- *Corresponding Author:
- Jian Xiao
Guangxi Key Laboratory of Translational Medicine of Integrated Traditional Chinese and Western Medicine for Commonly Infectious Diseases, Guangxi University of Chinese Medicine, Nanning, Guangxi 530200, China
E-mail: hy757266169@163.com
| Date of Received | 07 June 2024 |
| Date of Revision | 08 August 2024 |
| Date of Accepted | 15 August 2025 |
| Indian J Pharm Sci 2025;87(4):169-176 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
T-cell therapy has changed the treatment landscape for both haematological malignancies and solid tumors, achieving breakthroughs in areas such as relapsed/refractory acute lymphoblastic leukaemia, lymphoma, multiple myeloma, and melanoma. However, T-cell therapy still faces several limitations, including off-target effects, tumour antigen escape, and the immunosuppressive tumor microenvironment. Serious adverse reactions induced by T-cell therapy, such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and immunologic escape leading to drug resistance and relapse, should also be addressed. Exploring new combination therapy approaches is considered an effective strategy to further enhance the anti-tumor effects of T-cell therapy. Chimeric antigen receptor T-cell therapy and T-cell receptor-engineered T-cell therapy are two directions of T-cell therapy. Tyrosine kinase inhibitors, immune checkpoint inhibitors, monoclonal antibodies, and other targeted therapeutic drugs have been shown to have synergistic effects with T-cell therapy while reducing adverse reactions. Therefore, this article will review the combination therapy models of various targeted therapeutic drugs and T-cell therapy from the perspectives of chimeric antigen receptor T-cell therapy and T-cell receptor-engineered T-cell therapy, aiming to provide references for the basic research and quality control of T-cell immunotherapy.
Keywords
Immunology, cytokine-targeted drugs, chimeric antigen receptor T cells, T cell receptor engineered T cells, research progress
Tumors are a disease that seriously endangers human physiological and psychological health, and malignant tumors have developed into one of the serious public health problems that seriously affect social development [1]. Malignant tumors cause serious harm to the body, leading to organ dysfunction or even failure, seriously affecting quality of life and survival time [2]. In recent years, T cell immunotherapy has made significant progress in targeting various types of malignant tumors. T cells, as a type of white blood cell in the immune system, have the ability to recognize and attack tumor cells [3,4]. At the same time, T cells are an important part of the Tumor Microenvironment (TME) and play an indispensable role in killing tumors [5,6]. T cell therapy enhances the immune system's ability to recognize and attack cancer cells but faces challenges such as TME suppression, T cell functional exhaustion, and side effects in practical applications [7]. Combination therapy with drugs can improve efficacy by enhancing T cell activity, overcoming the TME, reducing adverse reactions, and enhancing T cell persistence [8,9]. Researchers are exploring combining T cell therapy with immune checkpoint inhibitors, tumor vaccines, targeted therapies, chemotherapy, radiotherapy, small molecule drugs, metabolic regulators, and gene editing techniques to enhance T cell anti-tumor functions [10]. To enable T cells to accurately identify tumor antigens and exert immune effects persistently, researchers have developed Chimeric Antigen Receptor T cell (CAR-T) Therapy and T Cell Receptor-Gene Engineered T Cell (TCR-T) therapy. Future research will continue to explore various combination strategies to find the optimal treatment regimen. This paper briefly reviews the mechanisms and clinical applications of targeted drug therapy combined with T cell therapy.
CAR-T
Structure and Principles of CAR-T:
CAR-T is a genetic engineering technology, through the introduction of chimeric antigen receptor (CAR) genes with specific antigen recognition function into the body’s own T-cells, so that they can acquire the ability to recognise and attack specific antigens [11]. CAR is a synthetic hybrid receptor generated through genetic engineering, comprising an extracellular antigen-binding domain consisting of a single-chain Variable Fragment (scFV) of an antibody, a hinge, a transmembrane domain, and intracellular signalling domains [12]. The antigen-binding domain, located extracellularly, is connected to the internal domains via a glycine-serine flexible linker peptide forming the scFv. This flexible linker peptide utilizes glycine to increase the spatial flexibility of the scFv, while serine residues enhance the solubility of the tightly folded scFv, facilitating recognition and binding of cancer cell surface antigens to exert cytotoxic effects [13]. Therefore, CAR-T cells can exert cytotoxic effects without the need for the Major Histocompatibility Complex (MHC) pathway.
