- Corresponding Author:
- Manisha Puranik
P.G. Department of Quality Assurance, Institute of Pharmaceutical Education and Research, Borgaon (Meghe), Wardha-442 001, India. E-mail: manisha68_12@yahoo.com
| Date of Submission | 29 December 2006 |
| Date of Revision | 20 December 2007 |
| Date of Acceptance | 19 June 2008 |
| Indian J Pharm Sci, 2008, 70 (3): 386-390 |
Abstract
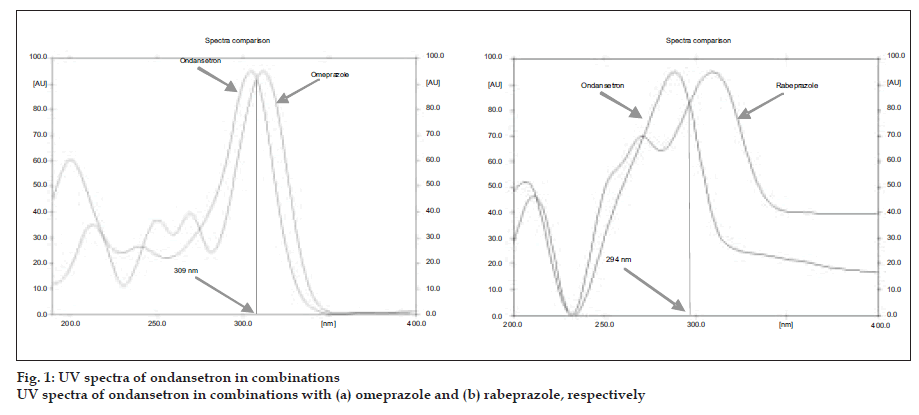
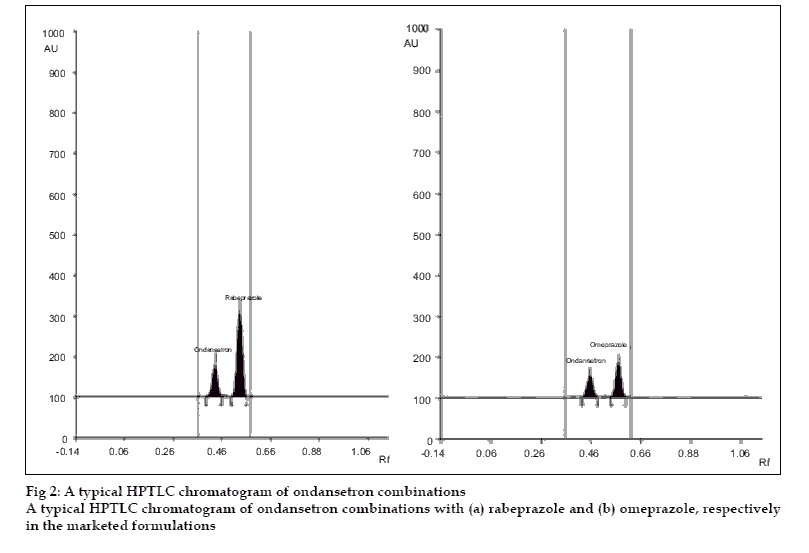
A simple, precise, accurate and rapid high performance thin layer chromatographic method has been developed for the simultaneous estimation of ondansetron combinations in solid dosage form with omeprazole and rabeprazole, respectively. The method involved separation of components by TLC on a precoated silica gel 60 F 254 using a mixture of dichloromethane:methanol (9:1) as a mobile phase. Detection of spots was carried out at 309 nm and 294 nm for ondansetron with omeprazole and ondansetron with rabeprazole combinations, respectively. The mean retardation factor for ondansetron and omeprazole were found to be 0.42±0.02, 0.54±0.03, respectively while for ondansetron and rabeprazole, 0.41± 0.02 and 0.51±0.02, respectively. The linearity and range was 0.1 to 0.5 µg/spot for three drugs. The method was validated for precision, accuracy and reproducibility.
