- *Corresponding Author:
- W. B. Jiang
Department of Cardiology, Wenzhou Central Hospital, The Second Affiliated Hospital of Shanghai University, Wenzhou, Zhejiang 325000, P. R. China
E-mail: jiangwb919@163.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “219-225” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ischemic heart disease, resulting from ischemic and anoxic cardiomyocytes, can lead to the abnormal metabolic activity which is able to result in some serious diseases including cardiac failure, thrombus and other deadly disease and it is also the bad factor to the health of human. Besides, oxidative stress resulting from ischemic heart disease is one of the most serious factors of this disease which can destroy the healthy tissue, protein and so on. Hypoxia, as a signal, is able to launch hypoxia-inducible factor 1-alpha which lives in cytoplasm. It transfers to cell nucleus to achieve its aim, which means hypoxia-inducible factor 1-alpha is going to bind to hypoxia-inducible factor 1-beta first, then start some downstream promoters to facilitate several target genes such as vascular endothelial growth factor, inducible nitric oxide synthase, necessary enzyme of glycolysis and erythropoietin. Reactive oxygen species has also been proved that, it can start hypoxia-inducible factor 1-alpha as the second messenger. At the moment, hypoxia-inducible factor 1-alpha has been proved that, it is influential for ischemic heart disease through some pathways such as the increased angiogenesis, promoting glycolysis and the decreased reactive oxygen species. In this review, we discussed the advances in hypoxiainducible factor 1-alpha and ischemic heart disease.
Keywords
Hypoxia-inducible factor-1, ischemic heart disease, oxidative stress, target genes
Ischemic Heart Disease (IHD) is one of the important causes of death around the world and there have been 1.7 million deaths in 2016 due to this disease which cannot be ignored and the characteristic of IHD is that coronary arteries can be narrow and blood flow volume can also be reduced, then myocardial cells can suffer from lack of blood and no oxygen, besides, due to the poor self-renewal of myocardial cells, in this wrong situation, cardiomyocytes can be injured and cannot be recovered in the future[1]. The origin of IHD can be known as atherosis, when the atherosclerotic plaque is cracked, it will able to add to vascular tasks and then worsen the condition of IHD. Due to the lack of blood, the change of cardiomyocyte environment can lead to ventricular remodeling and myocardial infarction, and more bad situation can also been induced[2]. According to a recent research in 2019, the number of deaths accounts for 16 % of the total deaths around the world, which is far more than the expected and the research also reports that IHD occurs in conjunction with diabetes, high blood pressure and some other diseases, but this kind of comorbidity rate is divided according to gender, age and race. In China, the incidence rate is increasing year after year[3] and another report shows that the incidence and trend of IHD is becoming younger in average age, and there is something more serious in young people. Social factors, economic factors and bad living habits all can accelerate the occurrence of this disease. So, it is necessary for us to find some solutions to cure it. Comparatively, we can quit some bad habits and take preventive measures to reduce the disease[4]. It is imperative to study and learn how to treat IHD.
Under hypoxia, Hypoxia-Inducible Factor 1-alpha (HIF-1α) has been regarded as the most necessary factor for downstream genes transcription. After moving into cell nucleus, HIF-1α binds to Hypoxia-Response Elements (HREs) (anoxic reaction element) which is regarded as a switch for next step and it can promote angiogenesis, glycolysis and redox homeostasis and it is essential for the treatment of IHD. And a recent report shows that Copper (Cu), as a kind of transition metal, has been proved that it is beneficial for human. When it comes to IHD, Cu is able to participate in a series of reactions caused by hypoxia. It can control HIF-1α to adjust oxygen steady-state and some other pathways to protect cardiomyocytes from ischemia, anoxia and inflammation. Besides, Cu is also protective against heart attacks. In this study about Cu, we have learned that Cu can increase the stability of HIF-1α through preventing the stability of Prolyl Hydroxylase (PHD) and Cu also can promote the binding of HIF-1α to downstream promoter sequence to protect heart[5]. Moreover, research from Zhou et al. also learns that HIF-1α has certain positive effects for IHD. During this study, Complex Congenital Heart Disease (CCHD) patients suffer from chronic hypoxia which is same as IHD and influence the activity of normal cells. This study specifically emphasizes that PHD2/HIF-1α pathway is good for the treatment of CCHD and a mutant gene decreases the expression of PHD and consolidates the stability of HIF-1α and then starts the downstream genes such as Vascular Endothelial Growth Factor (VEGF) and Erythropoietin (EPO). Above all, HIF-1α protect cardiomyocytes and it is valuable for us to discuss the function of HIF-1α for the treatment of IHD. The followings are some introductions of HIF-1α and IHD and their relationships[6].
