):
GC-MS is a powerful method for structural elucidation and chemical analysis of a thermally stable and volatile compound. Analyte separations can be achieved through coupling and spectral information with the help of GC and MS provided sensitivity. It has some significance like progressive chromatographic resolution and increases peak capability, requires a single mobile phase in separation, fewer problems with the solubility and separation that have been achieved through electronic controls such as heat programming [42]. The GC-MS combination is a compact instrument greatly cheaper and well-known in current laboratories of the pharmaceutical industry. In the determination of the residual solvent, GC-MS holds a vital function. The impurity profiling can be completed besides, the structure elucidation can also achieve, in a case where the peak is associated with solvent interference, it can be detected, thus providing information on the presence of the toxic and hazardous solvent of class I category [43]. Solid-phase micro-extraction of more interfering volatile from the sample in addition to the environment sample can be identified with static headspace; hence it is essential to combine solid-phase micro-extraction with MS detection. MS-MS is another alternative for detecting unknown volatile in a highly complex sample. Mass fragmentography is a prevailing means for precise and sensitive detection of residual solvent [44].
Online GC-MS has a great benefit it provides data simultaneously on some impurity at a level of less than 0.01 %. The key benefit of GC-MS is that it can give molecular mass evidence using a chemical ionization method and to elucidate the composite structure it provides information on fragmentation using electron impact (positive and negative charges) ionization technique [45].
Limitations: During the ionization procedure some compound gets extensively fragmented, hence provide more complex spectra which are difficult to interpret. However, in various cases, ions are less precise or fragment are too widespread which reduce the sensitivity [46].
High-performance liquid chromatography-Mass spectrometry (HPLC-MS):
The combination of LC-MS has several advantages over the individual instrumentation LC and MS spectrometry. Owing to the high selectivity, sensitivity, dynamic range and ruggedness of LC-MS, it has been extensively employed in the pharmaceutical research area. It has excellent sensitivity for the small amount of impurity and degrading products. The innovation in the current era, increase the use of the LC-MS/MS, which is also known as tandem LC-MS. It has wide applicability in the quantitative analysis and structure elucidation of the unknown impurity [47].
The LC can be skilfully couple with the MS, as there is less risk for the solvent and flow rate interference in the LC-MS interface. The important benefits of HPLCMS are that it can be coupled with a photodiode array UV detector. To furnish all information for the structure explication of the impurity the most extensively use is HPLC/UV/MS/MS. Operating the LC-MS in the multiple reaction monitoring modes that will repeat the experiment (n) number of times, where n in (LC-MSn) is the number of MS-MS experiments. This technique is extensively used for structure elucidation [48].
Limitations: The first-generation instrument employs a soft ionization method that gives molecular mass records only. In the LC-MS excipient interference is observed repeatedly. The drug product in solution form generally causes contamination of the tip of the capillary. The confirmation of the final structure of the compound can be incomplete without an NMR spectrometry study. Some of the factors that should be considered for optimization of the method such as mobile phase composition, flow rate, additives like a buffer or any ions pair and should be considered the ionization techniques factors such as spray voltage, the temperature of nebulizer, the voltage of cone in MS, nature of gas and its pressure should be considered as an essential factor to be considered.
Liquid chromatography-nuclear magnetic resonance spectrometry (LC-NMR):
LC-NMR is a viable commercial technology, since about the middle of 1990. LC-NMR has sensitivity issues; here sensitivity is the capability of the NMR spectrometer to acquire sufficient data to allow for the unambiguous structure elucidation of the trace intensity in a compound mixture analyzed by HPLC. Previously many limitations of LC-NMR had obstructed its widespread application; however, this limitation has been recently overcome. Since the cryoprobes are highly sensitive as compared to the regular probes [49,50].
The signal-to-noise ratio for the cryoprobes is four times that of regular probes, therefore regular probes require 0.5 μg of sample whereas; cryoprobe requires only 0.1 μg of the sample. Thus, it reduces the time for analysis, as well as reduce the sample required for the analysis [51,52]. The practice of LC-NMR would reduce the time and labor exhaustive isolation step, thus the identification and analysis become faster and easier [53,54].
