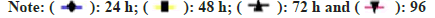- *Corresponding Author:
- P.P. Punj
Department of Zoology, Fish Biology and Fisheries Laboratory, Nagaland University, Lumami, Nagaland 798627, India
E-mail: pranaypunj@gmail.com
| Date of Received | 04 September 2022 |
| Date of Revision | 16 April 2023 |
| Date of Acceptance | 19 March 2024 |
| Indian J Pharm Sci 2024;86(2):546-555 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Epigallocatechin gallate and C-phycocyanin are natural food supplements, gaining a lot of popularity in biomedical researches. The lethal concentration 50 value for the zebrafish model was determined using a 96 h semi-static acute toxicity test. Zebrafish were transferred into a 1 l water tank starting with a minimal dose of 8 mg of epigallocatechin gallate and 1 g of C-phycocyanin, which further increased to the concentration of 30 mg for epigallocatechin gallate and 5 g for C-phycocyanin. Lethal concentration 50 of epigallocatechin gallate and C-phycocyanin was calculated to be 21.37 mg/l and 2.45 g/l respectively at 96 h in the zebrafish model. At high doses above 12 mg of epigallocatechin gallate and 1 g of C-phycocyanin, the test fish displayed various behavior responses like erratic and jerky swimming, attempts to jump out of the water, frequent surfacing and swallowing of air, normal skin color change, decreased opercular movement and finally leading to death. Histological observations showed hypertrophy, atrophy and vacuolar degeneration in the liver whereas tubular degeneration, glomerular hyper cellularity, dilation of Bowman’s space, hemorrhage, and necrosis were observed in the kidneys exposed to the high doses of epigallocatechin gallate and C-phycocyanin treated fish. The gills of the test animals also expressed lamellar synechiae, hyperplasia, curling of lamellae, mucus secretion and shortening of the secondary lamella. The results demonstrated that doses above 30 mg/l and 5 g/l in the case of epigallocatechin gallate and C-phycocyanin respectively gave 100 % mortality and expressed organ-specific toxicity. Overall results revealed that exposure to high concentrations of epigallocatechin gallate and C-phycocyanin uninterruptedly may lead to severe alterations in the tissue of experimental animals.
Keywords
Acute toxicity, behavior response, C-phycocyanin, zebrafish model, epigallocatechin gallate, histological observations
Toxicity is the leading cause of drug development failure and it is a key concentration and issue for current drug discovery[1]. People assume that allnatural products are safe to consume and that no more research is required on toxicity. Off late natural food supplements are gaining a lot of popularity; two such products are Epigallocatechin Gallate (EGCG) and C-Phycocyanin (C-PC). EGCG is one of the most studied and abundant catechins found in green tea extract. It is well known to exhibit antioxidant and anti-inflammatory properties and is marketed as a dietary supplement[2]. Studies in model animals have shown that it inhibits the development and growth of tongue cancer cells in Keratin 14 (KRT14)/ERT-Kirsten Rat Sarcoma Virus (Kras) transgenic mice[3]. It induces apoptosis and inhibits the Phosphatidylinositol- 3-Kinase (PI3K)/Protein Kinase B (Akt)/ mammalian Target of Rapamycin (mTOR) pathway progression in precancerous lesions of gastric carcinoma treated rats[4]. It protects and improves the colon from chemically induced agents like N, N′-Dimethylhydrazine (DMH) in Wistar rats and protects the lung against fluoride-induced oxidative injury[5,6]. However, findings suggest the use of EGCG as a pro-oxidant; a high dose of EGCG causes events of hepatotoxicity as well as suppresses the antioxidant enzyme in the liver [7].
C-PC is a light-harvesting, water-soluble, proteinbound pigment obtained from some microalgae such as Spirulina platensis that has been attracting a lot of importance as a nutritious supplement with its high protein content[8]. It is the primary pigment constituent component of Spirulina platensis, making up approximately 15 % of its total dry weight. They are extensively used as a dietary supplement in several countries due to their nutritional asset[9]. It exhibits a vast array of therapeutic potentials such as antiinflammatory, neuroprotective, hepatoprotective, immunomodulatory, antimutagenic, antiviral, antimalarial, anticancer, antioxidant and free radical scavenging agent and diabetic nephroprotective agent[10]. Thus, with EGCG and CPC exhibiting such strong and diverse potential for their application in various biomedical studies the need to understand the toxicity and nature of this compound arises.
Zebrafish (Danio rerio) is used as an excellent animal model to inspect samples of the bioactive compounds through toxicity assay. Commonly used mammalian models have some drawbacks with higher cost and a prolonged time for results, yet ethically questionable. It has been acknowledged as an effective animal model for assessing drug targets for toxicity and safety risks because of their sharing of common physiological, morphological and histological characteristics with mammals. It has short cycle, utilizing smaller amounts of test substance and high throughput over the classic in vivo model. Multiple organs may be monitored and pharmacodynamic, pharmacokinetic and metabolite activities can be studied at the same time. The toxic effect of drugs/compounds on the heart, liver, kidney, developmental stages, and behavior can be effectively studied using the zebrafish model system [11,12].
A toxicology test is necessary for allopathic treatment and alternative medicine to uncover any possible side effects that may not be apparent until they manifest. Currently, the use of natural products such as EGCG and C-PC is on the rise and their application in various biomedical researches. Although many findings show the capability of EGCG and C-PC to suppress the effect caused by different carcinogens and mutagens still only a few reports regarding the safe use of these compounds have been recorded[13,14]. As such, the need for proper knowledge of the toxicity of these two compounds is to be studied carefully. The assessment of toxicity using acute toxicity bioassay can prove the safety of traditional medicine using EGCG and C-PC and promote its consumption and other research activities. The purpose of this study was to examine the acute toxicity of EGCG and C-PC in the zebrafish models and observe the histological damages cause in the tissue of kidney, liver and gills of the adult zebrafish.