Action Characteristics of HIF-1α
HIF-1 was first found as a kind of protein that can bind to erythrogenin and HIF-1 can respond to oxygen levels. HIF-1 is a dimer that is made of HIF-1α and HIF-1 beta (β). HIF-1β is able to express steadily in cytoplasm but the content of HIF-1α is low in normoxic conditions[7]. HIF-1β is also known as Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT). There are three subtypes of HIF-α, but HIF-1α is the most important one in oxygen pathways and it comes to light through the study of Semenza[8]. In normoxic conditions, HIF-1α show instability and it can combine with the protein of von Hippel Lindau (VHL) for degradation process and α-ketoglutarate-dependent dioxygenase, PHD and asparaginyl hydroxylase all can participate in the whole process. When everything is ready, ubiquitination pathway can be started and then HIF-1α is going to be degraded. However, during the oxygen-deficient environment, PHD is inactive, hydroxylation of proline cannot be achieved and HIF-1α cannot be degraded and moves into cell nucleus. In cell nucleus, HIF-1α combines with HIF-1β forming a dimer and this dimer start downstream target genes through promoter and HRE to achieve its protective effect[9]. HIF-1α, as a factor of oxygen regulation, reveals different expression quantity at different oxygen concentration. At 20 % oxygen concentration, the expression of HIF-1α protein is at very low level, but when the oxygen concentration is below 5 %, the expression of HIF-1α protein increases rapidly and the expression of HIF-1α protein is associated with oxygen partial pressure. The expression of HIF-1α increased with the increase of oxygen partial pressure and the expression of HIF-1α decreased with the decrease of oxygen partial pressure[10]. Both ends of HIF-1α can sense hypoxia signals and the role of C-terminal is to regulate transcription and stabilize hypoxia-inducible proteins. Both the C and N terminals have Transactivation Domain (TAD) regions and the TAD region of the N-terminal is responsible for transcriptional activation[11].
Hypoxia Activates Downstream Genes of HIF-1α in IHD
Effect of HIF-1α on IHD through VEGF:
Coronary artery occlusion can cause irreversible damage to cardiomyocytes and the blood flow recover is necessary for the treatment of IHD. Angiogenesis not only participates in wound healing, embryogenesis, other physiological and pathological processes but also restores blood flow to coronary arteries and promotes regeneration of cardiac muscle cells which has been proved by previous experiments[12]. VEGF is a multipotent growth factor that plays an important role in the regulation of angiogenesis and vascular permeability barrier[13]. In the case of atherosclerosis and other IHD, VEGF can improve endothelial function, angiogenesis of ischemic myocardium and effectively reduce necrotic tissue, which proves the important role of VEGF in IHD and a research of Li et al. also verifies the function. In Li’s research, they first established the rat model of myocardial infarction by ligation of left anterior descending branch, then the adenovirus HIF-1α mutant Ad-HIF-1α-trip was constructed from the mutation site of adenovirus HIF-1α, where this kind can make HIF-1α stable in cells for a longer time. Through Reverse Transcription-Polymerase Chain Reaction (RT-PCR), Enzyme-Linked Immunosorbent Assay (ELISA) and Western blotting, it is found that the mutant could increase the expression of VEGF and other angiogenic factors and improve infarct size and cardiac function, and it is obvious that the stable HIF-1α is good for IHD through VEGF. Besides, this study not only detects the messenger Ribonucleic Acid (mRNA) expression of VEGF from the 1st d to the 7th d, but also detects the expression of VEGF from 7th d to the 28th d. It has been found that the stability of HIF-1α and VEGF can only stay for 7 d and from the 8th d, the stability of them can reduce little by little, which proves the previous study that the effect of VEGF cannot be stable for a long