The limitations of LC-NMR:
The sensitivity issues of the NMR are the main limitation in the application of LC-NMR [55]. In LC-NMR, the protonated solvent cannot be used as a mobile phase for HPLC. The protonated solvent shows a resonance signal that dominates 1H-NMR spectra and swamps the moderately weak signal from a small amount of analyte. Thus, to compensate for this issue deuterated solvent can be used, but it is cost expensive, result in peak broadening and Rt shift may be observed. Need solvent suppression technique, for using the deuterated solvent, this result in suppression of analyte proton signals near the suppression solvent line and result in damage of spectral information [56].
As HPLC contains metal and moving metal portions, the problem may be encountered if it is appearing close to the superconducting magnet of the NMR system. For unshielded magnet require the HPLC to be a minimum of 1.5-2 m away and 30-50 cm is requiring for the shielded magnet. The HPLC system should be overloaded with the analyte in demand to confirm that a suitable quantity of analyte is in the NMR active region [57].
LC-MS/NMR
The limitation of on-flow LC-NMR is the sensitivity issue, at the magnetic field strength of 500 MHz and 1ml/min flow rate the residence time of the analyte is reduce and the detection limit is 10 ppm. Thus, to increase the analyte residence time the flow rate should be reduced but this may increase the analysis time and also affect the resolution. Hence one of the recent advancements to increase the NMR sensitivity is LCMS/ NMR [58].
Directly coupled LC-MS/NMR is used universally in pharmaceutical laboratories. Coupling of the HPLC with that of MS and NMR is known as LC-MS/NMR, in this type of hyphenated technique the eluent from the HPLC column is split into the relatively small portion, from that one portion going to MS (ESI) and the remaining portion going to the NMR spectrometer as MS has advanced sensitivity as compared to that of NMR spectrometer. The coupling of both data types allows an unambiguous correlation of NMR spectra with a particular trace level analyte. Many a time the MS spectra are not sufficient for the full structural elucidation. Thus, by coupling of LC, MS and NMR will give additional information on the drug structural configuration, functional group and NMRsilent heteroatoms (N, Cl, O) present in unknown impurities [59].
Advantages:
Minimal sample requirement and it is a fully automated technique. Reduce the time for analysis with minimal sample degradation chances and sample can be recovered for further analysis.
Capillary zone electrophoresis-Mass spectrometry (CZE-MS):
The main reason to couple the capillary zone electrophoresis with the mass spectroscopy technique is that the ideal separation power of the capillary electrophoresis (CE) has been achieved whereas; mass spectra will provide sufficient structural information. There should be a high grade of orthogonality between the methods during the separation of impurity, to accomplish better resolution of separated impurities [60-65]. Different techniques for impurity profiling, include coupling of CE with varying MS ionization systems such as electrospray ionization (ESI-MS), atmospheric pressure chemical ionization (APCI-MS), atmospheric pressure photoionization (APPI-MS) and thermospray ionization (TSI-MS). The ESI-MS and TSI-MS are useful for detecting the ionic compound whereas; the APCI-MS and APPI-MS are not capable of detecting the ionic sample [66-72]. Accordingly, it can be also aid to differentiate between the ionic or non-ionic unknown impurity.
The sensitivity of the charge solution decreases in the following order ESI-MS≥TSI-MS≥APPI-MS≥APCIMS. For detection of the solution which is not changed and for less polar compounds, APCI is mostly employed. ESI-MS is mostly employed due to its sensitivity and softness. V Dora et al. reported a comparison study between three methods such as RP-HPLC, Capillary electrophoresis and also included CE-ESI-MS/MS for impurity profiling in galantamine hydrochloride in a stressed condition. Important information obtained regarding the impurity by comparison between three methods, two impurities were detected at a low level. It is a combination of separation and identification technique, since requiring a small volume of the sample as well as provide high resolution and sensitivity [73-77].
Limitations of CZE-MS:
Capillary electrophoresis is a very complicated technique since it requires the optimization of all the parameters that have an impact on the separation mechanism. The less sensitive technique, therefore cannot detect the trace quantity of impurity.