The intracellular signalling domain is a crucial component for the efficacy of CAR-T therapy. The first-generation CAR intracellular signalling domain contains only CD3 or FcR signalling domains [14]. Although the majority of CAR-T cells exert biological effects through dependence on the Immune Receptor Tyrosine-Based Activation Motif (ITAM) derived from Cluster of Differentiation (CD) 3, a single CD3 signalling domain seems insufficient to unleash the potent anti-tumor effects of CAR-T [15].
The second-generation CARs incorporate CD28 and CD137 co-stimulatory domains, demonstrating robust therapeutic effects in hematologic malignancies and showing some efficacy in solid tumors such as glioblastoma and advanced sarcoma [16]. To effectively utilize the biological effects of different co-stimulatory domains, the third-generation CAR combines CD137 and CD28 in the intracellular segment to promote their differentiation into central memory T cells, enhance CAR-T cell proliferation, and improve anti-tumor activity [17].
The fourth-generation CAR-T, building upon the third generation, utilizes genetic engineering to fuse the single-chain variable fragment recognizing the target antigen with spacers, transmembrane motifs, and T cell activation motifs, and incorporates an inducible Interleukin (IL)-12 gene, endowing CAR-T cells with dual anti-cancer activity induced by CAR-T cells and IL-12 [18]. In addition to enhancing CAR-T cell activation via autocrine secretion, IL-12 can attract and activate innate immune cells via paracrine secretion, after which the recombinant genes are transduced into T cells, enabling them to selectively kill target cells.
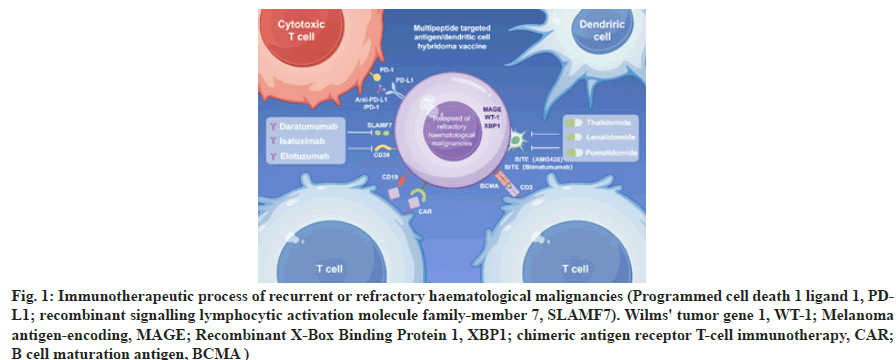
In summary, the efficacy mechanism of CAR-T involves steps such as specific antigen recognition, T cell activation, tumor cell killing, and immunological memory effects, making it an effective immunotherapy for cancer. The immunological process is depicted in Figure 1.
Fig. 1: Immunotherapeutic process of recurrent or refractory haematological malignancies (Programmed cell death 1 ligand 1, PDL1; recombinant signalling lymphocytic activation molecule family-member 7, SLAMF7). Wilms' tumor gene 1, WT-1; Melanoma antigen-encoding, MAGE; Recombinant X-Box Binding Protein 1, XBP1; chimeric antigen receptor T-cell immunotherapy, CAR; B cell maturation antigen, BCMA )
Combination therapy of CAR-T with small molecule inhibitors:
Small molecule inhibitors, such as dasatinib, effectively inhibit T cell activation and are considered switches for controlling the activation of T cells and CAR-T. Studies have shown that the addition of dasatinib can induce CAR-T into a dormant state, inhibiting its proliferation and the release of inflammatory factors [19]. The team led by Katrin Mestermann found that dasatinib effectively controlled CRS reactions in mice after CD19 CAR-T infusion, increasing the survival rate from 25% to 70%, confirming dasatinib as a reversible switch for CAR-T activity [20]. In addition to serving as a switch for CAR-T activation, adding dasatinib during CAR-T preparation can help produce higher quality cells. Clinical trial results using CD5 CAR-T to treat Acute Lymphoblastic Leukemia (ALL) showed that the addition of imatinib and dasatinib during CAR-T preparation enhanced the anti-tumor activity of CD5 CAR-T by blocking tonic signals [21]. Other specific Src kinase inhibitors such as ponatinib and saracatinib can also strongly inhibit CAR-T cytotoxic activity by inhibiting the signalling pathway mediated by Src kinase.