Keywords
Ondansetron, rabeprazole, omeprazole, HPTLC, validation
Ondansetron combination with proton pump inhibitors has recently been introduced in market for the treatment of peptic ulcer, gastroesophagal reflux disease (GERD) and to prevent nausea. Ondansetron antagonizes 5HT-3 receptor both peripherally as well as centrally and block the initiation of the reflux, so is used as antiematic. Ondansetron is official in USP1. Omeprazole and rabeprazole are benzimidazole proton pump inhibitors, which suppress gastric acid secretion by H+/K+-ATPase enzyme system at the secretory surface of the gastric parietal cell. These drugs are used for the treatment of duodenal, gastric and esophageal ulceration. Omeprazole is official in USP [1], IP [2], BP [3], where as rabeprazole is not official in any of the pharmacopoeia, but is reported in Merck Index [4].
Literature survey revealed HPLC [5,6] in human plasma, visible spectrophotometric method [7] for ondansetron in solid dosage form. For omeprazole methods reported are HPLC-MS and HPLC-UV in biological fluids [8,9], capillary electrophoresis [10], HPLC employing electrochemical and coulometric detection [11], TLC [12] and spectrophotometry [13]. For rabeprazole method reported are HPLC for detection in blood plasma [14,15] spectrophotometric methods [16], and LC-MS/MS [17] method. As no analytical method has so far been indicated for the ondansetron combinations with proton pump inhibitors, an attempt has been made to estimate them simultaneously by HPTLC.
Ondansetron reference standard was obtained as a gift sample from Neon Lab Ltd, Mumbai. Rabeprazole was obtained from Dr. Reddy′s Lab, Hyderabad and omeprazole obtained from Zydus Cadila Health Care, Ahmedabad. Silica gel 60 F254 TLC plates (20 × 10cm, layer thickness 0.2 mm, E. Merk, Mumbai) were used as a stationary phase. Dichloromethane:methanol (9:1) used as a mobile phase for both combinations. A Camag HPTLC system comprising of Camag linomat V semiautomatic sample applicator, Camag TLC scanner 3, Camag Win CATS software, Camag twin trough chamber and a sonicator were used. Tablets containing omeprazole (10 mg) and ondansetron (4 mg) (Ranidom-O, Mankind Pharmaceuticals Pvt. Ltd., Mumbai, India) and capsules containing rabeprazole (20 mg) and ondansetron (6 mg) (OND-R, Besto Chemical Ltd., New Delhi, India) were used during study.
Working standards of ondansetron, omeprazole and rabeprazole (25 mg each) were weighed and diluted with methanol to get the final concentration 0.05 µg/ µl for omeprazole and 0.02 µg/µl for ondansetron and 0.05 µg/µl for rabeprazole and 0.015 µg/µl for ondansetron. For ondansetron combination with rabeprazole, contents of twenty capsules was crushed to fine powder, quantity equivalent to 20 mg rabeprazole (6 mg ondansetron) was weighed accurately and transfer to 10 ml volumetric flask and for ondansetron combination with omeprazole, twenty tablets were crushed to fine powder, weight equivalent to 10 mg of omeprazole (4 mg of ondansetron) was transferred to 10 ml volumetric flask. Then to each flask about 5ml of methanol was added and sonicated for 15 min, finally volume was made to mark with methanol. The extracts were filtered through Whatman filter paper 41 and required dilutions were made to get the final concentration containing 0.05µg/µl rabeprazole, 0.015 µg/µl ondansetron and 0.05 µg/ µl omeprazole, 0.02 µg/µl ondansetron and 6 µl of standard and sample were applied as 5 mm band on the TLC plate.
TLC plates were prewashed with methanol and activated prior to use. The chromatographic conditions maintained were: Precoated Silica gel 60 F254 (20×10 cm) aluminum sheets as stationary phase. Dichloromethane:methanol (9:1 V/V) as mobile phase for both the ondansetron combinations. Samples were applied as bands 5 mm width at 14.1 mm intervals using Camag linomate V semiautomatic sample applicator and migration distance allowed was 80 mm, drying of plate done for 3 min at 600 temperatures. The plates were scanned at 309 nm for omeprazole and ondansetron and 294 nm for rabeprazole and ondansetron combination with Camag TLC scanner III, using Camag Win CATS software (fig. 1).
For calibration curve, 0.1, 0.2, 0.3, 0.4 and 0.5 µg/ µl standard solution of omeprazole, rabeprazole and ondansetron were applied on TLC plate. The TLC plates were dried, developed and analyzed as described earlier.