Conclusion
According to the regulatory guideline, the analytical monitoring of impurity in a new drug substance is a mandatory requirement for market authorization. Thus, pharmaceutical products should be analytically monitored for any impurity present in trace amounts. As APIs are not 100 % pure those trace amounts of impurity may affect the safety and efficacy of the final formulation and patient consuming those products. Even standard assay procedures are not sufficient to describe impurities both qualitatively and quantitatively. All the techniques specify for impurity profiling may contribute effectively to the identification and structural elucidation of the unknown impurity. The application of this technique will significantly depend on the nature of impurity and the type and origin of impurity present in the drug substance. For the separation of the impurities mainly liquid chromatography techniques are widely employed, but the isolated fraction should be enough to carry out further structural analysis in off-line mode. In the current era, many advancements have brought huge changes in the analysis technique and also faster the process of analysis and have reduced the efforts. The on-line mode of analysis has not only saves our time but also provide qualitative and quantitative data for the unknown impurity. The hyphenated technique has been extensively used for impurity profiling. For the monitoring of the impurity either one of the methods can be utilized or else a combination of the technique can be employed based upon the requirement.
Conflict of interest
The authors report no declarations of interest.
References
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Impurities in New Drug Substances Q3A (R2) 2006;15.
- International Conference on Harmonisation (ICH). Q3B (R2). Impurities in new drug products. ICH Harmon Tripart Guidel 2006;12.
- Görög S. The importance and the challenges of impurity profiling in modern pharmaceutical analysis. Trends Anal Chem 2006;25(8):755-7.
- Ingale SJ, Sahu CM, Paliwal RT, Vaidya S, Singhai AK. Advance approaches for the impurity profiling of pharmaceutical drugs: A review. Int J Pharm Life Sci 2011;2(7):955-62.
- Nagpal S, Upadhyay A, R Bhardwaj T, Thakkar A. A review on need and importance of impurity profiling. Curr Pharm Anal 2011;7(1):62-70.
- Rahman N, Azmi SNH, Wu HF. The importance of impurity analysis in pharmaceutical products: An integrated approach. Accredit Qual Assur 2006;11(1):69-74.
- Görög S. Critical review of reports on impurity and degradation of product profiling in the last decade. Trends Anal Chem 2018;101:2-16.
- Kaufmann B, Christen P. Recent extraction techniques for natural products: microwave‐assisted extraction and pressurised solvent extraction. Phytochem Anal 2002;13(2):105-13.
- Giergielewicz-Mozajska H, Dabrowski L, Namieśnik J. Accelerated solvent extraction (ASE) in the analysis of environmental solid samples - Some aspects of theory and practice. Crit Rev Anal Chem 2001;31(3):149-1\65.
- Conte E, Milani R, Morali G, Abballe F. Comparison between accelerated solvent extraction and traditional extraction methods for the analysis of the herbicide diflufenican in soil. J Chromatogr A 1997;765(1):121-5.
- Herrero M, Mendiola JA, Cifuentes A, Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J Chromatogr A 2010;1217(16):2495-511.
- King JW. Applications of capillary supercritical fluid chromatography-supercritical fluid extraction to natural products. J Chromatogr Sci 1990;28(1):9-14.
- Dispas A, Marini R, Desfontaine V, Veuthey JL, Kotoni D, Losacco LG, et al. First inter-laboratory study of a Supercritical Fluid Chromatography method for the determination of pharmaceutical impurities. J Pharm Biomed Anal 2018;161:414-24.
- De Bohner LS, Soto EF, De Cohan T. Quantitative analysis of phospholipids by thin-layer chromatography. J Chromatogr A 1965;17:513-9.
- Reich E, Schibli A. A standardized approach to modern high-performance thin-layer chromatography (HPTLC). J Planar Chromatogr-Mod TLC 2004;17(6):438-43.
- Shepherd RW, Bunting PS, Khan M, Hill JG, Soldin SJ, Gall DG. A rapid, sensitive method for accurate analysis of individual bile acids in biological fluids by high-performance thin-layer chromatography and densitometry. Clin Biochem 1978;11(3):106-11.
- Neumann H, Gloger M. Profiling of illicit heroin samples by high-resolution capillary gas chromatography for forensic application. Chromatographia 1982;16(1):261-4.
- Sun M, Bai L, Liu DQ. A generic approach for the determination of trace hydrazine in drug substances using in situ derivatization-headspace GC-MS. J Pharm Biomed Anal 2009;49(2):529-33.
- Orata F. Derivatization Reactions and Reagents for Gas Chromatography Analysis. Adv Gas Chromatogr - Prog Agric Biomed Ind Appl 2012:83-108.
