- *Corresponding Author:
- Y. X. Wang
Department of Cardiology, Yongkang First People’s Hospital, Yongkang, Zhejiang 321300, People’s Republic of China
E-mail: jdwyx@qq.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “93-102” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The Barthel index is a widely used assessment tool for evaluating physical performance in activities of daily living. The present study investigated the relationship between activities of daily living measured by Barthel Index at hospital admission and the occurrence of contrast-associated acute kidney injury among patients undergoing coronary angiography or percutaneous coronary intervention. In this retrospective cross-sectional study, 3148 patients undergoing coronary angiography or percutaneous coronary intervention were enrolled. Activities of daily living were stratified into 5 degrees according to Barthel index scores. Contrast-associated acute kidney injury was defined as an increase of either 25 % or 0.5 mg/dl (44.2 μmol/l) in basal serum creatinine level within 72 h, following the use of contrast agent. Univariable and multivariable linear and logistic regression analysis were used to determine the associations of Barthel index with the proportion of serum creatinine elevation and contrast-associated acute kidney injury, respectively. The exploratory subgroup analysis was further conducted. Totally, 16.7 % of patients suffered from contrast-associated acute kidney injury and the mean age was 67.07±10.71 y old, 65.7 % for males. Multivariable linear regression analysis demonstrated that Barthel index scores were significantly associated with the proportion of serum creatinine elevation (beta=-10.150, 95 % confidence interval=-11.788 to -8.511, p<0.001). Multivariable linear regression analysis demonstrated that Barthel index scores were independent predictors of contrast-associated acute kidney injury incidence (p<0.001). With the increase in Barthel index scores, the incidence of contrast-associated acute kidney injury showed a significant downward tendency. Subgroup analysis showed consistent results. Activities of daily living measured by Barthel index at hospital admission was an independent prediction indicator to assess contrast-associated acute kidney occurrence among patients undergoing coronary angiography or percutaneous coronary intervention.
Keywords
Activities of daily living, Barthel index, contrast-associated acute kidney injury, coronary angiography, percutaneous coronary intervention
Contrast-Associated Acute Kidney Injury (CA-AKI), known as Contrast-Induced Acute Kidney Injury (CIAKI), is one of the major complication after Coronary Angiography (CAG) or Percutaneous Coronary Intervention (PCI) and the incidence is about 2.7 %-20.6 %[1-3]. CA-AKI pathophysiology includes both direct and indirect damages caused by contrast agents, mainly involving tubular injury, vascular dysfunction and inflammation[4]. The occurrence of CA-AKI directly or indirectly causes prolonged hospital stays, more expensive hospitalizations and increased mortality[5,6]. Setting up a facile and economic prediction model to identify patients at high risk is crucial. Currently, several models for predicting CA-AKI occurrence have been developed based on traditional risk factors, mainly involving test results and procedural characteristics[7-9]. However, few studies have been conducted to explore the impact of a patient’s physical function on the occurrence of CA-AKI.
Activities of Daily Living (ADL) are defined as the ability of physical performance required for independent living, such as feeding, dressing, bathing and toileting. The decline of ADL leads to functional dependence, a condition in which a person cannot complete basic activities without assistance, which is thought to be an intuitive external presentation of the adverse effects of various diseases on physical function. Developed in 1965[10], the Barthel Index (BI) is still widely used today as a wellestablished score to assess the functional capacity for ADL on a clinical basis because of its simplicity, communicability and ease of scoring[11,12]. Although healthcare professionals routinely evaluate patients at hospital admission through BI scores, research has been mainly exclusive to neurological disorders or terminal cancers[13,14] and BI’s role in CAG-related complications remains unclear.
The present study aimed to investigate the relationship between ADL measured by BI at hospital admission and CA-AKI occurrence among patients undergoing CAG or PCI.