Materials and Methods
Chemicals:
EGCG (>95 %), hematoxylin-eosin stain, xylene and absolute alcohol were purchased from Sigma Aldrich. Commercially available purified C-PC was procured from Algallio Biotech, India. All other chemicals were of reagent grade. Solutions were prepared with deionized water. All glassware and aquariums used in the experiment were washed and thoroughly rinsed with distilled water before use.
Acclimatization of zebrafish:
Zebrafish (5-7 mo; 3-5 cm long; weighing about 300-500 mg) with an equal gender ratio were purchased from Aqua fish and pets, Jorhat Assam, India. Fishes were purchased and then treated with 0.05 % Potassium permanganate solution (KMnO4) for 5 min to avoid any cutaneous infection. It was cultured in a zebrafish housing system (Model- NT-ZB-11; Make-Narshi Technologies) to ensure constant temperature (28°±2°), persistent chemical, biological and mechanical water filtration and aeration (7.20 mg Oxygen (O2)/l). Polycarbonate fish tanks were maintained under a 14 h per day and 10 h per night photoperiod cycle. Adult zebrafish were fed three times a day with feed that is commercially available containing protein 28 %, crude fat 3 %, fiber 4 % and moisture 10 %.
Acute toxicity study and behavioral study:
The 96 h semi-static acute toxicity test of zebrafish exposed to EGCG and C-PC was performed according to guideline 203 of Organisation for Economic Co-operation and Development[15]. Adult zebrafish were randomly exposed to 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28 and 30 mg of EGCG and 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25, 3.5, 3.75, 4, 4.25, 4.5, 4.75 and 5 g of C-PC. The different concentrations of EGCG and C-PC were dissolved in 1 l dechlorinated water. The test performed consists of ten adult zebrafish. The fresh solution was prepared and changed at 48 h after the first exposure. The test animals were fed with commercially available feed and were not fed 24 h before the experiment. Test animals were observed periodically for 4 d. Dead animals and feces were timely removed from the experimental jar to ensure non-interference in the system. Behavioral changes were recorded such as gulping activity where the zebrafish were observed according to the piping and gasping movement of their opercular at the surface of the experimental solution, hyperactivity like skittering and erratic swimming movements, and hypo activity such as lethargy, inactivity, weakness, quiescent, ceased swimming and immobility were recorded individually in the experimental subjects. In order to observe the morphological change, the wholebody image of the experimental zebrafish was captured by using a Nikon D3500 digital camera.
Histopathological study:
Histopathological techniques were followed based on the method of Abdelhamid et al.[16]. The organs were dissected and immediately rinsed with the physiological saline solution to remove blood and debris adhering to the tissue then; they were then fixed in 10 % buffer formalin for 24 h. The fixative was removed by washing through running tap water overnight. After dehydrating through an ascending graded series of ethyl alcohols, the fixative was then cleared using two change of xylene and embedded in paraffin wax. The tissues were cut at the 4-5 μ thickness and stained with Harris-hematoxylin, and counterstained with eosin, dissolved in 95 % alcohol. After dehydration and clearing, sections were mounted in Canada balsam. The prepared slides were observed and photographed under a CXL microscope attached to a Sony digital camera (model E31SPM20000KPA; USB 2.0) and analyzed using image J software.
Statistical analysis:
The recorded mortality data were corrected for natural response by Abbott’s formula which was further subjected to probit analysis for calculating Lethal Concentration 50 (LC50) and other statistics at 95 % confidence limits of upper confidence limits[17], and lower confidence limits were calculated using Statistical Package for the Social Sciences (SPSS) 22.0 software (SPSS Inc., United States of America (USA)). Mortality percentage graph of the test animal was prepared using graph pad prism version 5.0. The result of the behavioral responses for the adult zebrafish were presented as mean±standard error of mean and represented using an error bar.