Given the close association of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway with various cytokine signalling pathways, JAK inhibitors are believed to reduce the risk of CRS after CAR-T therapy. Over a dozen JAK inhibitors have been approved for marketing, including ruxolitinib and fedratinib. Kenderian et al. [22] first confirmed in an AML animal model that concomitant administration of ruxolitinib with CD123 CAR-T effectively reduced the release of inflammatory factors without affecting the anti-leukaemia effect of CAR-T. Compared to the control group, the symptoms of weight loss in mice were improved, and survival rates were increased. Combination culture of ruxolitinib and CD19 CAR-T in vitro inhibited the proliferation and expansion of CAR-T cells, but did not affect their cytotoxic function. Xu et al. [23] similarly confirmed that ruxolitinib temporarily inhibited CAR-T proliferation and cytotoxicity, and although the cytotoxic capacity of CAR-T was restored after ruxolitinib withdrawal, cytokine release capacity remained suppressed. Although ruxolitinib has been shown to reduce the risk of CRS after CAR-T therapy, its inhibitory effect on CAR-T proliferation and the reduction of Granzyme B and Interferon-Gamma (IFN-γ) levels still warrant attention. Therefore, ruxolitinib is not recommended for people with poor CAR-T expansion. Moreover, potential complications such as thrombocytopenia and bleeding risk during ruxolitinib treatment should also be considered.
Mechanistic Target of Rapamycin (mTOR) is involved in the regulation of T cells including development, activation, and migration. In T cell differentiation, mTOR is a regulator of memory CD8 T cell differentiation, and inhibition of mTOR activity significantly increases the generation of memory T cells. In CD4+ T cells, mTOR signalling activation promotes the differentiation of Th1, Th2, and Th17 cells and inhibits the differentiation of Treg cells. Food and Drug Administration (FDA) approved mTOR inhibitors include rapamycin, everolimus, and temsirolimus. Leclercq et al.[24] demonstrated in a lymphoma mouse model that mTOR inhibitors significantly reduced the release of cytokines by CD19-T cells without affecting their anti-tumor activity, suggesting that mTOR inhibitors are more suitable for preventing CRS compared to hormonal JAK inhibitors or Src inhibitors.
Rapamycin is the earliest approved mTOR inhibitor. Besides its direct anti-tumor cell killing ability, it also inhibits the activation of T cells and B cells. Huye et al.[25] designed rapamycin-resistant CD19 CAR-T and found that combination with rapamycin improved the killing of lymphoma and acute B-Cell Leukemia (B-ALL) tumor cells in vitro. Additionally, a team led by Wei Haiming from the University of Science and Technology of China reported that the TOR signal during CAR-T activation can downregulate the expression of CXCR4, impairing the migration ability of CAR-T. Pre-treatment with rapamycin enhances the bone marrow migration ability of CAR-T, enhancing the therapeutic effect[26].
In summary, the combination of mTOR inhibitors and CAR-T can effectively intervene in CRS reactions and enhance the anti-tumor ability of CAR-T.
Immune checkpoint inhibitors:
When immune checkpoints such as PD-1, PDL1, and CTLA-4 are blocked, T cells can more effectively exert their cytotoxic effects. Although CAR-T is not restricted by the MHC, enhancing the ability of CAR-T to resist tumor escape, overexpression of immune checkpoints in the tumor immunosuppressive microenvironment can still restrict the normal cytotoxicity of CAR-T. Persistent stimulation of tumor antigens can lead to CAR-T exhaustion through the overexpression of immune checkpoint molecules such as CTLA-4, PD-1, and TIM-3, making the combination of immune checkpoint inhibitors a new option to improve the anti-tumor ability of CAR-T therapy. The main immune checkpoints currently under study include PD-1, CTLA-4, TIM-3, etc.