Materials and Methods
Study population:
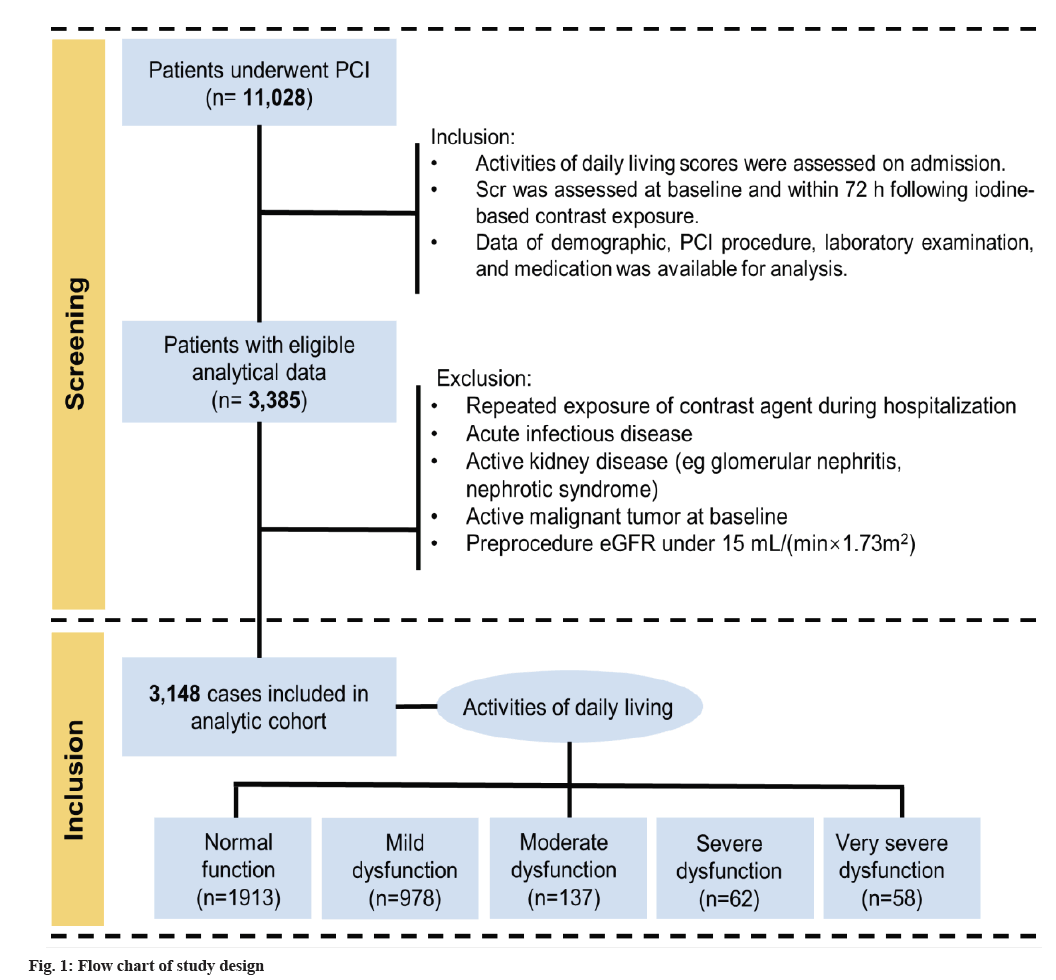
This study was a retrospective review of all consecutive eligible patients undergoing CAG or PCI from December 2006 to December 2019 at Sir Run Run Shaw Hospital and its medical consortium hospitals. Additional details about the study subjects are listed in fig. 1.
Selection criteria: The inclusion criteria include patients with ADL scores assessed at hospital admission; patients with documented Serum creatinine (Scr) before the procedure and at 72 h after the procedure; patients with complete data of demographics, angiographic procedure, laboratory examination and medication. Exclusion criteria include patients with repeated contrast exposure within 1 w or less from the procedure; patients with a pre-existing end-stage renal disease requiring hemodialysis, estimated Glomerular Filtration Rate (eGFR)<15 ml/min/1.73 m2; patients with active kidney disease (e.g., glomerular nephritis, nephrotic syndrome); patients with an acute infectious disease or active malignant tumor. Finally, 3148 patients were enrolled. The study was carried out according to the Declaration of Helsinki and was approved by the Ethics Committee of Sir Run Run Shaw Hospital (No. 20201217-36).
Data collection and assessment of clinical parameters:
Different parameters involving patient demographics, clinical features, laboratory results, medications and angiographic characteristics were obtained from the hospital information system.
Healthcare professionals evaluate BI score routinely at the patient’s hospital admission. The BI score is recommended as an assessment of ADL, consisting of 10 items about feeding, dressing, bathing, grooming, toileting, bowel control, bladder control, ambulation, chair transferring and stair climbing, with scoring from 0 to 100 (0=completely dependent; 100=completely independent)[10]. ADL were stratified according to BI scores into the following 5 degrees, normal function (BI=100), mild dysfunction (60-99), moderate dysfunction (40-59), severe dysfunction (20-39) and very severe dysfunction (BI<20).
The concentrations of Scr were measured at hospital admission and the postoperative Scr concentrations recorded were the highest for at least three measurements within a 72 h timeframe. CA-AKI was defined as an increase of either 25 % or 0.5 mg/ dl (44.2 μmol/l) in basal Scr concentrations within 72 h following the implementation of the contrast agent[15].
Statistical analysis:
Statistical analyses were performed using R version 4.0.5 (The R Foundation for Statistical Computing, Vienna, Austria) and Statistical Package for the Social Sciences (SPSS) software version 22.0 (SPSS Inc., Chicago, Illinois (IL), United States). Continuous variables were expressed as mean±Standard Deviation (SD) and compared using Student t-test in case of normal distributed, while expressed as median (Interquartile Range [IQR]) and compared using Mann-Whitney U-test in case of non-normal distributed. Categorical variables were expressed as counts (percentage) and compared using Chi-Square test or Fisher’s exact test.
Univariant and multivariate linear regression analyses was done to evaluate the relationship between BI on admission and the proportion of Scr elevation and Locally Estimated Scatterplot Smoothing (LOESS) curve was used to fit the relationship between BI score and the proportion of Scr elevation. Univariant and multivariate logistic regression were used to determine the association of BI on admission with CA-AKI and Restricted Cubic Spline (RCS) curve was performed for visualization analysis. The previously recognized risk factors variables of CA-AKI from previous studies were included as covariates in the multivariate regression analysis[16]. Tests for trend (p for trend) were carried out by the predefining ordered categorical variable (BI: 100, 60-99, 40-59, 20-39, <20) acting as a continuous variable in the logistic regression analysis. Finally, the exploratory subgroup analysis was conducted to assess the relationship between BI and CA-AKI. The p-values<0.05 were considered statistically significant.