Results and Discussion
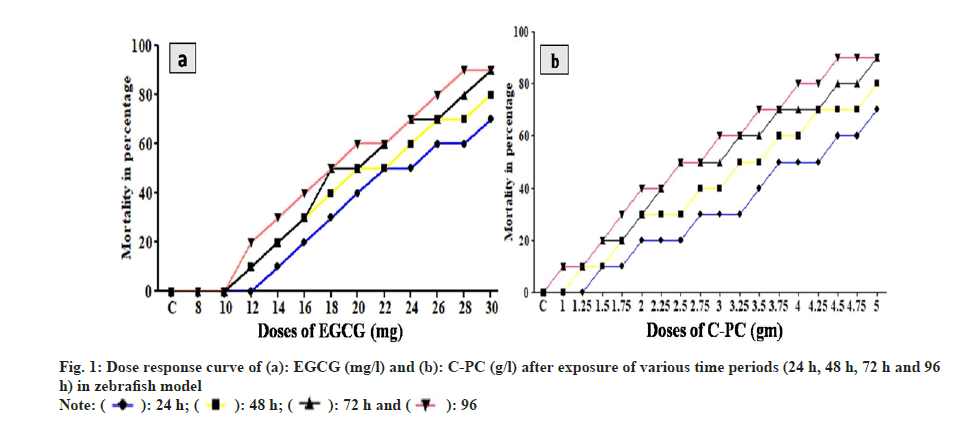
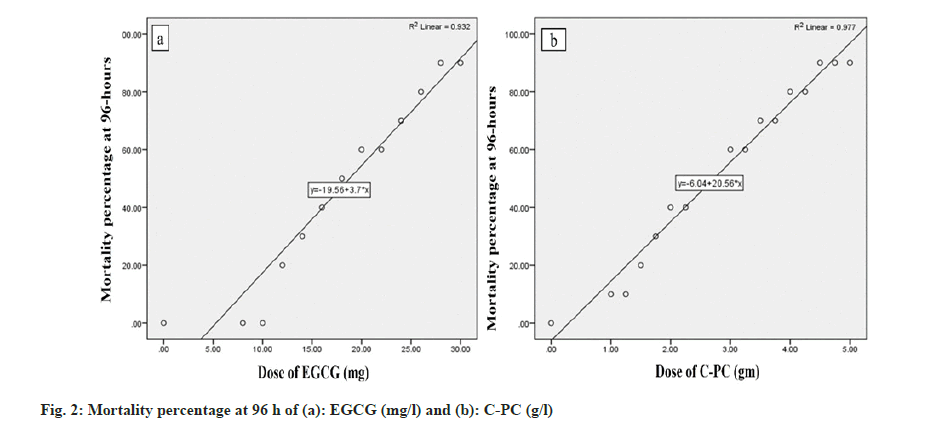
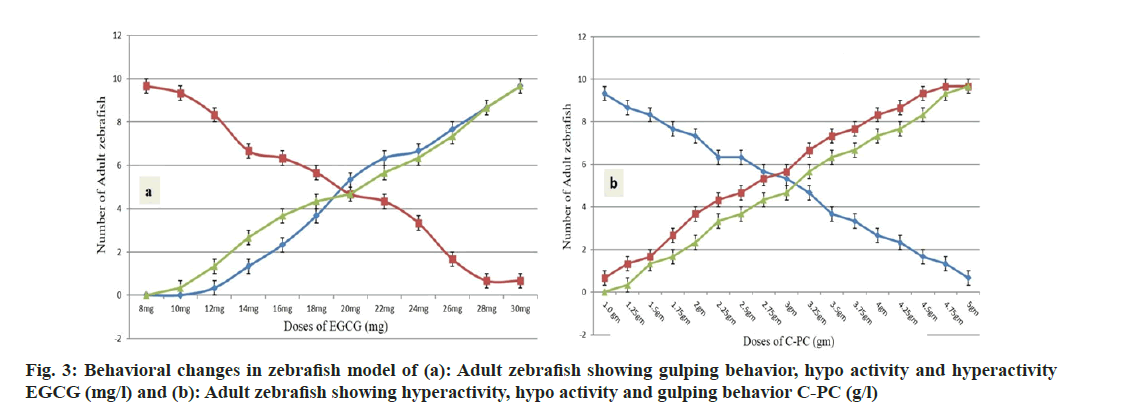
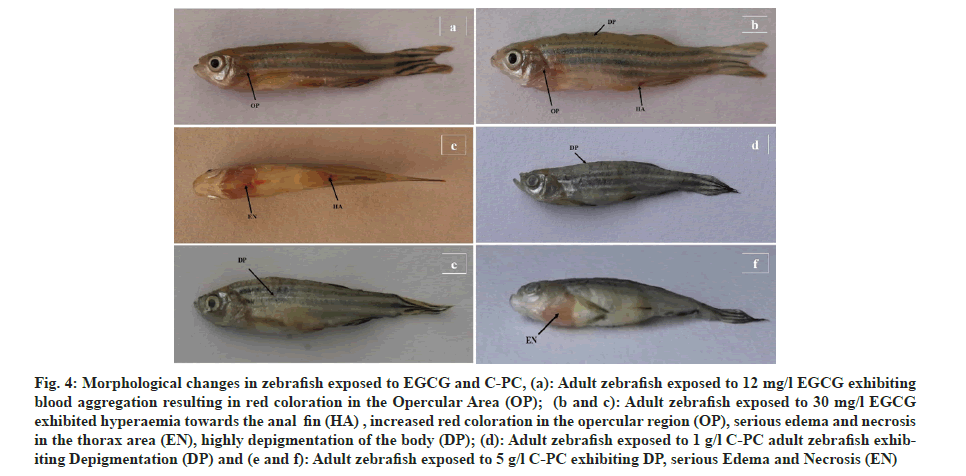
The experimental zebrafish displayed mortality in a dose- and time-dependent manner (fig. 1). The LC50 was calculated to be 26.91 mg/l, 25.70 mg/l, 23.44 mg/l and 21.37 mg/l for EGCG whereas the LC50 value for C-PC was 3.71 g/l, 3.31 g/l, 2.88 g/l and 2.69 g/l at 24 h, 48 h, 72 h and 96 h respectively (Table 1). The linear curve between mortality of test fish against the different concentrations of EGCG and C-PC treated for 96 h showed a highly positive correlation (r=0.932 for EGCG and r=0.977 for C-PC) (fig. 2). The mortality was found to be 100 % at doses above 30 mg/l in the case of EGCG and above 5 g/l for C-PC. Test animals responded to the treatment with rapid movement swimming, swallowing, jumping activity and immobile behavior as the concentration increased (fig. 3). At higher doses, fins became harder and stretched. A large amount of mucus was present over the fish body. The dead zebrafish showed reddish skin coloration in the opercular region due to hemorrhage. There was a decrease in the pigments of the zebrafish body as exposure to time and dose were increased. Severe necrosis and edema were observed in the chest area of the fishes exposed to EGCG and C-PC (fig. 4a-fig. 4f). The magnitude of damage caused to the organs was found to be a time- and dose-dependent factor. Longer duration and higher doses were found to inflict significantly more damage.