Under the persistent stimulation of tumor antigens, CAR-T overexpresses PD-1, leading to exhaustion and treatment failure. John et al.[27] first used PD-1 antibodies in combination with CAR-T targeting human epidermal growth factor receptor 2 in a mouse breast cancer tumor model and found a significant increase in IFN-γ secretion in CAR-T cells. Meanwhile, the combined use of PD-1 antibodies reduced the proportion of Myeloid-Derived Suppressor Cells (MDSCs) in the TME. Cherkassky et al.[28] reported in 2016 that when the tumor burden was too high, mesothelin CAR-T became exhausted, but reactivation of CAR-T and restoration of its anti-tumor ability were achieved by using PD-1 inhibitors. In addition to directly combining PD-1 antibodies with CAR-T therapy, scholars have developed CAR-T cells that continuously secrete PD-1 antibodies using the non-viral vector piggy Bac transposon system.
By constructing two recombinant plasmids encoding PD-1 and chimeric antigen receptors and then co-transfecting T cells, in vivo CART achieved self-amplification, with only mild adverse reactions such as grade 1 hypertension and fatigue. The combination of PD-1 inhibitors and CAR-T therapy can effectively improve CAR-T exhaustion, enhance T cell cytotoxicity and proliferation, reduce the immunosuppressive microenvironment, and thus enhance the efficacy of CAR-T.
In addition to PD-1, immune checkpoint pathways such as CTLA-4, TIM-3, and LAG-3 also affect the function of CAR-T cells, but research on the combination of these immune checkpoints with CAR-T is still in the experimental stage in vitro and in vivo. Liu et al.[29] reported that CAR-T cells with PD-1 or TIM-3 knockout exhibited stronger anti-tumor effects both in vitro and in vivo. Kenderian et al.[22] similarly confirmed that after stimulating CAR-T with tumor antigens for 1 w, PD-1 and TIM-3 were up-regulated, and inhibiting TIM-3 and PD-1 increased the secretion of cytokines by CAR-T cells. Condomines et al.[30] utilized RNA interference technology to downregulate the expression of CTLA-4 on CD19CAR-T cells, resulting in enhanced proliferation and anti-tumor capabilities in vivo.
Monoclonal antibody drugs:
Blinatumomab is the first CD19/CD3 bispecific antibody that can simultaneously target CD19 on the surface of malignant proliferating B cells and CD3 on the surface of T cells. It is mainly used in the treatment of relapsed/refractory B-ALL. CD20 is a differentiation antigen expressed by most B cells starting from the pre-B cell stage, making it a specific target for B-cell tumors. Rituximab (CD20 monoclonal antibody) is the first targeted monoclonal antibody drug used in cancer treatment, initially applied in the treatment of non-Hodgkin lymphoma and chronic lymphocytic leukaemia. Previous studies have suggested that rituximab can enhance the function of CD19 CAR-T cells and exert synergistic anti-tumor effects. This may be attributed to the effective enhancement of CAR-T cytotoxic activity by rituximab pre-treatment of tumor cells, as well as the effective reduction of CART exhaustion.
TCR-T
Mechanism of action of TCR-T:
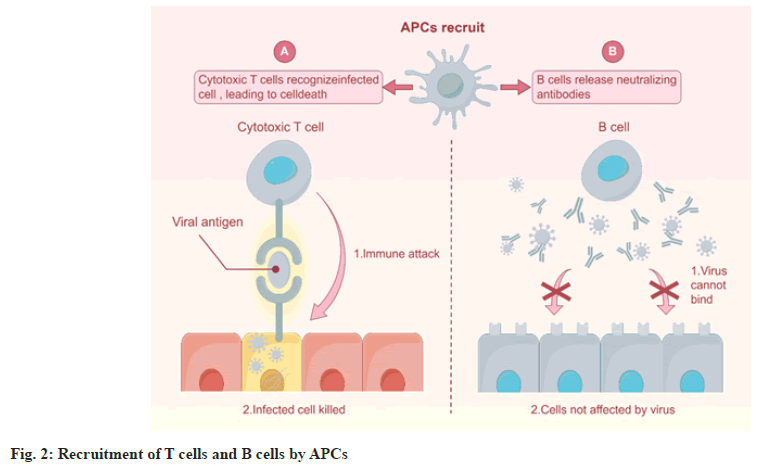
TCR-T therapy is a form of cancer immunotherapy similar to CAR-T cell therapy, but it utilizes TCR instead of CAR [31]. T cells modified through genetic engineering carry TCRs on their surface, enabling them to recognize specific antigens present on the surface of tumor cells, such as Tumor-Associated Antigens (TAAs) or Tumor-Specific Antigens (TSAs) [32]. During the process of recognizing tumor antigens, TCR-T relies on the antigen presentation role of Antigen-Presenting Cells (APCs). Only when the antigen is processed by APCs to form an antigen-MHC complex can it be recognized by TCR-T cells [33]. APCs recruit T cells and B cells to activate specific immune responses, regulating the intensity and nature of immune responses through complex intercellular interactions and cytokine networks (Figure 2). The types of antigens that TCR-T can recognize include all HLA-I and II antigens that can be processed by APCs into MHC complexes, as well as antigens distributed on the cell membrane and within the cytoplasm [34].