time[14]. Another study from Hernan proves that overexpression of HIF-1α in mesenchymal cells enhances angiogenesis which is beneficial for IHD. During this study, mesenchymal cells overexpressing HIF-1α can promote angiogenesis more strongly than normal mesenchymal cells and this phenomenon is achieved through Jagged 1 (JAG1, uniquely encapsulated in Mesenchymal Stem Cell (MSC) exosomes) and Notch ligand. Although its mechanism is not clear, the growth factor such as VEGF and Platelet-Derived Growth Factor (PDGF) has become the focus[15]. The research results of Wang et al. show that microRNA (miR)-29 inhibitors are beneficial for IHD through Phosphatidylinositol-3-Kinase (PI3K)/mammalian Target of Rapamycin (mTOR)/HIF-1α/VEGF pathway. miR-29 works by increasing the contractile load of cardiac myocytes and results in more serious disease, and it can combine with PI3K so that, it can initiate the phosphorylation pathway which leads to PI3K to degrade. In other words, after the use of inhibitors, PI3K/mTOR/HIF-1α/VEGF pathway can be activated and the increase of the VEGF expression promotes and improves the function of myocardial cell[16]. Gonzalez-king et al.[15] show that injection of Hydroxyapatite Electret (HAE) into mice can significantly reduce myocardial infarction area and fibrosis in the distal unaffected area is also significantly reduced. HIF-1α binds and activates transcription of VEGF and C-X-C Motif Chemokine Ligand 12 (CXCL12), resulting in increased VEGF activity, increased angiogenesis and increased myocardial survival area in mice. In addition, polarization of HAE may also play a protective role through VEGF induction of angiogenesis. Wang et al.[16] also show that injection of muscone in mice increases angiogenesis around the infarct area, as well as increased phosphorylated VEGF Receptor (P-VEGFR2) in vascular endothelial cells. Western blot and PCR results show increased expression of HIF-1α and VEGF, which proves that muscone can protect myocardial infarction by up-regulating VEGF through HIF-1α. Therefore, VEGF can play an effective role in the treatment of IHD.
Effect of HIF-1α on IHD through glycolysis:
Glycolysis is the main way to maintain the energy source of cardiomyocytes during acute ischemia and coronary artery occlusion, and it can protect the contractility of heart and damage of cardiomyocytes. Under hypoxia, glycolysis is activated and some key enzymes, such as hexose kinase ? and pyruvate dehydrogenase-1, regulate glycolysis pathway to alleviate myocardial ischemia-reperfusion injury, which has been proved that it is beneficial for the disease[17]. Currently, targeting myocardial glucose metabolism during myocardial ischemia is considered to be an effective treatment to reduce myocardial infarction area and protect myocardial cells. Glucose Transporter (GLUT) protein in plasma membrane mediates glucose uptake in myocardial cells, which is an important mechanism for regulating glucose uptake[18]. Glycolysis is an energy metabolism pathway independent of oxygen and in general, increased glycolysis is a way of protecting the body except for the bad effect of energy metabolism. HIF-1α stimulates and enhances the glycolysis pathway, and the main way is to activate some key enzymes such as GLUT1, Hexokinase 2 (HK2), Lactate Dehydrogenase A (LDHA) and 3-Phosphoinositide-Dependent Kinase-1 (PDK1)[19]. One research shows that the injection of pinocembrin can reduce the size of myocardial infarction. During the experiment, the protective effect of pinocembrin is because of the increase of Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase 3 (PFKFB3) which is the important enzyme in glycolysis. To increase the accuracy of the casting, the research also knocks out PFKFB3 and the result shows that the protective effect is removed. Above all, all of the research proves that the injection of pinocembrin prior to myocardial ischemia-reperfusion can protect the myocardium or glycolytic mediated energy conversion is beneficial for myocardial protection in IHD[20].