Filtered solutions (6 µl) of the marketed formulations were spotted on to the plate followed by development scanning. The analysis was repeated six times, the spot was resolved into two peaks in the chromatogram of drug samples. The contents were calculated from the peak areas of standards and samples recorded.
A solvent system that would give dense and compact spots with appropriate and significantly different Rf values was desired for quantification of ondansetron combinations. The mobile phase consisting of dichloromethane: methanol (9:1 V/V) gave Rf value of 0.42±0.02, 0.54±0.03, respectively for ondansetron and omeprazole while for ondansetron and rabeprazole, 0.41± 0.02 and 0.51±0.02, respectively (fig. 2).
The developed method was validated in terms of linearity and range, limit of detection, limit of quantification, recovery study, inter days study, intra day study and study by different analysts. The limit of detection for omeprazole, ondansetron and rabeprazole, ondansetron was found to be 99 ng/spot, 39.9 ng/spot, and 90.3 ng/spot, 54.2 ng/spot, respectively. The limit of quantification for omeprazole, ondansetron and rabeprazole, ondansetron was found to be 302.8 ng/ spot, 121.1 ng/spot and 273.9 ng/spot, 164.4 ng/spot, respectively.
The linear regression data (n=6, Table 1) showed a good linear relationship over a concentration range 100 to 500 ng/spot for omeprazole, ondansetron and rabeprazole. Repeatability of method was determined by 6 times spotting 10 µl of standard drug solution on TLC plate, measurement of peak areas was performed and from the peak areas the % RSD was determined. For omeprazole and ondansetron % RSD was found to be 0.35 and 0.40, respectively and for rabeprazole and ondansetron % RSD was 0.19 and 0.45, respectively. Repeatability of measurement was determined by spotting 10µl of standard drug solution on TLC plate, after development the separated spots were scanned six times without changing position and % RSD for measurement of peak areas of omeprazole and ondansetron was found to be 0.64 and 0.80, respectively and for rabeprazole and ondansetron % RSD was 0.31 and 0.42, respectively.
| Parameters* | Omeprazole | Ondansetron | Rabeprazole | Ondansetron |
|---|---|---|---|---|
| Rf (±SD) | 0.54±0.03 | 0.42±0.02 | 0.51±0.02 | 0.41±0.02 |
| Linearity and range (ng/spot) | 100-500 | 100-500 | 100-500 | 100-500 |
| Limit of detection (ng/spot) | 99 | 39.9 | 90.3 | 54.2 |
| Limit of quantification (ng/spot) | 302.8 | 121.1 | 273.9 | 164.4 |
| Repeatability of application (%RSD) | 0.35 | 0.4 | 0.19 | 0.45 |
| Repeatability of measurement (%RSD) | 0.64 | 0.8 | 0.31 | 0.42 |
| Intra day (%RSD) | 0.12 | 0.14 | 0.66 | 0.29 |
| Inter day (%RSD) | 0.77 | 0.58 | 0.23 | 0.54 |
| Different analysts (%RSD) | 0.21 | 0.61 | 0.14 | 0.72 |
*Average of 6 determinations
Table 1: Validation parameters
The assay value for the marketed formulation was found to be within the limits as listed in Table 2. The low RSD value indicates suitability of the method for routine analysis of omeprazole, ondansetron and rabeprazole, ondansetron in pharmaceutical dosage form. Recovery studies were carried out to study accuracy and precision of the method. These studies were carried out at three levels i.e. multiple level recovery studies. To the powder formulations the pure standard drug were added at 80%, 100% and 120% levels, dilutions were made and analyzed by the method, the % recovery was calculated by using formula, % recovery = (T-A)/S × 100 where, T is total amount of the drug estimated, A is the amount of drug contributed by tablet powder and S is the amount of pure drug added. The results of recovery studies for both the ondansetron combinations were found to be around 99-100%, indicating that the method is free from interference from excipients.