- Rao RN, Nagaraju V. An overview of the recent trends in development of HPLC methods for determination of impurities in drugs. J Pharm Biomed Anal 2003;33(3):335-77.
- Vassort A, Barrett DA, Shaw PN, Ferguson PD, Szucs R. A generic approach to the impurity profiling of drugs using standardized and independent capillary zone electrophoresis methods coupled to electrospray ionization mass spectrometry. Electrophoresis 2005;26(9):1712-23.
- Mallampati S, Pauwels J, Hoogmartens J, Van Schepdael A. 12 CE in impurity profiling of drugs. Sep Sci Technol 2008;9(07):259-315.
- D. Tzanavaras P. Recent Advances in the Analysis of Organic Impurities of Active Pharmaceutical Ingredients and Formulations: A Review. Curr Org Chem 2010;14(19):2348-64.
- Karljikovic-Rajic K, Novovic D, Marinkovic V, Agbaba D. First-order UV-derivative spectrophotometry in the analysis of omeprazole and pantoprazole sodium salt and corresponding impurities. J Pharm Biomed Anal 2003;32(4-5):1019-27.
- Nicolas EC, Scholz TH. Active drug substance impurity profiling: Part I. LC/UV diode array spectral matching. J Pharm Biomed Anal 1998;16(5):813-24.
- Dong C, Morita S, Goto M, Zhou H. Space-resolved extreme ultraviolet spectrometer for impurity emission profile measurement in large helical device. Rev Sci Instrum 2010;81(3):033107.
- Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int 2000;107(1-3):5-12.
- Ronowicz J, Kupcewicz B, Mydłowska J, Budzisz E. Impurity profile analysis of drug products containing acetylsalicylic acid: A chemometric approach. Cent Eur J Chem 2013;11(7):1091-1100.
- Kupcewicz B, Ronowicz J, Balcerowska-Czerniak G, Walasek A, Budzisz E. Evaluation of impurities in simvastatin drug products with the use of FT-IR spectroscopy and selected chemometric techniques. Open Chem 2013;11(8):1320-9.
- Rajawat J, Jhingan G. Mass spectroscopy. InData Processing Handbook for Complex Biological Data Sources 2019:1-20.
- Nagy ZK, Balogh A, Vajna B, Farkas A, Patyi G, Kramarics Á, et al. Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci 2012;101(1):322-32.
- Mák M, Czira G, Brlik J. 2.3. Mass spectrometry in impurity profiling. Prog Pharm Biomed Anal 2000;4:97-108.
- Zhou H, Zheng Z, Wu S, Tai Y, Cao X, Pan Y. Separation and characterization of clindamycin and related impurities in bulk drug by high-performance liquid chromatography-electrospray tandem mass spectrometry. J Pharm Biomed Anal 2006;41(4):1116-23.
- Chitturi SR, Bharathi C, Reddy AR, Reddy KC, Sharma HK, Handa VK, et al. Impurity profile study of lopinavir and validation of HPLC method for the determination of related substances in lopinavir drug substance. J Pharm Biomed Anal 2008;48(5):1430-40.
- Berendsen BJ, Zuidema T, De Jong J, Stolker LA, Nielen MW. Discrimination of eight chloramphenicol isomers by liquid chromatography tandem mass spectrometry in order to investigate the natural occurrence of chloramphenicol. Anal Chim Acta 2011;700(1-2):78-85.
- Blinov KA, Elyashberg ME, Molodtsov SG, Williams AJ, Martirosian ER. An expert system for automated structure elucidation utilizing 1H-1H, 13C-1H and 15N-1H 2D NMR correlations. Fresenius J Anal Chem 2001;369(7):709-14.
- Ramachandra B. Development of impurity profiling methods using modern analytical techniques. Crit Rev Anal Chem 2017;47(1):24-36.
- Balogh G. NMR spectroscopy. Prog Pharm Biomed Anal 2000;4:441-7.
- Holzgrabe U. Quantitative NMR spectroscopy in pharmaceutical applications. Prog Nucl Magn Reson Spectrosc 2010;57(2):229-40.
- Niessen WMA, Consultancy HM. Hyphenated Techniques, Applications of in Mass Spectrometry. Encycl Spectrosc Spectrom 2017;59(1982):174-80.