During the process of immune recognition, APCs recruit all co-stimulatory molecules associated with TCR, leading to stronger activation signals transmitted to T cells within the body [35]. TCR recognizes MHC-peptide complexes presented on the cell surface after intracellular processing, thus TCR-T therapy can select tumor cell antigens as targets, expanding the range of antigen recognition [36]. Additionally, it can enhance the anti-tumor activity of T cells to achieve therapeutic goals. Treatment with tumor antigen-specific TCR-T cells has shown promising clinical activity in people with metastatic melanoma, as well as certain efficacy in cancers such as cholangiocarcinoma, renal cell carcinoma, colorectal cancer, and multiple myeloma [37]. MAGE-A4 TCR-T cell therapy has demonstrated remission in five solid tumor indications (lung cancer, bladder cancer, gastric esophageal cancer, head and neck cancer, and ovarian cancer) [38]. TCR-T therapy shows unprecedented prospects in the treatment of solid tumors, becoming a highly promising therapeutic modality. Compared to other T cell therapies, the advantages of TCR-T cell immunotherapy mainly include the following aspects. Firstly, TCR-T can recognize TSAs or TAAs; secondly, the probability of severe cytokine release syndrome is lower. Additionally, TCR-T cell immunotherapy possesses immunological memory function and can survive in the body for a longer period. However, TCR-T cells lack homing receptors, resulting in few effector cells infiltrating tumor tissues, leading to limited efficacy in treating solid tumors.
cbased ex vivo engineered CAR-T or TCR-T technologies have the advantage of generating high levels of CAR or TCR encoded on the surface of effector cells, the engineered effector cells produced in this way are personalized cell therapy products that require extraction of T cells from people, and the manufacturing process is expensive and time-consuming. To overcome these challenges, T cells targeted with mRNA encoding CAR or TCR can be administered intravenously in the form of nanoparticles, resulting in the generation of CAR or TCR-engineered T cells in vivo. Studies have injected nanoparticles carrying mRNA encoding CAR or TCR targeting T cells into mice, reprogramming circulating T cells to transiently express CAR or TCR, for the treatment of lymphoma, prostate cancer, and hepatitis B virus-induced hepatocellular carcinoma in mouse models [39]. Immune inhibitory cells such as Tregs and MDSCs in the TME secrete cytokines such as IL-10 and Transforming Growth Factor-Beta (TGF-β), which induce malignant tumor growth, migration, evasion of the immune system, and resistance to T cell killing, making it difficult for TCR-T to completely eliminate tumor cells [40]. The combination of immune checkpoint inhibitors with TCR-T has been shown to have clinical activity [41], but more extensive research results are awaited.