Results and Discussion
Totally, 3148 patients were enrolled in the study and the mean age was 67.07±10.71 y old, 65.7 % for males. The mean BI among the whole population was 87.98±20.99 scores. The incidence of CA-AKI was 16.9 % (533/3148). Table 1 shows the details of the baseline clinical and procedural characteristics.
| Characteristics | CA-AKI | |||||
|---|---|---|---|---|---|---|
| Overall (n=3148) | No (n=2615) | Yes (n=533) | p value | |||
| Demographic features | ||||||
| Age, years old | 67.07±10.71 | 66.55±10.73 | 69.61±10.25 | <0.001* | ||
| Male, n (%) | 2067 (65.7) | 1746 (66.8) | 321 (60.2) | 0.004* | ||
| Diabetes, n (%) | 760 (24.1) | 611 (23.4) | 149 (28.0) | 0.028* | ||
| Hypertension, n (%) | 2005 (63.7) | 1652 (63.2) | 353 (66.2) | 0.198 | ||
| BMI, kg/m2 | 24.42±5.31 | 24.31±5.32 | 24.97±5.21 | 0.023* | ||
| EF, % | 59.36±12.97 | 60.04±12.76 | 56.03±13.53 | <0.001* | ||
| BI, score | 87.98±20.99 | 90.31±18.39 | 76.56±28.05 | <0.001* | ||
| Laboratory data | ||||||
| eGFR, ml/(min×1.73 m2) | 78.78±23.34 | 79.57±22.15 | 74.89±28.16 | <0.001* | ||
| Low-Density Lipoprotein Cholesterol (LDL-C), mmol/l | 2.22±0.90 | 2.23±0.91 | 2.19±0.87 | 0.409 | ||
| cTnI, μg/l | 0.78±2.59 | 0.61±2.24 | 1.62±3.75 | <0.001* | ||
| CK-MB, U/l | 25.05±38.77 | 23.54±32.90 | 32.72±59.81 | <0.001* | ||
| HbA1c, % | 6.42±1.33 | 6.38±1.28 | 6.65±1.60 | 0.001* | ||
| PCI procedure data | ||||||
| Volume of contrast agent, mg | 102.07±71.53 | 101.35±71.70 | 105.61±70.64 | 0.21 | ||
| Chronic Total Occlusion (CTO), n (%) | 136 (16.4) | 110 (16.1) | 26 (17.8) | 0.708 | ||
| Multivessel lesions, n (%) | 50 (6.6) | 40 (6.4) | 10 (7.9) | 0.673 | ||
| Direct PCI, n (%) | 105 (14.1) | 85 (13.7) | 20 (15.7) | 0.644 | ||
| Medication | ||||||
| Statin, n (%) | 2626 (83.4) | 2209(84.5) | 417 (78.2) | 0.001* | ||
| BB, n (%) | 1588 (50.4) | 1298 (49.6) | 290 (54.4) | 0.05 | ||
| ACEI/ARB, n (%) | 1398 (44.4) | 1181 (45.2) | 217 (40.7) | 0.066 | ||
| CCB, n (%) | 886 (28.1) | 740 (28.3) | 146 (27.4) | 0.711 | ||
Note: Categorical data are presented as n (%) and continuous data are expressed as mean±SD, *p<0.05
Table 1: Baseline Characteristics of the Study
Compared with non-CA-AKI, CA-AKI patients were significantly older (69.61±10.25 vs. 66.55±10.25 y old, p<0.001), had fewer male sex (60.2 % vs. 66.8 %, p=0.004) and a higher proportion of diabetes mellitus (28.0 vs. 23.4%, p=0.028). CAAKI patients had higher levels of Body Mass Index (BMI), Hemoglobin A1c (HbA1c), Creatine Kinase- Myocardial Band (CK-MB), cardiac Troponin I (cTnI) and lower levels of eGFR and Ejection Fraction (EF) (all p-values<0.05). Besides, the medication characteristics of CA-AKI patients were reflected in a lower proportion of use of statin (p-value<0.05). Additionally, CA-AKI patients had a significantly lower BI than non-CA-AKI (76.56±28.05 vs. 90.31±18.39 scores, p<0.001).