Hou et al.[21] show that PDK-1 can maintain the normal life activities of cells under the condition of hypoxia by promoting the cells transition from aerobic oxidation to glycolysis. In this experiment, we have known that sevoflurane preconditioning is beneficial for IHD, but the specific mechanism is not very clear. Injection of sevoflurane before myocardial ischemia-reperfusion can protect cardiomyocytes and the expression of Phosphofructokinase (PFK) and HIF-1α increase through the results of Western blot which means the protective effect of sevoflurane may be through HIF-1α/PDK-1 signal pathway to be beneficial for IHD. Zhang et al.[22] have found that Danqi Pill (DQP) can improve glucose metabolism in heart failure rats through myocardial glucose uptake. After the treatment of DQP, not only both GLUT4, Pyruvate Kinase M2 (PKM2) and key enzymes of glucose metabolism are promoted to keep the cardiomyocytes energized, but also attenuated PHD and enhanced HIF-1α expression, which proves that the effect of DQP may be accomplished through HIF-1α/Peroxisome Proliferator-Activated Receptor-Gamma Coactivators-1 alpha (PGC-1α) signaling pathway. In other words, after ischemia, HIF-1α activates key enzymes of the glycolysis pathway to respond to hypoxia stress response, suggesting that HIF-1α can provide a small amount of energy to protect cells by regulating the glycolysis pathway.
Effect of HIF-1α on IHD via inducible Nitric Oxide Synthase (iNOS):
The role of iNOS in IHD is still controversial. Inflammation and leukocytosis are necessary after myocardial infarction in mice and leukocytosis accompanied by the increase of iNOS expression is the key to the formation of tissue oxidative stress, which means that loss of iNOS expression in leukocytes was beneficial for heart failure mice. On the other hand, the loss of iNOS in leukocytes may facilitate the activation of monocytes recruited by M2 and promote tissue repair and these effects are independent of whole cardiomyocytes. Targeting leukocytes or macrophages may be a future therapeutic strategy. However, some researches show that iNOS may be profitable, which results from the source of iNOS. The iNOS from cardiocytes is bad for IHD because it can increase the mortality, promote ventricular remodeling and cancel the protective effect of ischemic preconditioning. But there are some opposite researches which show that specific overexpression of iNOS is good for IHD and has improved fibrosis in myocardium of pigs with heart failure. As for the iNOS from macrophage, it is accompanied by the production of superoxide and toxic substances[23]. In a study by Wilmes et al. in response to hypoxia, iNOS induces the production of large amounts of Nitric Oxide (NO) which plays a positive role in heart failure, IHD and arteriosclerosis. The results show that the production of iNOS and NO could induce the production of peroxynitrite in myocardial infarction, which is the key factor that further leads to myocardial injury. Expect for the infarction area, an increase in iNOS is also found in non-infarcted areas, which means that iNOS is expected to be an early marker of myocardial infarction in the future[24]. In the study of Wilmes et al. it has been proved in previous studies that the increase of iNOS is the result of tissue damage and inflammation, and the activation of iNOS by HIF-1α may be a pathological effect. In this experiment, the expression of iNOS was increased in both the affected and non-affected areas of myocardial infarction. However, the increase of iNOS expression in the non-affected area of myocardial infarction indicates that myocardial cells have undergone pathological changes before the disease completely occurs, which may be the basis for forensic diagnosis of myocardial infarction in the future[25]. Cheng et al. show that PKM2 can bind HIF-1α to recruit its downstream promoter element (HRE) and in the experiment, omega (ω)-alkynyl arachidonic acid inhibits the binding of HIF-1α to PKM2, thus inhibiting the binding of HIF-1α to iNOS and reducing the activity of iNOS, which means that it has a protective effect on acute myocardial infarction. Therefore, the effect of iNOS on IHD needs to be further studied[26].