| Brand name | Drug | Label claim (mg/tab) | Amount found* (mg/tab) | % drug found* | % RSD |
|---|---|---|---|---|---|
| Ranidom-O | Ondensetron | 4 | 3.99 | 99.9 | 0.45 |
| Omeprazole | 10 | 10.039 | 100.39 | 0.49 | |
| OND-R | Ondensetron | 6 | 5.99 | 99.96 | 0.82 |
| Rabeprazole | 20 | 19.96 | 99.83 | 0.72 |
*Average value of 6 observations
Table 2: Analysis of ondensetron with omeprazole (ranidom-o) and ondensetron with rabeprazole (ond-r)
The ruggedness of the method was evaluated by studying analyst to analyst, intra day and inter days variations and the % RSD was calculated, that was found to be within range. From the above results it can be concluded that the HPTLC method is accurate, precise, specific and reproducible and can be used for routine analysis of ondansetron combinations with proton pump inhibitors in solid dosage form.
Acknowledgements
The authors thank the Principal, Institute of Pharmaceutical Education and Research, Wardha and Anchrom Lab, Mumbai for providing facilities for the research work and Neon Lab Ltd for providing ondansetron, Dr. Reddy′s Lab for providing rabeprazole and Zydus Cadila Health Care for providing omeprazole as gift sample for analysis.
References
- The United States pharmacopoeia XXIV and National Formulary XIX, Asian ed. Rockville, MD: US Pharmacopoeial Convention, Inc.; 2000. p. 1219.
- Indian Pharmacopoeia, Vol. II, Govt. of India, Ministry of Health and Family Welfare. New Delhi; Published by The Controller of Publications; 1996. p. 532-3.
- British Pharmacopoeia. 15th ed. Published on the recommendation of the Medicines Commission Pursuant to the Medicines Act 1968, London: HMSO; 1993. p. 1590.
- The Merck Index, 13th ed. Whitehouse Station, NJ: Merck and Co.; Inc.,; 2001. p. 8181.
- Deport M, Leroux S, Caille G. High resolution liquid chromatographic method using ultraviolet detection for the determination of ondensetron in human plasma. J Chromatogr B 1997;693:399-404.
- Colthup P, Felgat CC, Palmar J, Scully N. Determination of ondensetron in plasma and its pharmacokinetics in young and elderly. J Pharm Sci 1991;868:71-4.
- Sastry SP, Rao T. Spectophotometric determination of amiodarone and ondansetron in pharmaceutical dosage forms with citric acid-acetic acid anhydride reagent. Indian J Pharm Sci 2002;63:482-5.
- Kange Z, Huijuan J, Wei L. Determination of omeprazole in rat plasma by HPLC without solvent extraction. J Chromatogr B 2006; 837:112-6.
- Yuch K, Wai PC, Huey YT, Jia WW. Improved high performance liquid chromatographic analysis of omeprazole in human plasma. J Pharm Biomed Anal 2001;24:715-9.
- Berzas JJ, Castanda G. Method development and validation for the separation and determination of omeprazole enantiomers in pharmaceutical preparation by capillary electrophoresis. Anal Chem Acta 2005;533:127-32.
- Gregory WS, John DS, James HA, Zheng Z. Omeprazole determination using HPLC with coulometric detection. J Pharm Biomed Anal 2001;25:357-62.
- Agbaba D, Novovic D, Karaljikovic K, Maronqovic V. Densitometric determination of omeprazole, pantoprazole and their impurities in pharmaceuticals. J Planar Chrom Modern TLC 2004;17:169-72.
- Lakshmi S, Venkatesan M. Simultaneous estimation of omeprazole and domperidone in solid oral dosage form using spectrophotometric method. Indian Drugs 2003;40:589-92.
- Ramakrishna NV, Vishwottam KN, Wishu S, Koteshwara M, Suresh Kumar S. High performance liquid chromatography method for the quantification of rabeprazole in human plasma using solid phase extraction. J Chromatogr B 2005;816:209-16.
- Mehta BR, Mehta RS, Bhatt KK. RP-HPLC method for the estimation of rabeprazole sodium in bulk and tablet dosage form. Indian Drugs 2005;42:39-42..
- Gindy AE, Fawzy EY, Moustafa M. Spectrophotometric and chromatographic determination of rabeprazole in presence of its degradation products. J Pharm Biomed Anal 2003;31:229-32.
- Yong Z, Xiaoyan C, Defang Z. Quantification of rabeprazole in human plasma by liquid chromatography- tandem mass spectrometry. Anal Chem Acta 2004;523:171-5.