| Duration | LC50 of EGCG (mg/l) | LC50 of C-PC (g/l) |
|---|---|---|
| 24 h | 26.91 | 3.71 |
| 48 h | 25.7 | 3.31 |
| 72 h | 23.44 | 2.88 |
| 96 h | 21.37 | 2.69 |
Table 1: LC50 of EGCG and C-PC for Various Duration (24 h, 48 h, 72 h AND 96 h) in Zebrafish Model
Fig 4: Morphological changes in zebrafish exposed to EGCG and C-PC, (a): Adult zebrafish exposed to 12 mg/l EGCG exhibiting blood aggregation resulting in red coloration in the Opercular Area (OP); (b and c): Adult zebrafish exposed to 30 mg/l EGCG exhibited hyperaemia towards the anal fin (HA) , increased red coloration in the opercular region (OP), serious edema and necrosis in the thorax area (EN), highly depigmentation of the body (DP); (d): Adult zebrafish exposed to 1 g/l C-PC adult zebrafish exhibiting Depigmentation (DP) and (e and f): Adult zebrafish exposed to 5 g/l C-PC exhibiting DP, serious Edema and Necrosis (EN)
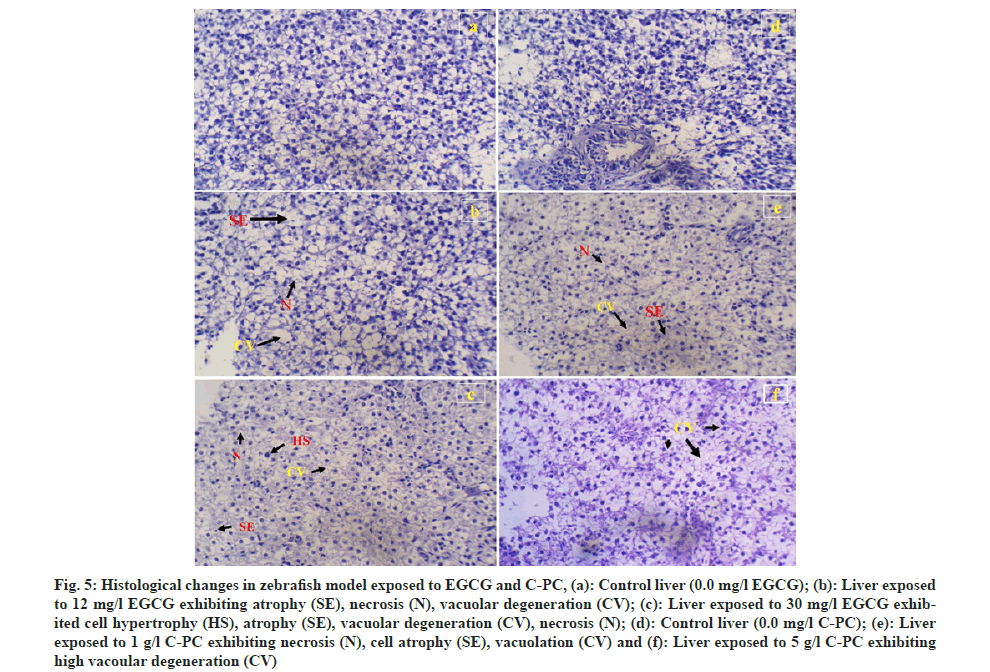
A comparative study was done to further understand the histopathological changes of the control group, with the minimum dose (12 mg of EGCG and 1 g of C-PC) where the test animal started showing lethargy and the highest dose (30 mg of EGCG and 5 g of C-PC) in which the degree of toxicity showed to be harmful, resulting in increased mortality. The test zebrafish showed changes in the liver tissue compared to the control group depending on the dose incorporated. Exposure to 12 mg/l of EGCG displayed cell dead or necrosis, atrophy and vacuolar degeneration, whereas higher vacuolar degeneration in different areas of the liver, atrophy, necrosis, hypertrophy, and sinusoids space was noticed at 30 mg/l of EGCG. Hepatic tissue exposed to 1 g/l of C-PC showed vacuolar degeneration, hepatocyte necrosis and atrophy, whereas severe vacuolar degeneration was observed when exposed to 5 g/l C-PC (fig. 5a-fig. 5f).