Effect of HIF-1α on IHD via EPO:
EPO can play a protective role through hematopoietic, anti-apoptotic and other mechanisms and the anti-apoptotic effect is achieved through the production of NO. On the one hand, in animal studies, we have known that EPO can promote hemoglobin production, which gives the positive effect to cardiocytes. On the other hand, from the previous experiment and the most recent experiment, EPO has been proved that it can reduce the degree of myocardial fibrosis through reducing collagen deposition, but does not protect ventricular function, which is thought to be a further damage caused by oxidative stress and inflammation[27]. Although the positive effect of EPO is beneficial for patients with myocardial infarction, EPO must be injected prior to reperfusion and it relies on the time of injection. However, as for human, EPO’s effect is just opposite to that seen in animal studies. In a trial collecting EPO effects over the last 5 y, it has also shown that EPO cannot improve cardiac function after Percutaneous Coronary Intervention (PCI) and the use of EPO also resulted in increased mortality and side effects[28]. In an experiment from Minamino et al. it show that low dose EPO injection has some protective effects on IHD which can reduce the size of myocardial infarction, prevent heart remodeling and enhance new blood vessels. In contrast, due to EPO’s similarity to thrombopoietin, high concentrations of EPO may promote platelet production and increase cardiovascular prevalence[29]. Previous studies on the effects of HIF-1α on IHD via EPO have been limited, but a research from Jang et al. shows some results. During the experiment of Obstructive Sleep Apnea-Hypopnea Syndrome (OSAHS), the mRNA and protein expression of HIF-1α increases and downstream genes including VEGF and EPO increases as well, which can result in thrombus. After the use of Mandibular Advancement Devices (MAD), the expression of HIF-1α and its downstream target gene EPO was decreased, and heart function is improved[30]. In conclusion, the research from Minamino et al. proves that low dose EPO has certain protective effects on the new function directly and the research from Jang et al. also shows that the high dose EPO has certain damage to cardiac function indirectly[29,30].
Reactive Oxygen Species (ROS) activates HIF-1α role in IHD
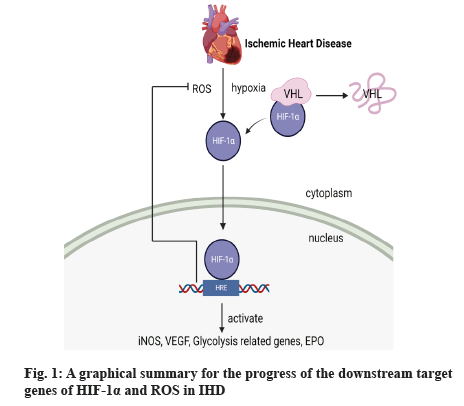
At present, we have known that mitochondria are the main source of ROS including superoxide radicals, hydroxyl radical and so on, which can result in some wrong effects to nucleic acids, lipids and proteins, and these effects can flare up after blood reperfusion. When ROS is produced, the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) of neighboring mitochondria becomes open and ROS will increase further and furthermore, mitochondrial inclusions are released including Cytochrome complex (Cyt c) and proapoptotic factor which can cause severe myocardial cell damage. Besides, oxidative stress caused by mitochondria leads to heart failure, ventricular remodeling and some others[31]. ROS production is involved in ventricular remodeling and electrical remodeling, leading to the formation of fibrosis and thrombosis. In a research study of atrial fibrosis it briefly mentions the relationship between HIF-1α and ROS. Under hypoxia, ROS, as a second messenger, is important for HIF-1α production, which means the production of ROS is able to promote the generation of HIF-1α and some other pathways can be started such as Mitogen-Activated Protein Kinase (MAPK) pathway which is bad for health[32]. Reperfusion is currently the only treatment for IHD, but the damage caused by ROS is inestimable. Clinically, preconditioning, which increases myocardial cell tolerance through transient ischemia prior to the onset of myocardial infarction, is currently considered effective, but myocardial infarction can be unpredictable and the other effective method is to use medication, which can use several drugs to treat the disease because of intricate redox homeostasis in animals, and the order of administration also determines the effect of treatment. However, these methods are not really applied clinically[33]. One research reveals the role of HIF-1α in cardiac fibroblasts when the heart is starved of oxygen. When cardiac muscle cells are deprived of oxygen, cardiac fibroblasts proliferate under ROS stimulation which is regarded as a signal. Interestingly, this experiment has found that the use of mitochondrial antioxidants reduces ROS production in HIF-1α knockout mice, but not in wild-type