- Jong D, áde Jong GJ, TháBrinkman UA. Investigation of on-line reversed-phase liquid chromatography–gas chromatography–mass spectrometry as a tool for the identification of impurities in drug substances. Analyst 1996;121(1):61-6.
- Wu CH, Feng CT, Lo YS, Lin TY, Lo JG. Determination of volatile organic compounds in workplace air by multisorbent adsorption/thermal desorption-GC/MS. Chemosphere 2004;56(1):71-80.
- McEwen CN, McKay RG. A Combination Atmospheric Pressure Lc/Ms: Gc/Ms Ion Source: Advantages of Dual Ap-Lc/Ms: Gc/Ms Instrumentation. J Am Soc Mass Spectrom 2005;16(11):1730-8.
- Bertram J. 3.2. Gas chromatography and GC/MS. Prog Pharm Biomed Anal 2000;4:409-40.
- Kumar A, Zhang K, Wigman L. Analytical technologies for genotoxic impurities in pharmaceutical compounds. LC-GC North Am 2015;33(5):344-59.
- Portolés T, Mol JG, Sancho JV, Hernández F. Advantages of atmospheric pressure chemical ionization in gas chromatography tandem mass spectrometry: pyrethroid insecticides as a case study. Anal chem 2012;84(22):9802-10.
- Ermer J. The use of hyphenated LC–MS technique for characterisation of impurity profiles during drug development. J Pharm Biomed Anal 1998;18(4-5):707-14.
- Görög S, Babjak M, Balogh G, Brlik J, Csehi A, Dravecz F, et al. Drug impurity profiling strategies. Talanta 1997;44(9):1517-26.
- Lonappan L, Rouissi T, Laadila MA, Brar SK, Hernandez Galan L, Verma M, et al. Agro-industrial-produced laccase for degradation of diclofenac and identification of transformation products. ACS Sustain Chem Eng 2017;5(7):5772-81.
- Iwasa K, Takahashi T, Nishiyama Y, Moriyasu M, Sugiura M, Takeuchi A, et al. Online structural elucidation of alkaloids and other constituents in crude extracts and cultured cells of Nandina domestica by combination of LC-MS/MS, LC-NMR and LC-CD analyses. J Nat Prod 2008;71(8):1376-85.
- Russell DJ, Hadden CE, Martin GE, Gibson AA, Zens AP, Carolan JL. Rapid Communications 2000;63(8):3-5.
- Rinaldi F, Fan J, Pathirana C, Palaniswamy V. Semi‐preparative LC‐SPE‐cryoflow NMR for impurity identifications: use of mother liquor as a better source of impurities. Magn Reson Chem 2013;51(9):517-22.
- Feng W, Liu H, Chen G, Malchow R, Bennett F, Lin E, et al. Structural characterization of the oxidative degradation products of an antifungal agent SCH 56592 by LC–NMR and LC–MS. J Pharm Biomed Anal 2001;25(3-4):545-57.
- Pan C, Liu F, Ji Q, Wang W, Drinkwater D, Vivilecchia R. The use of LC/MS, GC/MS, and LC/NMR hyphenated techniques to identify a drug degradation product in pharmaceutical development. J Pharm Biomed Anal 2006;40(3):581-90.
- Norwood DL, Mullis JO, Feinberg TN. 7 Hyphenated techniques. Sep Sci Technol 2007;8:189-235.
- Wolfender JL, Rodriguez S, Hostettmann K. Liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectroscopy for the screening of plant constituents. J Chromatogr A 1998;794(1-2):299-316.
- Mo H, Balko KM, Colby DA. A practical deuterium-free NMR method for the rapid determination of 1-octanol/water partition coefficients of pharmaceutical agents. Bioorg Med Chem Lett 2010;20(22):6712-5.
- Spraul M, Freund AS, Nast RE, Withers RS, Maas WE, Corcoran O. Advancing NMR sensitivity for LC-NMR-MS using a cryoflow probe: application to the analysis of acetaminophen metabolites in urine. Anal Chem 2003;75(6):1536-41.
- Lin Y, Schiavo S, Orjala J, Vouros P, Kautz R. Microscale LC-MS-NMR platform applied to the identification of active cyanobacterial metabolites. Anal Chem 2008;80(21):8045-54.