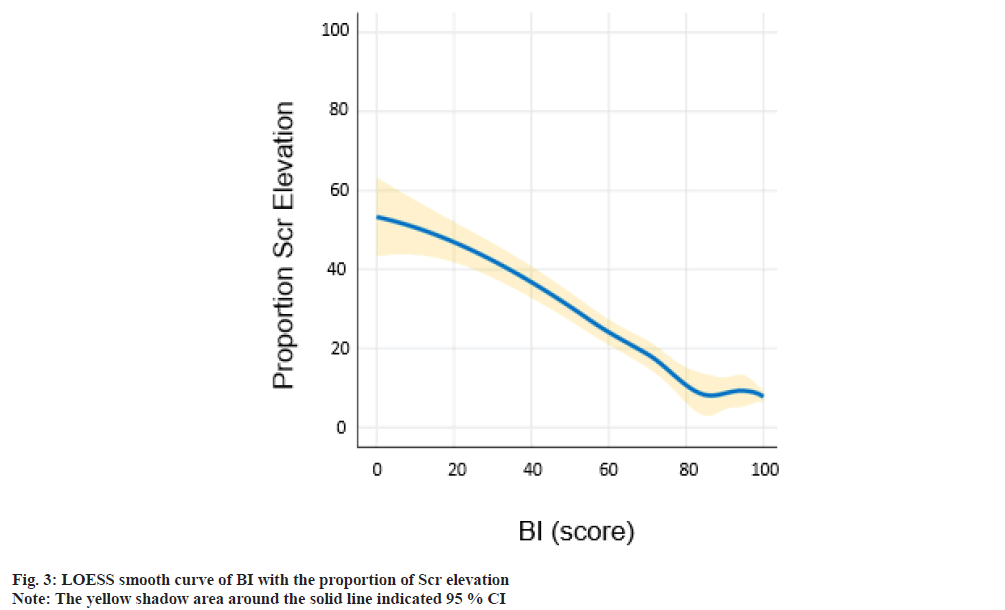
The population was divided into 5 degrees according to BI scores (predefined cut points: 100, 60-99, 40-59, 20-39, <20). Fig. 2 shows the population distribution and the incidence of CA-AKI in 5 groups. With the decrease in BI score, a decline in population counts and elevation of CA-AKI incidence was observed in the present study. The blue histograms described the overall distribution in each BI group. The gold dashed line depicts the trend in incidence of CAAKI. The relationship between BI and proportion of Scr elevation was shown here. As shown in fig. 3, LOESS smooth curve was conducted to fit the relationship between BI score and the proportion of Scr elevation. With the elevation of the BI score, the proportion of Scr elevation showed an overall downward tendency.
Linear regression analysis done to assess the relationship between BI score and the proportion of Scr elevation was shown in Table 2. After adjusting for age, gender, diabetes mellitus, hypertension, EF, eGFR, volumes and type of contrast agent, and medications (statin, Beta-Blockers (BB), Angiotensin Converting Enzyme Inhibitors (ACEI)/ Angiotensin Receptor Blockers (ARBs) and Calcium Channel Blockers (CCBs), multivariable linear regression analysis demonstrated that BI scores were independent predictors for the proportion of Scr elevation (beta (β)=-10.150, 95 % Confidence Interval [CI]: -11.788 to -8.511, p<0.001). BI scores were negatively correlated with the proportion of Scr elevation.
| Models | β-coefficient [95 % CI] | p value |
|---|---|---|
| Model 1 | -10.921 [-12.48 to -9.363] | <0.001* |
| Model 2 | -10.119 [-11.738 to -8.499] | <0.001* |
| Model 3 | -10.150 [-11.788 to -8.511] | <0.001* |
Note: *p<0.05
Table 2: Linear Regression Analyses of Bi on the Proportion of SCR Elevation
As shown in Table 2, model 1 is adjusted for none; model 2 is adjusted for age (years old), gender (male or female), diabetes (yes or no), hypertension (yes or no), EF (%), eGFR (ml/min/1.73 m2), contrast volume (mg) and contrast type (isotonic or hypotonic); model 3 is based on model 2, additionally adjusted for medications (administration of statin, BB, ACEI/ ARB and CCB) (yes or no).
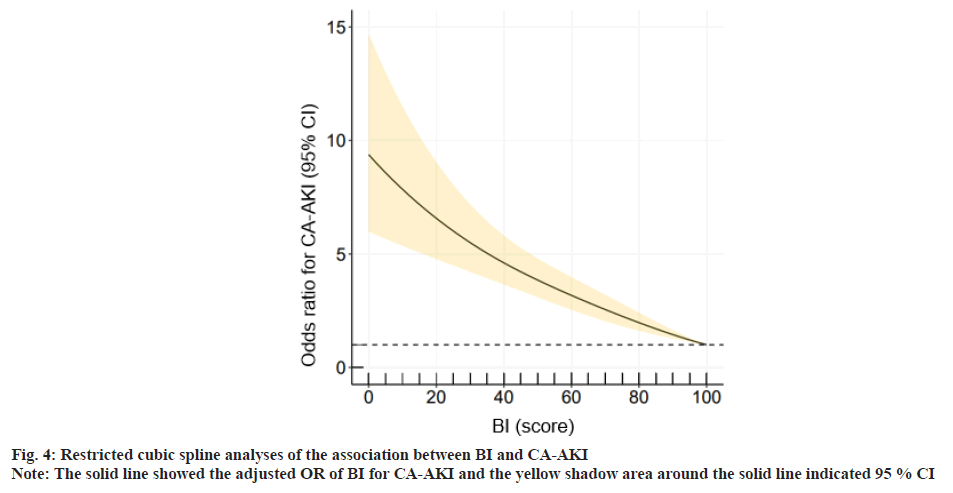
The relationship between BI and the incidence of CAAKI was explained here. As shown in fig. 4, restricted cubic spline analysis was conducted to explore the relationship of BI score with CA-AKI. BI scores, the incidence of CA-AKI showed a downward tendency.
The univariable and multivariable logistic regression analysis were used to determine the relationship between BI and the incidence of CA-AKI (Table 3). Model 1 showed BI scores which were negatively correlated with the incidence of CA-AKI (p for trend<0.001; each grade compared with the reference: 60-99 vs. 100: 21.4 % vs. 11.4 %, Odds Ratio (OR)=2.102, 95 % CI [1.708 to 2.588], p<0.001; 40-59 vs. 100: 32.8% vs. 11.4%, OR=3.784, 95 % CI [2.579 to 5.551], p<0.001; 20-39 vs. 100: 48.4 % vs. 11.4 %, OR=7.252, 95 % CI [4.322 to 12.168], p<0.001; <20 vs. 100: 51.7 % vs. 11.4 %, OR=8.288, 95 % CI [4.859 to 14.135], p<0.001).