Clinical trials of TCR-T immunotherapy were first reported in 1998, but the number of clinical trials increased significantly after MART-1-specific TCR-T cells were successfully used to treat melanoma in 2006. Although TCR-T therapy based on TCRpeptide/ MHC interaction is the most common and widely used immunotherapy, other TCR-based immunotherapy strategies have also been explored in clinical trials. Fusion proteins composed of a soluble TCR on one end and an immune cell activation domain on the other have been studied in recent years. In 2022, the FDA approved Kimmtrak (tebentafusptebn, IMC gp100) for the treatment of HLA-A*0201- positive unrespectable or metastatic uveal melanoma in adults [42]. This is the first approved bispecific T cell engager therapy for solid tumors and the only approved therapy for unrespectable or metastatic uveal melanoma. Current research targets mainly include TAA targets such as NY-ESO-1 [43], MAGE family proteins [44], and MART-1 [45]. Afamitresgene autoleucel, for the treatment of advanced synovial sarcoma, has also received priority review qualification from the United States (US) FDA in January 2024 and is expected to become the first globally approved TCR-T cell therapy product for solid tumors. In addition, other HLA-A2-restricted gp100-specific TCs are also being investigated as candidate drugs for melanoma TCR-T cell therapy.
Although TCR-T has great potential in the treatment of solid tumors, there is currently a relative lack of literature on combination therapy with drugs. We believe this may be due to several reasons. First, compared to CAR-T cell therapy, clinical research and application of TCR-T cell therapy are relatively new, which may result in fewer studies and clinical practices on combination therapy with drugs. Secondly, the development and production of TCR-T cell therapy are relatively complex, and technical challenges such as finding appropriate tumor antigens, modifying TCRs of T cells, cell expansion, and quality control may slow down the research progress on combination therapy with drugs.
In summary, after modification of T cell surface receptors and intracellular domains, CAR-T and TCR-T can target and recognize tumor antigens, further amplifying immune activation signals and generating persistent immune responses. Current T cell therapies cannot improve the immunosuppressive TME, which may require further validation of the efficacy of T cell therapy for solid tumors. Researchers have begun to modify innate immune cells with CARs, including NKT cells, γδT cells, as well as NK cells, macrophages, etc., but these studies are still in the early stages of clinical development, and more immunomodulatory drugs are urgently needed.
Ethical approval
This study posed a low risk to the study participants and no formal ethical approval was given, but the entire study process followed ethical principles and the data were anonymized.
Author contribution
Kaiji Li was responsible for the overall design and organization of the article, writing, and revising the main content, particularly the sections on T cell therapy and the mechanisms of combined drug therapy. Zhanshuai Wu conducted literature research, wrote the sections on the combined application of immune checkpoint inhibitors and tumor vaccines with T cell therapy, and revised the initial draft. Xian Liu gathered and organized research data on the combination of targeted therapy, chemotherapy, and radiotherapy with T cell therapy, and wrote the corresponding chapters. Jian Ding contributed primarily to the research on the combined application of small molecule drugs and metabolic regulators with T cell therapy, writing the relevant sections and assisting with overall proofreading. Jian Xiao, as the corresponding author, provided guidance and supervision, ensuring the accuracy and completeness of the research direction and content, and performed the final review and revision. All authors participated in data analysis and discussion, ensuring the rigor and scientific integrity of the article.
Funding
This study was supported by Guangxi Natural Science Foundation (2020GXNSFAA297162) and Doctoral Research Initiation Grant, Guangxi University of Traditional Chinese Medicine (2018BS015).
Acknowledgements
This study was supported by Guangxi Natural Science
Conflicts of interest
The authors declare that the study was conducted in the absence of any business or financial relationship that could be interpreted as a potential conflict of interest.
References
- Zeng Z, Wang J, Zhao S, Zhang Y, Fan J, Wu H, et al. A bioinspired flexible sensor for electrochemical probing of dynamic redox disequilibrium in cancer cells. Adv Sci 2023;10(36):2304079.
[Crossref] [Google Scholar] [PubMed]
- Ellsworth RE, Field LA, Love B, Kane JL, Hooke JA, Shriver CD. Differential gene expression in primary breast tumors associated with lymph node metastasis. Int J Breast Cancer 2011;2011(1):142763.
[Crossref] [Google Scholar] [PubMed]
- Stromberg SP, Carlson J. Robustness and fragility in immunosenescence. PLoS Comput Biol 2006;2(11):e160.
[Crossref] [Google Scholar] [PubMed]
- Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, et al. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes 2023;14(3):130.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Qiu Y, Lu W, Jiang Y, Wang J. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med 2018;16(1):147.
[Crossref] [Google Scholar] [PubMed]
- Behnan J, Finocchiaro G, Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain 2019;142(4):847-66.