- Stutz H. Advances in the analysis of proteins and peptides by capillary electrophoresis with matrix‐assisted laser desorption/ionization and electrospray‐mass spectrometry detection. Electrophoresis 2005;26(7‐8):1254-90.
- Visky D, Jimidar I, Van Ael W, Vennekens T, Redlich D, De Smet M. Capillary electrophoresis‐mass spectrometry in impurity profiling of pharmaceutical products. Electrophoresis 2005;26(7‐8):1541-9.
- Van Wijk AM, Muijselaar PG, Stegman K, De Jong GJ. Capillary electrophoresis-mass spectrometry for impurity profiling of basic pharmaceuticals using non-volatile background electrolytes. J Chromatogr A 2007;1159(1-2):175-84.
- Scheffel U, Rhodes BA, Natarajan TK, Wagner HN. Albumin microspheres for study of the reticuloendothelial system. J Nucl Med 1972;13(7):498-503.
- Guntupalli S, Ray UK, Murali N, Gupta PB, Kumar VJ, Satheesh D, et al. Identification, isolation and characterization of process related impurities in ezetimibe. J Pharm Biomed Anal 2014;88:385-90.
- Prakash L, Malipeddi H, Subbaiah BV, Lakka NS. Impurity profiling and stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05 % (w/w) cream. J Chromatogr Sci 2015;53(1):112-21.
- Lajin B, Steiner O, Fasshold L, Zangger K, Goessler W. The identification and chromatographic separation of a new highly analogous impurity of the active pharmaceutical ingredient icatibant. Eur J Pharm Sci 2019;132:121-124.
- Kumar V, Bhurta D, Sharma A, Kumar P, Bharate SB, Vishwakarma RA, et al. Impurity profiling of anticancer preclinical candidate, IIIM-290. J Pharm Biomed Anal 2019;166:1-5.
- Prakash L, Himaja M, Subbaiah BV, Vasudev R, Srinivasulu C, Haribabu R. Isolation, identification and characterization of degradant impurities in Tolterodine tartrate formulation. J Pharm Biomed Anal 2014;90:215-221.
- Zhuang T, Zhang W, Cao L, He K, Wang Y, Li J, et al. Isolation, identification and characterization of two novel process-related impurities in olanzapine. J Pharm Biomed Anal 2018;152:188-96.
- Kumar N, Devineni SR, Gajjala PR, Dubey SK, Kumar P. Synthesis, isolation, identification and characterization of new process-related impurity in isoproterenol hydrochloride by HPLC, LC/ESI-MS and NMR. J Pharm Anal 2017;7(6):394-400.
- Karthikeyan K, Arularasu GT, Murali V, Pillai KC. Identification, isolation, characterization and response factor determination of process-related impurity in meprobamate drug substance. J Pharm Biomed Anal 2011;54(1):208-212.
- Reddy RB, More KR, Gupta L, Jha MS, Magar L. Identification, synthesis, isolation and characterization of new impurity in metoprolol tartrate tablets. J Pharm Biomed Anal 2016;117:104-8.
- Darcsi A, Tóth G, Kökösi J, Béni S. Structure elucidation of a process-related impurity of dapoxetine. J Pharm Biomed Anal 2014;96:272-277.
- Reddy GV, Reddy BV, Haque SW, Gautam HD, Kumar P, Kumar AP, et al. Development and validation of a stability-indicating UPLC method for rosuvastatin and its related impurities in pharmaceutical dosage forms. Quimica Nova 2011;34(2):250-5.
- Reddy GR, Kumar AP, Ram Reddy BV, Sreeramulu J. Application of ion-trap mass spectrometry for identification and structural determination of an unknown impurity in simvastatin. Pharmazie 2009;64(10):638-41.
- Ram Reddy GV, Kumar AP, Venkateswara Reddy B, Sreeramulu J, Park JH. Separation, identification and structural elucidation of a new impurity in the drug substance of amlodipine maleate using LC-MS/MS, NMR and IR. Croat Chem Acta 2010;83(4):443-9.
- Venkateswara Reddy B, Kumar AP, Ram Reddy GV, Sahai M, Sreeramulu J, Park JH. Stability indicating reversed-phase high performance liquid chromatography method for determination of impurities in ofloxacin tablet formulations. Anal Lett 2010;43(17):2653-62.