After adjusting for age, gender, diabetes mellitus, hypertension, EF, eGFR, volumes and type of contrast agent, (shown in model 2), and further adjusting for medications (statin, BB, ACEI/ARB and CCB) (shown in model 3), multivariable linear regression analysis demonstrated that BI scores were independent predictive indicators for the incidence of CA-AKI (all p for trend<0.001).
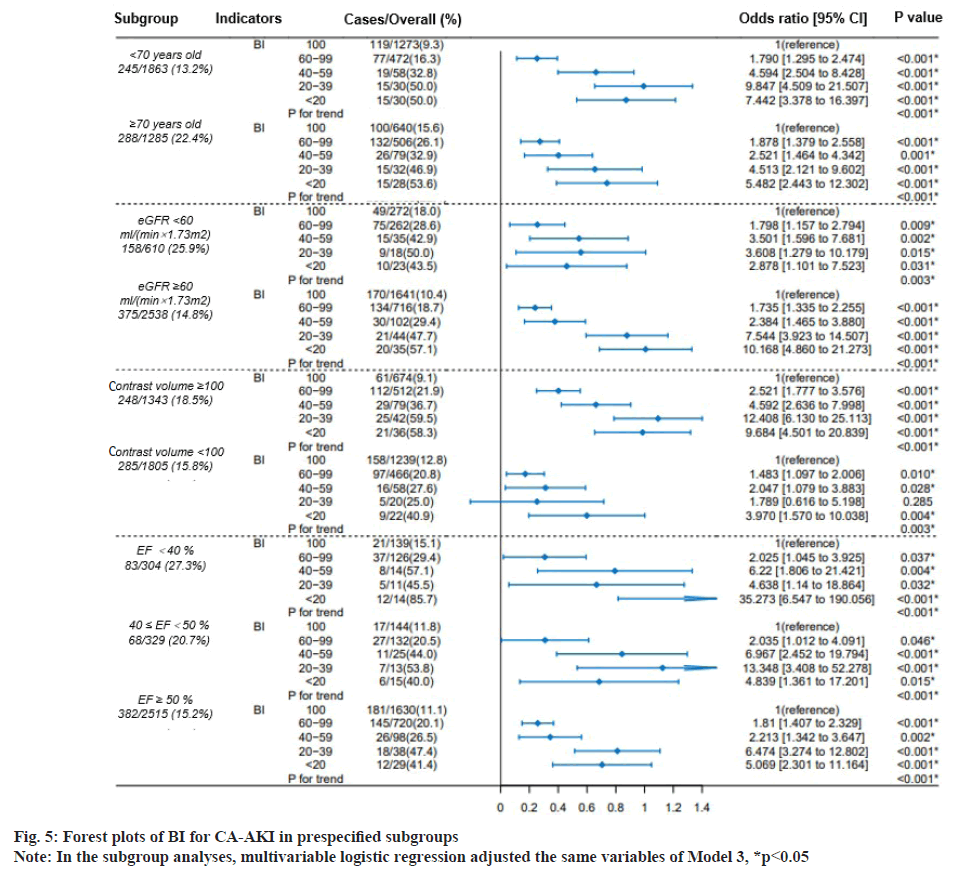
Fig. 5 showed the exploratory analysis performed in subgroups, based on age (<70 or ≥70 y.), eGFR (<60 or ≥60 ml/min/1.73 m2), contrast volumes (<100 or ≥100 mg) and EF (<40, 40≤EF<50, ≤50%). Compared with the reference (BI=100), the incidence of CA-AKI increased with the decreasing BI, which remained consistent in all subgroups (all p for trend<0.001).
The morbidity of CA-AKI was about 16.9 % in this multicenter retrospective study. The results demonstrated that BI scores were negatively correlated with the proportion of Scr elevation. ADL measured by BI at hospital admission was an independent prediction indicator to assess the incidence of CA-AKI among patients undergoing CAG or PCI, that was, the incidence of CA-AKI increased with decreasing BI scores. Similar results were observed in subgroups by exploratory analysis.
The association of better exercise capacity with fewer complications and better prognosis is well established[17]. A nationwide cohort study from the Danish national geriatric database demonstrated that ADL provided independent evaluation information on the expected survival time of elderly hospitalized patients[18]. The research of ADL have been mostly restricted to neurological disorders or terminal cancers[19,20]. With great reliability, validity and repeatability, the BI score is a standard routine assessment procedure for inpatients[11]. The BI scores can be assessed orally by inquiring the patient or a family member. With rapid and simple properties, BI score is applicable to evaluate patients early at hospital admission. Higuchi et al. adapted this valuable indicator in cerebrovascular diseases field to assess the long-term prognosis of patients with Acute Coronary Syndrome (ACS)[21]. This smallscale retrospective study showed that ADL at discharge was a valuable predictive indicator for the long-term prognosis and 1 y mortality among elderly ACS patients after PCI[21]. Another multi-center retrospective cohort study further supported that BI score at hospital admission was associated with mortality during follow-up in ACS patients[22]. It has not been investigated in depth for the association between ADL and the short-term complications of cardiovascular disease.