mice. And experimental data indicates that HIF-1α can protect myocardial cells from ROS damage and this may result from Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) and some other pathways[34]. Research from Chaudhuri et al. investigates the relationship between HIF-1α and cell apoptosis through nanotargeting technology which can make HIF-1α overexpression. After oxidative stress, the expression of B-Cell Lymphoma 2 (BCL2)/Adenovirus E1B 19 kDa Protein-Interacting Protein 3 (BNIP3) and apoptosis increases, or we can say oxidative stress and apoptosis activity can reduce after the overexpression of HIF-1α. Besides, in the case of HIF-1α overexpression, Heme Oxygenase-1 (HO-1) can also express to rise and the damage from oxidative stress goes down, and this beneficial effect is through down-regulation of BNIP3. And this anti-apoptotic effect is also through Nuclear Factor kappa B (NF-κB) which can be started by HIF-1α and then NF-κB combines with promoter of BNIP3 to regulate its activity[35]. Another research from Chaudhuri et al. studies the association between degree of hypoxia, duration of hypoxia and apoptosis. The research has found that cells undergo apoptosis at 8 % oxygen, but this disappears over a longer period of time because of Heat Shock Protein 90 (HSP90)-Inositol-Requiring Enzyme 1 (IRE1) and at 14 % oxygen, cells do not undergo apoptosis, but at 1 %-4 % oxygen, apoptosis is severely upregulated which is not influenced by length of time. At different times and at different levels of hypoxia, the expression level of HIF-1α changes with ROS, but compared to 5 h at 8 % oxygen, the expression of HIF-1α increase obviously though the ROS is low. HIF-1α, which can be activated by ROS, has revealed that they can reduce apoptosis through different kinds of pathways[36]. A graphical summary for the progress of the downstream target genes of HIF-1α and ROS in IHD is shown in fig. 1.
Conclusion
In this review, we studied the progress of the downstream target genes of HIF-1α and ROS in IHD. IHD is a kind of disease that occurs when myocardial cells are ischemic and hypoxic. The incidence is increasing year by year in China. Therefore, we must pay more attention to this disease. HIF-1α is translocated into the nucleus under hypoxia and binds to HIF-1β to initiate transcription of downstream target genes, including VEGF, key enzyme genes in glycolysis, production of EPO and large expression of iNOS. In response to oxidative stress, HIF-1α regulates downstream substances to reduce oxidative stress-induced damage. In a large number of previous experiments, we have proved that HIF-1α plays a certain role in IHD under hypoxia through different pathways. However, it is not difficult to find that the specific mechanism of some effects is not completely clear and the experimental results may be contrary to the previous conclusions. It has been known that ROS can activate HIF-1α to some extent, but whether the downstream genes of HIF-1α mentioned in this paper are involved in ROS production or whether there is a certain correlation has not been mentioned in the present paper. In the future, it is necessary to further study the relationship between HIF-1α and IHD, so as to truly benefit patients with IHD in clinic.
Author’s contributions:
Anwu Huang and Zhaolin Wang contributed equally to this work.
Acknowledgements:
This research was supported by Natural Science Funds of Zhejiang Province, China (No.Y22H024180)
Conflict of interests:
The authors declared no conflict of interest.
References
- Chen H, Xue R, Huang P, Wu Y, Fan W, He X, et al. Modified exosomes: A good transporter for miRNAs within stem cells to treat ischemic heart disease. J Cardiovasc Transl Res 2022;15(3):514-23.
[Crossref] [Google scholar] [PubMed]
- Guo X, Shi Q, Zhang W, Qi Z, Lv H, Man F, et al. Lipid droplet-A new target in ischemic heart disease. J Cardiovasc Transl Res 2022;15(4):730-9.
[Crossref] [Google scholar] [PubMed]
- Zhou D, Wang L, Ding S, Shen M, Qiu H. Phenotypic disease network analysis to identify comorbidity patterns in hospitalized patients with ischemic heart disease using large-scale administrative data. Healthcare 2022;10(1):80.
[Crossref] [Google scholar] [PubMed]
- Prasad DS, Kabir Z. Editorial comment: Focus on the global burden of IHD from big data to precision public health. Eur J Prev Cardiol 2022;29(2):417-9.
[Crossref] [Google scholar] [PubMed]
- Sacco A, Martelli F, Pal A, Saraceno C, Benussi L, Ghidoni R, et al. Regulatory miRNAs in cardiovascular and Alzheimer’s disease: A focus on copper. Int J Mol Sci 2022;23(6):3327.