Fig 5: Histological changes in zebrafish model exposed to EGCG and C-PC, (a): Control liver (0.0 mg/l EGCG); (b): Liver exposed to 12 mg/l EGCG exhibiting atrophy (SE), necrosis (N), vacuolar degeneration (CV); (c): Liver exposed to 30 mg/l EGCG exhibited cell hypertrophy (HS), atrophy (SE), vacuolar degeneration (CV), necrosis (N); (d): Control liver (0.0 mg/l C-PC); (e): Liver exposed to 1 g/l C-PC exhibiting necrosis (N), cell atrophy (SE), vacuolation (CV) and (f): Liver exposed to 5 g/l C-PC exhibiting high vacoular degeneration (CV)
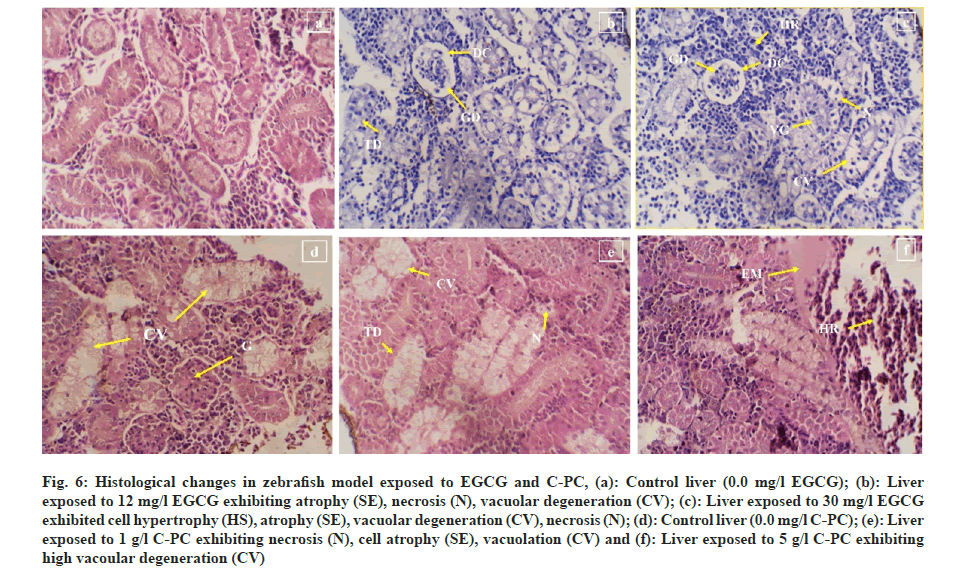
12 mg of EGCG exposure showed histoarchitectural changes in the kidney such as focal renal tubular degeneration, glomerular shrinkage, and dilation of Bowman’s space whereas exposure to 30 mg EGCG showed glomerular shrinkage, acute cellular degeneration, vacuolar degeneration, hemorrhage, and Bowman's space dilation. Test zebrafish exposed to 1 g of C-PC showed vacuolation and hyper cellularity of glomerulus whereas a dose of 5 g showed necrosis, tubular degeneration, vacuolation, hemorrhage and edematous fluid (fig. 6a-fig. 6f).
Fig 6: Histological changes in zebrafish model exposed to EGCG and C-PC, (a): Control liver (0.0 mg/l EGCG); (b): Liver exposed to 12 mg/l EGCG exhibiting atrophy (SE), necrosis (N), vacuolar degeneration (CV); (c): Liver exposed to 30 mg/l EGCG exhibited cell hypertrophy (HS), atrophy (SE), vacuolar degeneration (CV), necrosis (N); (d): Control liver (0.0 mg/l C-PC); (e): Liver exposed to 1 g/l C-PC exhibiting necrosis (N), cell atrophy (SE), vacuolation (CV) and (f): Liver exposed to 5 g/l C-PC exhibiting high vacoular degeneration (CV)
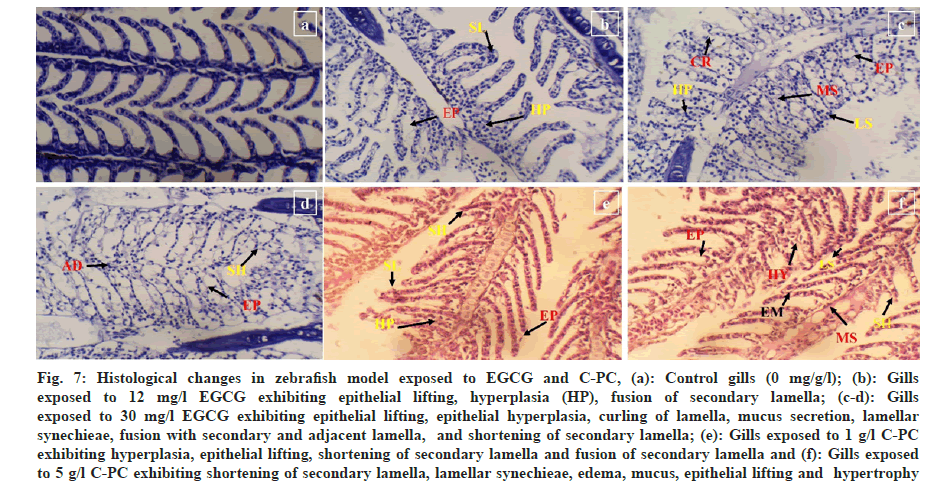
In the experimental group treated with 30 mg/l of EGCG and 5 g/l of C-PC, gills exhibited hypertrophy, shortening of secondary lamellae, edema, curling of lamellae, epithelial hyperplasia, epithelial lifting, and lamellar synechiae. Further, with a higher dosage of EGCG 30 mg/l caused mucus secretion and fusion of secondary lamellae as well as fusion with the adjacent sides can be clearly seen. A lower concentration of EGCG 12 mg/l and 1 g/l of C-PC showed lesser lamellar fusion and hyperplasia compared to the high dosage, slight epithelial lifting was observed, and curling of the lamellae was observed in EGCG as well as in C-PC (fig. 7a-fig. 7f).
Fig 7: Histological changes in zebrafish model exposed to EGCG and C-PC, (a): Control gills (0 mg/g/l); (b): Gills exposed to 12 mg/l EGCG exhibiting epithelial lifting, hyperplasia (HP), fusion of secondary lamella; (c-d): Gills exposed to 30 mg/l EGCG exhibiting epithelial lifting, epithelial hyperplasia, curling of lamella, mucus secretion, lamellar synechieae, fusion with secondary and adjacent lamella, and shortening of secondary lamella; (e): Gills exposed to 1 g/l C-PC exhibiting hyperplasia, epithelial lifting, shortening of secondary lamella and fusion of secondary lamella and (f): Gills exposed to 5 g/l C-PC exhibiting shortening of secondary lamella, lamellar synechieae, edema, mucus, epithelial lifting and hypertrophy
In recent years, numerous researches have shown the effects of nutraceuticals on animal models however very little researches have been done on the LC50 activity of EGCG and C-PC in zebrafish model. The toxicity of test compounds was determined by immersing adult zebrafish in the solution of EGCG and C-PC and observing their behavior as well as undergoing histological changes. Doses above the LC50 of EGCG and C-PC have been found to be harmful to different target organs. Study on several exposure routes of pyraclostrobin in adult zebrafish, mortality was found to be increased in a dose- and time-dependent way[18]. The assessment of behavioural response is a useful tool for determining the toxicological influence of the test compounds on the test animals[19]. When animals are exposed to a stressful environment, such as through chemical compounds, their defense mechanisms are activated, as their homeostasis is disrupted, resulting in behavioral changes such as escape behavior, settling down at the bottom of the tank, and rapid movement to avoid hazardous substances.