As predicted, the incidence of CA-AKI is increasing with the decline in BI scores. The decline in ADL is often accompanied by old age, organ dysfunction and increased comorbidities, such as diabetes and hypertension[23-25]. Advanced age is well recognized as a negative prognostic marker for CA-AKI[26,27]. Patients with preoperative cardiac or renal dysfunction are also more likely to occur CA-AKI[28,29]. With the decline in ADL, patients with impaired swallowing and digestion function can adversely occur nutritional risks to affect their prognosis[30]. Our research center has set up a retrospective cross-sectional study to demonstrate that high nutritional risks will increase CA-AKI morbidity among patients after CAG[31]. Moreover, favorable ADL may be beneficial for cardiovascular health through inducing anti-inflammatory response[32], while low ADL levels may facilitate chronic inflammatory response[33]. Wesolowska et al. reported that there was an inverse relationship between ADL and inflammatory markers, particularly in elderly adults[34]. Lian et al. also supported that the decline in ADL resulted in the upregulation of inflammatory markers[35]. Inflammatory markers upregulation, involving C-Reactive Protein (CRP), etc., is closely related to the occurrence of CAAKI[ 36,37]. In the current research, the predictive value of BI score on the occurrence of CA-AKI was still observed after adjustment for these well-established risk factors.
Furthermore, as shown in model 3 in Table 3, after adjusting covariates, the incidence of CA-AKI significantly increased when BI score<40 (that was, when the degree of ADL reached severe dysfunction or above), while there is no significant difference for morbidity risk between the group of BI 20-39 and the group of BI<20 ([severe dysfunction vs. reference] vs. [Very Severe dysfunction vs. reference], OR=6.622 vs. 6.591). The patients of group of BI<40 may represent few physical activities or be bedridden for a long time and easily develop infections and other comorbidities, further increasing the incidence of CA-AKI.
| BI score | ADL grade | Patients/Overall (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | p value | OR (95 % CI) | p value | OR (95 % CI) | p value | |||
| 100 | Normal function | 219/1913 (11.4 %) | (Reference) | (Reference) | (Reference) | |||
| 60-99 | Mild dysfunction | 209/978 (21.4 %) | 2.102 [1.708 to 2.588] | <0.001* | 1.828 [1.469 to 2.273] | <0.001* | 1.869 [1.499 to 2.330] | <0.001* |
| 40-59 | Moderate dysfunction | 45/137 (32.8 %) | 3.784 [2.579 to 5.551] | <0.001* | 3.041 [2.048 to 4.516] | <0.001* | 3.131 [2.095 to 4.680] | <0.001* |
| 20-39 | Severe dysfunction | 30/62 (48.4 %) | 7.252 [4.322 to 12.168] | <0.001* | 6.176 [3.624 to 10.523] | <0.001* | 6.591 [3.838 to 11.318] | <0.001* |
| <20 | Very severe dysfunction | 30/58 (51.7 %) | 8.288 [4.859 to 14.135] | <0.001* | 6.553 [3.769 to 11.394] | <0.001* | 6.622 [3.771 to 11.628] | <0.001* |
| p value | <0.001* | <0.001* | <0.001* | |||||
Note: BI score=100 was set as reference category, where *p<0.05
Table 3: Logistic Regression Analyses of Bi on the CA-AKI
These results stress out the importance of evaluating BI scores at hospital admission of patients who intend to undergo CAG or PCI. The significance of this study lies on the one hand, the study confirms that the BI score is a simple, economical and easy to operate model to predict the occurrence of CA-AKI. The morbidity of CA-AKI increases with the decline in BI scores. The low BI scores warn physicians to be alert to the occurrence of CA-AKI. On the other hand, we speculate that the appropriate increase of rehabilitation exercises and the improvement of ADL, for patients with decreased ADL, especially those with severe dysfunction, may reduce the incidence of CA-AKI.
This study also had some limitations that require to be discussed. First, the inherent bias cannot completely avoid as a retrospective observational study, although statistical correction was performed for confounders to the greatest extent. Therefore, the validity of the results needs to be further verified by carrying out large-scale prospective studies. Second, one of the inclusion criteria is patients with documented Scr before and 72 h after CAG or PCI. Patients in stable conditions were discharged the next day and this group of patients was excluded. This might lead to selection bias. Third, operators might be partially subjective, which could result in error or bias. ADL measured by BI at hospital admission was a useful, easily accessible and independent prediction indicator to assess the incidence of CA-AKI among patients undergoing CAG or PCI.
Ethical approval:
The study was approved by the Ethics Committee of Sir Run Run Hospital, College of Medicine, Zhejiang University (202012217-36).
Author’s contributions:
Yunxiang Wang conceived and designed the study. Qirong Jiang organized these data and drafted the manuscript with the help of Siwei Yang, Qirong Jiang, Changchun Lai and Zakareya Moayed Ali Alsalman analyzed the data. Zakareya Moayed Ali Alsalman, Peng Wang and Xiaolong Hu drew the pictures. Yunxiang Wang detected any errors in the whole process. All authors have read and approved the manuscript for submission.
Conflict of interests:
The authors declare that they have no competing interests.