[Crossref] [Google scholar] [PubMed]
- Zhou Y, Ouyang N, Liu L, Tian J, Huang X, Lu T. An EGLN1 mutation may regulate hypoxic response in cyanotic congenital heart disease through the PHD2/HIF-1A pathway. Genes Dis 2019;6(1):35-42.
[Crossref] [Google scholar] [PubMed]
- West JB. Physiological effects of chronic hypoxia. N Engl J Med 2017;376(20):1965-71.
[Crossref] [Google scholar] [PubMed]
- Zheng J, Chen P, Zhong J, Cheng Y, Chen H, He Y, et al. HIF?1α in myocardial ischemia?reperfusion injury (Review). Mol Med Rep 2021;23(5):1-9.
[Crossref] [Google scholar] [PubMed]
- Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab 2018;27(2):281-98.
[Crossref] [Google scholar] [PubMed]
- Hirota K. HIF-α prolyl hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines 2021;9(5):468.
[Crossref] [Google scholar] [PubMed]
- Fang Z, Zhang Y, Zhao X, Jin W, Yu L. The role of PKC and HIF-1 and the effect of traditional Chinese medicinal compounds on cerebral ischemia-reperfusion injury. Evid Based Complement Alternat Med 2022;2022:1-12.
[Crossref] [Google scholar] [PubMed]
- Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, et al. VEGF?A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress?mediated autophagy. J Cell Physiol 2019;234(10):17690-703.
[Crossref] [Google scholar] [PubMed]
- Chen ZZ, Gong X, Guo Q, Zhao H, Wang L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J Ethnopharmacol 2019;228:70-81.
[Crossref] [Google scholar] [PubMed]
- Li M, Cui Y, He W, Deng X, Wang Y, Cai M, et al. Effects of triple-mutated hypoxia-inducible factor-1α on angiogenesis and cardiac function improvement in rats with myocardial infarction. Cell Physiol Biochem 2018;50(6):2329-40.
[Crossref] [Google scholar] [PubMed]
- Gonzalez-King H, Garcia NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 2017;35(7):1747-59.
[Crossref] [Google scholar] [PubMed]
- Wang X, Liu Y, Hou H, Shao W, Huang D, Hao Z, et al. miRNA-29 aggravates myocardial infarction via inhibiting the PI3K/mTOR/HIF1α/VEGF pathway. Aging 2022;14(7):3129-42.
[Crossref] [Google scholar] [PubMed]
- Gao J, Feng W, Lv W, Liu W, Fu C. HIF-1/AKT signaling-activated PFKFB2 alleviates cardiac dysfunction and cardiomyocyte apoptosis in response to hypoxia. Int Heart J 2021;62(2):350-8.
[Crossref] [Google scholar] [PubMed]
- Yuan Y, Liu X, Miao H, Huang B, Liu Z, Chen J, et al. PEDF increases GLUT4-mediated glucose uptake in rat ischemic myocardium via PI3K/AKT pathway in a PEDFR-dependent manner. Int J Cardiol 2019;283:136-43.
[Crossref] [Google scholar] [PubMed]
- Sun R, Meng X, Pu Y, Sun F, Man Z, Zhang J, et al. Overexpression of HIF-1a could partially protect K562 cells from 1, 4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol In Vitro 2019;55:18-23.
[Crossref] [Google scholar] [PubMed]
- Zheng Y, Wan G, Yang B, Gu X, Lin J. Cardioprotective natural compound pinocembrin attenuates acute ischemic myocardial injury via enhancing glycolysis. Oxid Med Cell Longev 2020;2020:1-13.
[Crossref] [Google scholar] [PubMed]
- Hou T, Ma H, Wang H, Chen C, Ye J, Ahmed AM, et al. Sevoflurane preconditioning attenuates hypoxia/reoxygenation injury of H9c2 cardiomyocytes by activation of the HIF-1/PDK-1 pathway. PeerJ 2020;8:e10603.
[Crossref] [Google scholar] [PubMed]
- Zhang Q, Guo D, Wang Y, Wang X, Wang Q, Wu Y, et al. Danqi pill protects against heart failure post-acute myocardial infarction via HIF-1α/PGC-1α mediated glucose metabolism pathway. Front Pharmacol 2020;11:458.