The treatment group displayed behavioral changes similar to those described by other researchers, where zebrafish exposed to Kandhamal haladi demonstrated irregular, erratic, and aberrant swimming movements[20]. Fin hardening occurs as a result of muscular stretching, making it difficult for the fish to swim. When zebrafish are exposed to a harmful environment, inflammation of the skin causes excessive mucus secretion. Mucus establishes a barrier between harmful surroundings and lowers irritation caused by the agents, which is a common defense strategy of fishes[21]. The red coloration in the test animal which is considered to be a result of massive hemorrhage was also recorded when studying the zebrafish response after exposure to propylthiouracil[22]. Further, when the toxic substances in the environment are high the test fishes exhibit hyperemia on various parts of the body similar results were seen when treating Nile tilapia with deltamethrin[23]. Disruption of the endocrine gland causes depigmentation, which alters the number of chromatophores in the thoracic area of zebrafish under toxic stress, including necrosis and edema when exposed to the venom of Bothrops alternatus[24].
The liver is the organ that detoxifies fatal chemical enzymes, but it is negatively affected when exposed to a higher toxicity dose. The extent of organ damage was in a time- and dose-dependent manner, with longer duration and larger dosages inflicting much greater harm. Cytoplasmic vacuole and necrosis in the cell in juvenile fish Colossoma macropomum were reported when testing the acute toxicity of a hot aqueous extract from Terminalia catappa leaves similarly cellular hypertrophy[25], atrophy, cytoplasmic vacuolation, and necrosis were found in common carp subjected to chlorpyrifos[26]. Cytoplasmic vacuolation and dilation of the sinusoid space were observed in zebrafish when subjected to Silver oxide (Ag2O) and Silver carbonate (Ag2CO3) doped Titanium dioxide (TiO2) nanoparticles and pure TiO2 particles[27]. Hypertrophy in the liver tissue is the result of a biological reaction to stress in the body environment. Poor hepatocyte metabolism caused by an obstruction of sinusoidal blood flow eventually results in cell atrophy. The creation of vacuoles in the liver cell was thought to signal an early stage of necrosis[28,29]. Hepatocyte necrosis is a probable cause of lethal compounds invading the cell and building up a toxic material inside the cell over time[30].
The zebrafish kidney is found in the ventral section of the vertebral column and has a distinct head and trunk area. It has nephrons with glomeruli, proximal and distal tubules and a collecting duct, similar to mammalian kidneys (fig. 6a). It helps filtration of blood residues, as well as salt and water absorption and maintains tissue homeostasis. The occurrence of degeneration in the tubules, vacuolation of tubules, change in the glomerulus and the Bowman’s capsule are considered to be the most consequent alterations in the kidney due to contamination in the environment[31]. Aluminum concentrations caused histological changes in the kidney, including cellular degeneration, hemorrhage and edematous effusion in Tilapia zillii[32]. Tubular degeneration in adults and embryos of zebrafish was reported while investigating the acute toxicity of Libidibia ferrea, a traditional anti-inflammatory source[33]. Tubular degeneration, vacuolation, glomerular shrinkage with dilated Bowman’s space, and necrosis were observed in freshwater fish Catla catla when treated with cypermethrin[34]. The striped catfish Pangasianodon hypophthalmus exposed to chromium also caused structural changes such as renal corpuscle shrinkage, Bowman’s space dilation, and necrosis[35]. Tubular alteration in the kidney is thought to be an indirect cause of metabolic failure in zebrafish caused by toxic chemical exposure. These modifications are supposed to lead to necrosis in the long run[36].
Fish gills are crucial in determining the impact of toxicants since they are easily impacted by foreign compounds in the water[37]. The chemicals had a considerable impact on the gills of treated groups, causing hypertrophy, hyperplasia, secondary lamellae shortening and fusion, edema, lamellae curling, and epithelial lifting. Tissue-specific toxicity of clothianidin on rainbow trout caused fusion and shortening of secondary lamellae, hypertrophy of secondary lamellae, and necrosis of secondary lamellae[38]. Changes in the structure including secondary lamellae curling, epithelial lifting, hyperplasia, and lamella fusing were reported in Sparus aurata subjected to erythromycin and oxytetracycline[39]. As a defense mechanism, this alteration in the structure of the gills occurs to minimize the interaction between the blood and the environment, so inhibiting and reducing the impact of the hazardous material. An animal protects its underlying tissue from damaging toxicants; hyperplasia increases the number of cells that are typically suited[30]. Edema and epithelial cell lifting in the gills are also thought to be a defense mechanism used by the fish to defend itself from xenobiotics in the environment[40]. Lamellar synechiae and mucus secretion was observed after short-term coexposure to TiO2 and CuO in the gills of Cyprinus carpio. Cell proliferation happens quickly, increasing the size of the epithelial layer and causing fusion between adjacent lamellae, maximizing the oxygen diffusion distance between blood and water[41]. Furthermore, the area available for gas exchange is substantially reduced due to the fusion of the respiratory epithelia, resulting in hypoxia and stress[42]. The preceding actions harm to the physiology of fish, resulting in death. In conclusion, we observed that zebrafish were very sensitive to EGCG and C-PC as evidenced from the behavioral responses, demonstrating the utility of this animal as a model for studying the effects of exposure to EGCG and C-PC. A high dose of EGCG and C-PC clearly showed to be very toxic causing severe damage to the gills, kidneys and liver, thus harming the normal physiological function of the experimental zebrafish. The result demonstrated that a higher concentration of EGCG above 30 mg/l and C-PC above 5 g/l gave 100 % mortality and expressed organ-specific toxicity but lower doses showed the least toxic effect. Therefore, using EGCG and C-PC in relatively high quantities is not safe considering the number of tissue damage caused to the animal.