References
- Pistolesi V, Regolisti G, Morabito S, Gandolfini I, Corrado S, Piotti G, et al. Contrast medium induced acute kidney injury: A narrative review. J Nephrol 2018;31:797-812.
[Crossref] [Google scholar] [PubMed]
- Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: A randomized, controlled trial. Circ Cardiovasc Interv 2011;4(5):456-62.
[Crossref] [Google scholar] [PubMed]
- Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): A prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389(10076):1312-22.
[Crossref] [Google scholar] [PubMed]
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011;7(4):189-200.
[Crossref] [Google scholar] [PubMed]
- Azzalini L, Kalra S. Contrast-induced acute kidney injury-definitions, epidemiology and implications. Interv Cardiol Clin 2020;9(3):299-309.
[Crossref] [Google scholar] [PubMed]
- Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 2015;11(2):88-101.
[Crossref] [Google scholar] [PubMed]
- Yin WJ, Yi YH, Guan XF, Zhou LY, Wang JL, Li DY, et al. Preprocedural prediction model for contrast‐induced nephropathy patients. J Am Heart Assoc 2017;6(2):e004498.
[Crossref] [Google scholar] [PubMed]
- Zheng H, Wang G, Cao Q, Ren W, Xu L, Bu S. A risk prediction model for contrast-induced nephropathy associated with gadolinium-based contrast agents. Ren Fail 2022;44(1):741-7.
[Crossref] [Google scholar] [PubMed]
- Jiang H, Li D, Xu T, Chen Z, Shan Y, Zhao L, et al. Systemic immune-inflammation index predicts contrast-induced acute kidney injury in patients undergoing coronary angiography: A cross-sectional study. Front Med 2022;9.
[Crossref] [Google scholar] [PubMed]
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J 1965;14:61-5.
[Google scholar] [PubMed]
- Bouwstra H, Smit EB, Wattel EM, van der Wouden JC, Hertogh CM, Terluin B, et al. Measurement properties of the Barthel index in geriatric rehabilitation. J Am Med Dir Assoc 2019;20(4):420-5.
[Crossref] [Google scholar] [PubMed]
- Hopman-Rock M, van Hirtum H, de Vreede P, Freiberger E. Activities of daily living in older community-dwelling persons: A systematic review of psychometric properties of instruments. Aging Clin Exp Res 2019;31:917-25.
[Crossref] [Google scholar] [PubMed]
- dos Santos Barros V, Bassi-Dibai D, Guedes CL, Morais DN, Coutinho SM, de Oliveira Simões G, et al. Barthel index is a valid and reliable tool to measure the functional independence of cancer patients in palliative care. BMC Palliat Care 2022;21(1):124.
[Crossref] [Google scholar] [PubMed]
- Duffy L, Gajree S, Langhorne P, Stott DJ, Quinn TJ. Reliability (inter-rater agreement) of the Barthel index for assessment of stroke survivors: Systematic review and meta-analysis. Stroke 2013;44(2):462-8.
[Crossref] [Google scholar] [PubMed]
- Fähling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol 2017;13(3):169-80.
[Crossref] [Google scholar] [PubMed]
- Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: Systematic review. BMJ 2015;351:4395.
[Crossref] [Google scholar] [PubMed]
- Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, et al. Exercise capacity and the risk of death in women: The St James women take heart project. Circulation 2003;108(13):1554-9.
[Crossref] [Google scholar] [PubMed]
- Ryg J, Engberg H, Anru PL, Pedersen SG, Jorgensen MG, Vinding KL, et al. Activities of daily living at hospital admission and estimated survival time of older patients. Age Ageing 2021;50(4):1200-7.
[Crossref] [Google scholar] [PubMed]
- D'Olhaberriague L, Litvan I, Mitsias P, Mansbach HH. A reappraisal of reliability and validity studies in stroke. Stroke 1996;27(12):2331-6.
[Crossref] [Google scholar] [PubMed]
- Bisson JI, Roberts NP, Andrew M, Cooper R, Lewis C. Psychological therapies for chronic Post-Traumatic Stress Disorder (PTSD) in adults. Cochrane Database Syst Rev 2013(12).
[Crossref] [Google scholar] [PubMed]
- Higuchi S, Kabeya Y, Matsushita K, Taguchi H, Ishiguro H, Kohshoh H, et al. Barthel index as a predictor of 1‐year mortality in very elderly patients who underwent percutaneous coronary intervention for acute coronary syndrome: Better activities of daily living, longer life. Clin Cardiol 2016;39(2):83-9.
[Crossref] [Google scholar] [PubMed]
- Li F, Li D, Yu J, Jia Y, Jiang Y, Chen T, et al. Barthel index as a predictor of mortality in patients with acute coronary syndrome: Better activities of daily living, better prognosis. Clin Interv Aging 2020:1951-61.
[Crossref] [Google scholar] [PubMed]
- Bier N, Belchior PD, Paquette G, Beauchemin É, Lacasse-Champagne A, Messier C, et al. The instrumental activity of daily living profile in aging: A feasibility study. J Alzheimers Dis 2016;52(4):1361-71.
[Crossref] [Google scholar] [PubMed]
- Kim DY, Kim MJ, Seo J, Cho I, Shim CY, Hong GR, et al. Predictors of subsequent heart failure after left atrial appendage closure. Circ J 2022;86(7):1129-36.