[Crossref] [Google scholar] [PubMed]
- Kingery JR, Hamid T, Lewis RK, Ismahil MA, Bansal SS, Rokosh G, et al. Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol 2017;112(2):1-7.
[Crossref] [Google scholar] [PubMed]
- Wilmes V, Scheiper S, Roehr W, Niess C, Kippenberger S, Steinhorst K, et al. Increased inducible Nitric Oxide Synthase (iNOS) expression in human myocardial infarction. Int J Legal Med 2020;134(2):575-81.
[Crossref] [Google scholar] [PubMed]
- Wilmes V, Lux C, Niess C, Gradhand E, Verhoff MA, Kauferstein S. Changes in gene expression patterns in postmortem human myocardial infarction. Int J Legal Med 2020;134(5):1753-63.
[Crossref] [Google scholar] [PubMed]
- Cheng Y, Feng Y, Xia Z, Li X, Rong J. ω-Alkynyl arachidonic acid promotes anti-inflammatory macrophage M2 polarization against acute myocardial infarction via regulating the cross-talk between PKM2, HIF-1α and iNOS. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862(12):1595-605.
[Crossref] [Google scholar] [PubMed]
- Pessoa FG, Mady C, Fonseca KC, de Oliveira-Fonoff AM, Salemi VM, Jordão MR, et al. Erythropoietin reduces collagen deposition after myocardial infarction but does not improve cardiac function. Can J Physiol Pharmacol 2018;96(6):541-9.
[Crossref] [Google scholar] [PubMed]
- Steppich B, Groha P, Ibrahim T, Schunkert H, Laugwitz KL, Hadamitzky M, et al. Effect of erythropoietin in patients with acute myocardial infarction: Five-year results of the REVIVAL-3 trial. BMC Cardiovasc Disord 2017;17(1):1-8.
[Crossref] [Google scholar] [PubMed]
- Minamino T, Higo S, Araki R, Hikoso S, Nakatani D, Suzuki H, et al. Low-dose erythropoietin in patients with ST-segment elevation myocardial infarction (EPO-AMI-II)-A randomized controlled clinical trial. Circ J 2018;82(4):1083-91.
[Crossref] [Google scholar] [PubMed]
- Jang KJ, Jeong S, Kang DY, Sp N, Yang YM, Kim DE. A high ATP concentration enhances the cooperative translocation of the SARS coronavirus helicase nsP13 in the unwinding of duplex RNA. Sci Rep 2020;10(1):1-3.
[Crossref] [Google scholar] [PubMed]
- Bugger H, Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta 2020;1866(7):165768.
[Crossref] [Google scholar] [PubMed]
- Tsai CF, Yang SF, Lo CH, Chu HJ, Ueng KC. Role of the ROS-JNK signaling pathway in hypoxia-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int J Mol Sci 2021;22(6):3249.
[Crossref] [Google scholar] [PubMed]
- Jiang L, Zeng H, Ni L, Qi L, Xu Y, Xia L, et al. HIF-1α preconditioning potentiates antioxidant activity in ischemic injury: The role of sequential administration of dihydrotanshinone I and protocatechuic aldehyde in cardioprotection. Antioxid Redox Signal 2019;31(3):227-42.
[Crossref] [Google scholar] [PubMed]
- Janbandhu V, Tallapragada V, Patrick R, Li Y, Abeygunawardena D, Humphreys DT, et al. HIF-1A suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction. Cell Stem Cell 2022;29(2):281-97.
[Crossref] [Google scholar] [PubMed]
- Chaudhuri RD, Banik A, Mandal B, Sarkar S. Cardiac-specific overexpression of HIF-1α during acute myocardial infarction ameliorates cardiomyocyte apoptosis via differential regulation of hypoxia-inducible pro-apoptotic and anti-oxidative genes. Biochem Biophys Res Commun 2021;537:100-8.
[Crossref] [Google scholar] [PubMed]
- Chaudhuri RD, Banerjee D, Banik A, Sarkar S. Severity and duration of hypoxic stress differentially regulates HIF-1α-mediated cardiomyocyte apoptotic signaling milieu during myocardial infarction. Arch Biochem Biophys 2020;690:108430.
[Crossref] [Google scholar] [PubMed]