Acknowledgments:
The authors gratefully acknowledge the financial support from DST-SERB, New Delhi, India, for an early career research award (ECR/2016/001398).
Authors contributions:
Ajungla Jamir and Sentiyanger Longkumer executed all the experimental work and drafted the manuscript. Pranay Punj Pankaj planned the concept of the work, prepared the final manuscript and supervised the work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Shyam M, Shilkar D, Rakshit G, Jayaprakash V. Approaches for targeting the mycobactin biosynthesis pathway for novel anti-tubercular drug discovery: Where we stand. Exp Opin Drug Discov 2022;17(7):699-715.
[Crossref] [Google Scholar] [PubMed]
- Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, Gallelli L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: From dietary sources to human microRNA modulation. Molecules 2019;25(1):63.
[Crossref] [Google Scholar] [PubMed]
- Wei H, Ge Q, Zhang LY, Xie J, Gan RH, Lu YG, et al. EGCG inhibits growth of tumoral lesions on lip and tongue of K-Ras transgenic mice through the Notch pathway. J Nutr Biochem 2022;99:108843.
[Crossref] [Google Scholar] [PubMed]
- Zhu F, Xu Y, Pan J, Li M, Chen F, Xie G. Epigallocatechin gallate protects against MNNG-induced precancerous lesions of gastric carcinoma in rats via PI3K/Akt/mTOR pathway. Evid Based Complement Altern Med 2021;2021.
[Crossref] [Google Scholar] [PubMed]
- Afzal SM, Vafa A, Rashid S, Shree A, Islam J, Ali N, et al. Amelioration of N, N′-dimethylhydrazine induced colon toxicity by epigallocatechin gallate in Wistar rats. Hum Exp Toxicol 2021;40(9):1558-71.
[Crossref] [Google Scholar] [PubMed]
- Shanmugam T, Selvaraj M, Poomalai S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in vivo and in silico study. Int Immunopharmacol 2016;39:128-39.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Wang Y, Wan X, Yang CS, Zhang J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pharmacol 2015;283(1):65-74.
[Crossref] [Google Scholar] [PubMed]
- Oliveira EG, Rosa GS, Moraes MA, Pinto LA. Characterization of thin layer drying of Spirulina platensis utilizing perpendicular air flow. Bioresource Technol 2009;100(3):1297-303.
- Chu WL, Lim YW, Radhakrishnan AK, Lim PE. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med 2010;10:53.
[Crossref] [Google Scholar] [PubMed]
- Jaeschke DP, Teixeira IR, Marczak LD, Mercali GD. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res Int 2021;143:110314.
[Crossref] [Google Scholar] [PubMed]
- Choi TY, Choi TI, Lee YR, Choe SK, Kim CH. Zebrafish as an animal model for biomedical research. Exp Mol Med 2021;53(3):310-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Xia Q, Wang J, Zhuang K, Jin H, Liu K. Progress in using zebrafish as a toxicological model for traditional Chinese medicine. J Ethnopharmacol 2022;282:114638.
[Crossref] [Google Scholar ] [PubMed]
- Alam M, Ali S, Ashraf GM, Bilgrami AL, Yadav DK, Hassan MI. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem 2022;379:132135.
[Crossref] [Google Scholar] [PubMed]
- Salgado MT, e Silva EF, Matsumoto AM, Mattozo FH, de Amarante MC, Kalil SJ, et al. C-phycocyanin decreases proliferation and migration of melanoma cells: In silico and in vitro evidences. Bioorgan Chem 2022;122:105757.
[Crossref] [Google Scholar] [PubMed]
- Organisation for Economic Co-operation and Development. Test Guideline 203. Fish, Acute Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris, France 1992.
- Abdelhamid FM, Mahgoub HA, Ateya AI. Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ Sci Pollut Res 2020;27:10950-65.
[Crossref] [Google Scholar] [PubMed]
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925;18(2):265-7.
[Google Scholar] [PubMed]
- Huang X, Yang S, Li B, Wang A, Li H, Li X, et al. Comparative toxicity of multiple exposure routes of pyraclostrobin in adult zebrafish (Danio rerio). Sci Total Environ 2021;777:145957.
[Crossref] [Google Scholar] [PubMed]
- Hong X, Zha J. Fish behavior: A promising model for aquatic toxicology research. Sci Total Environ 2019;686:311-21.
[Crossref] [Google Scholar] [PubMed]
- Satpathy L, Parida SP. Acute toxicity assessment and behavioral responses induced by kandhamal Haladi in adult zebrafish (Danio rerio). Biointerface Res Appl Chem 2020;11(1):7368-81.
- Leris I, Kalogianni E, Tsangaris C, Smeti E, Laschou S, Anastasopoulou E, et al. Acute and sub-chronic toxicity bioassays of olive mill wastewater on the Eastern mosquitofish Gambusia holbrooki. Ecotoxicol Environ Safety 2019;175:48-57.