[Crossref] [Google scholar] [PubMed]
- Kitamura M, Izawa KP, Taniue H, Mimura Y, Ikeda Y, Nagashima H, et al. Activities of daily living at different levels of renal function in elderly hospitalized heart failure patients. Aging Clin Exp Res 2018;30:45-51.
[Crossref] [Google scholar] [PubMed]
- van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury. Part 2: Risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients: Recommendations for updated ESUR contrast medium safety committee guidelines. Eur Radiol 2018;28:2856-69.
[Crossref] [Google scholar] [PubMed]
- Hoste EA, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018;14(10):607-25.
[Crossref] [Google scholar] [PubMed]
- Duan C, Cao Y, Liu Y, Zhou L, Ping K, Tan MT, et al. A new preprocedure risk score for predicting contrast-induced acute kidney injury. Can J Cardiol 2017;33(6):714-23.
[Crossref] [Google scholar] [PubMed]
- Xu T, Lin M, Shen X, Wang M, Zhang W, Zhao L, et al. Association of the classification and severity of heart failure with the incidence of contrast-induced acute kidney injury. Sci Rep 2021;11(1):1-11.
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009-18.
[Crossref] [Google scholar] [PubMed]
- Li D, Chen Z, He W, Lin L, Xu T, Jiang H, et al. The association between nutritional risk and contrast-induced acute kidney injury in patients undergoing coronary angiography: A cross-sectional study. Nutr J 2022;21(1):56.
[Crossref] [Google scholar] [PubMed]
- Duggal NA, Niemiro G, Harridge SD, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol 2019;19(9):563-72.
[Crossref] [Google scholar] [PubMed]
- Noz MP, Hartman YA, Hopman MT, Willems PH, Tack CJ, Joosten LA, et al. Sixteen‐week physical activity intervention in subjects with increased cardiometabolic risk shifts innate immune function towards a less proinflammatory state. J Am Heart Assoc 2019;8(21):e013764.
[Crossref] [Google scholar] [PubMed]
- Wesolowska K, Czarkowska‐Paczek B, Wirkowska A. Activities of daily living decrease serum levels of C‐reactive protein in elderly adults but not in young and middle-aged adults. J Am Geriatr Soc 2016;64(4):883-4.
- Lian XQ, Zhao D, Zhu M, Wang ZM, Gao W, Zhao H, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med 2014;58:64-9.
[Crossref] [Google scholar] [PubMed]
- Gao F, Zhou YJ, Zhu X, Wang ZJ, Yang SW, Shen H. C-reactive protein and the risk of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Am J Nephrol 2011;34(3):203-10.
[Crossref] [Google scholar] [PubMed]
- Ma K, Qiu H, Zhu Y, Lu Y, Li W. Preprocedural SII combined with high-sensitivity C-reactive protein predicts the risk of contrast-induced acute kidney injury in STEMI patients undergoing percutaneous coronary intervention. J Inflamm Res 2022:3677-87.
[Crossref] [Google scholar] [PubMed]
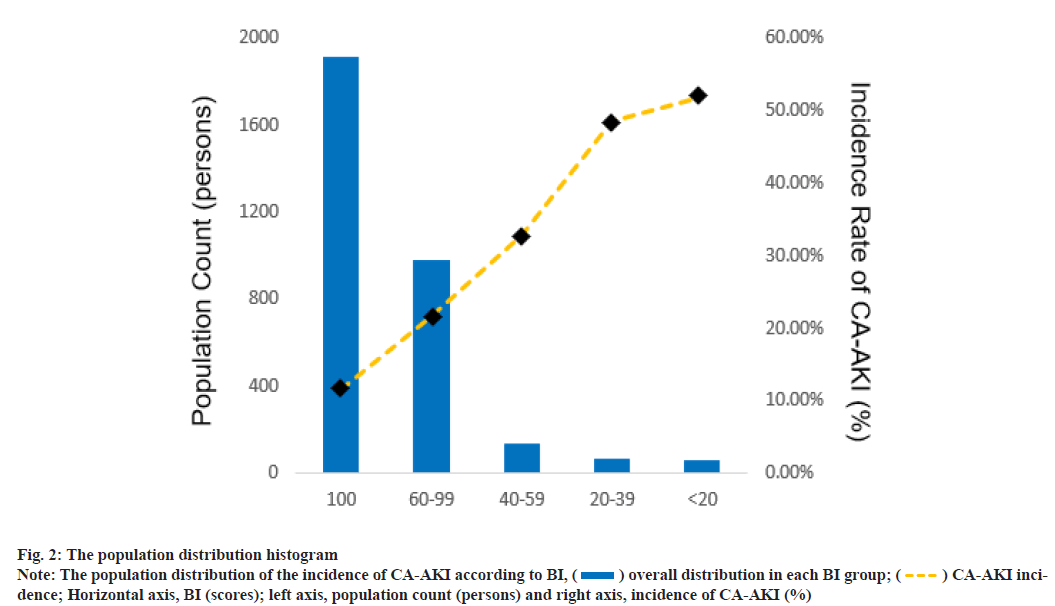
 ) overall distribution in each BI group; (
) overall distribution in each BI group; ( ) CA-AKI incidence;
Horizontal axis, BI (scores); left axis, population count (persons) and right axis, incidence of CA-AKI (%)
) CA-AKI incidence;
Horizontal axis, BI (scores); left axis, population count (persons) and right axis, incidence of CA-AKI (%)