[Crossref] [Google Scholar] [PubMed]
- Schmidt F, Braunbeck T. Alterations along the hypothalamic-pituitary-thyroid axis of the zebrafish (Danio rerio) after exposure to propylthiouracil. J Thyroid Res 2011;2011.
[Crossref] [Google Scholar] [PubMed]
- Yildirim MZ, Benlı AC, Selvı M, Ozkul A, Erkoc F, Kocak O. Acute toxicity, behavioral changes, and histopathological effects of deltamethrin on tissues (gills, liver, brain, spleen, kidney, muscle, skin) of Nile tilapia (Oreochromis niloticus L.) fingerlings. Environ Toxicol 2006;21(6):614-20.
[Crossref] [Google Scholar] [PubMed]
- Carvalho JC, Keita H, Santana GR, de Souza GC, Dos Santos IV, Amado JR, et al. Effects of Bothrops alternatus venom in zebrafish: A histopathological study. Inflammopharmacology 2018;26:273-84.
[Crossref] [Google Scholar] [PubMed]
- Meneses JO, dos Santos Cunha F, Dias JA, da Cunha AF, dos Santos FJ, da Costa Sousa N, et al. Acute toxicity of hot aqueous extract from leaves of the Terminalia catappa in juvenile fish Colossoma macropomum. Aquacul Int 2020;28:2379-96.
- Pal S, Kokushi E, Koyama J, Uno S, Ghosh AR. Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. J Environ Sci Health Part B 2012;47(3):180-95.
[Crossref] [Google Scholar] [PubMed]
- Mahjoubian M, Naeemi AS, Sheykhan M. Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on zebrafish (Danio rerio). Chemosphere 2021;263:128182.
[Crossref] [Google Scholar] [PubMed]
- Linhua HA, Zhenyu WA, Baoshan XI. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci 2009;21(10):1459-66.
[Crossref] [Google Scholar] [PubMed]
- Mai Y, Peng S, Li H, Lai Z. Histological, biochemical and transcriptomic analyses reveal liver damage in zebrafish (Danio rerio) exposed to phenanthrene. Comp Biochem Physiol Part C Toxicol Pharmacol 2019;225:108582.
[Crossref] [Google Scholar] [PubMed]
- Mansouri B, Maleki A, Davari B, Johari SA, Shahmoradi B, Mohammadi E, et al. Histopathological effects following short-term coexposure of Cyprinus carpio to nanoparticles of TiO2 and CuO. Environ Monitoring Assessment 2016;188(10):1-2.
[Crossref] [Google Scholar] [PubMed]
- Badroo IA, Nandurkar HP, Khanday AH. Toxicological impacts of herbicide paraquat dichloride on histological profile (gills, liver, and kidney) of freshwater fish Channa punctatus (Bloch). Environ Sci Pollut Res 2020;27:39054-67.
[Crossref] [Google Scholar] [PubMed]
- Hadi AA, Alwan SF. Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminum. Int J Pharm Life Sci 2012;3(11):2071-81.
- Ferreira DQ, Ferraz TO, Araujo RS, Cruz RA, Fernandes CP, Souza GC, et al. Libidibia ferrea (juca), a traditional anti-inflammatory: A study of acute toxicity in adult and embryos zebrafish (Danio rerio). Pharmaceuticals 2019;12(4):175.
[Crossref] [Google Scholar] [PubMed]
- Sharma D, Gandotra R, Dhiman SK, Arif M, Dogra A, Lone A, et al. Ultrastructural biomarker responses in gill tissues of Cirrhinus mrigala (Hamilton, 1822) through SEM after exposure to zinc sulphate. Egyptian J Aquatic Res 2021;47(2):157-62.
- Suchana SA, Ahmed MS, Islam SM, Rahman ML, Rohani MF, Ferdusi T, et al. Chromium exposure causes structural aberrations of erythrocytes, gills, liver, kidney, and genetic damage in striped catfish Pangasianodon hypophthalmus. Biol Trace Element Res 2021;199:3869-85.
[Crossref] [Google Scholar] [PubMed]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 2005;4(1):35-44.
[Crossref] [Google Scholar] [PubMed]
- Luzio A, Parra S, Costa B, Santos D, Alvaro AR, Monteiro SM. Copper impair autophagy on zebrafish (Danio rerio) gill epithelium. Environ Toxicol Pharmacol 2021;86:103674.
[Crossref] [Google Scholar] [PubMed]
- Dogan D, Nur G, Deveci HA. Tissue-specific toxicity of clothianidin on rainbow trout (Oncorhynchus mykiss). Drug Chem Toxicol 2022;45(4):1851-61.
[Crossref] [Google Scholar] [PubMed]
- Rodrigues S, Antunes SC, Nunes B, Correia AT. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ Sci Pollut Res 2019;26(15):15481-95.
[Crossref] [Google Scholar] [PubMed]
- Oliveira HH, Reis-Filho JA, Nunes JA, Dos Santos RM, de F Esteves Santiago E, Aguilar L, et al. Gill histopathological biomarkers in fish exposed to trace metals in the Todos os Santos Bay, Brazil. Biol Trace Elem Res 2022;200(7):3388-99.
[Crossref] [Google Scholar] [PubMed]
- Sharma R, Jindal R, Faggio C. Impact of cypermethrin in nephrocytes of freshwater fish Catla catla. Environ Toxicol Pharmacol 2021;88:103739.
[Crossref] [Google Scholar] [PubMed]
- Lakra KC, Banerjee TK, Lal B. Coal mine effluent-induced metal bioaccumulation, biochemical, oxidative stress, metallothionein, and histopathological alterations in vital tissues of the catfish, Clarias batrachus. Environ Sci Pollut Res 2021;28(20):25300-15.
[Crossref] [Google Scholar] [PubMed]