[Crossref] [Google Scholar] [PubMed]
- Chen ML, Yan BS, Lu WC, Chen MH, Yu SL, Yang PC, et al. Sorafenib relieves cell‐intrinsic and cell‐extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer 2014;134(2):319-31.
[Crossref] [Google Scholar] [PubMed]
- Sconocchia T, Sconocchia G. Regulation of the immune system in health and disease by members of the bone morphogenetic protein family. Front Immunol 2021;12:802346.
[Crossref] [Google Scholar] [PubMed]
- Wolf P, Alzubi J, Gratzke C, Cathomen T. The potential of CAR T cell therapy for prostate cancer. Nat Rev Urol 2021;18(9):556-71.
[Crossref] [Google Scholar] [PubMed]
- Bruxiola G, Cejalvo JM, Gambardella V, Cervantes A. In the literature: April 2019. ESMO Open 2019;4(2):e000513.
[Crossref] [Google Scholar] [PubMed]
- Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses-pre-clinical evidence and ongoing clinical applications. Front Immunol 2015;6:505.
[Crossref] [Google Scholar] [PubMed]
- Rossetti R, Brand H, Lima SC, Furtado IP, Silveira RM, Fantacini DM, et al. Combination of genetically engineered T cells and immune checkpoint blockade for the treatment of cancer. Immunother Adv 2022;2(1):ltac005.
[Crossref] [Google Scholar] [PubMed]
- Shebbo S, Binothman N, Darwaish M, Niaz HA, Abdulal RH, Borjac J, et al. Redefining the battle against colorectal cancer: A comprehensive review of emerging immunotherapies and their clinical efficacy. Front Immunol 2024;15:1350208.
[Crossref] [Google Scholar] [PubMed]
- Beatty GL, O'Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: defining the challenges and next steps. Pharmacol Ther 2016;166:30-9.
[Crossref] [Google Scholar] [PubMed]
- Testa U, Leone G, Pelosi E, Castelli G, Hohaus S. CAR-T cell therapy in large B cell lymphoma. Mediterr J Hematol Infect Dis 2023;15(1):e2023066.
[Crossref] [Google Scholar] [PubMed]
- Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol 2016;13(6):370-83.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Wang M, Chen Y, Liu Y. Current challenges and therapeutic advances of CAR-T cell therapy for solid tumors. Cancer Cell Int 2024;24(1):133.
[Crossref] [Google Scholar] [PubMed]
- He Y, Vlaming M, van Meerten T, Bremer E. The implementation of TNFRSF co-stimulatory domains in CAR-T cells for optimal functional activity. Cancers 2022;14(2):299.
[Crossref] [Google Scholar] [PubMed]
- Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv 2019;3(5):711-7.
[Crossref] [Google Scholar] [PubMed]
- Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med 2019;11(499):eaau5907.
[Crossref] [Google Scholar] [PubMed]
- Watanabe N, Mo F, Zheng R, Ma R, Bray VC, van Leeuwen DG, et al. Feasibility and preclinical efficacy of CD7-unedited CD7 CAR T cells for T cell malignancies. Mol Ther 2023;31(1):24-34.
[Crossref] [Google Scholar] [PubMed]
- Kenderian SS, Ruella M, Shestova O, Kim MY, Klichinsky M, Chen F, et al. Ruxolitinib prevents cytokine release syndrome after CART cell therapy without impairing the anti-tumor effect in a xenograft model. Blood 2016;128(22):652.
- Xu N, Yang XF, Xue SL, Tan JW, Li MH, Ye J, et al. Ruxolitinib reduces severe CRS response by suspending CAR-T cell function instead of damaging CAR-T cells. Biochem Biophys Res Commun 2022;595:54-61.
[Crossref] [Google Scholar] [PubMed]
- Leclercq G, Haegel H, Toso A, Zimmermann T, Green L, Steinhoff N, et al. JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy. J Immunother Cancer 2022;10(1):e003766.
[Crossref] [Google Scholar] [PubMed]
- Huye LE, Nakazawa Y, Patel MP, Yvon E, Sun J, Savoldo B, et al. Combining mTor inhibitors with rapamycin-resistant T cells: A two-pronged approach to tumor elimination. Mol Ther 2011;19(12):2239-48.
[Crossref] [Google Scholar] [PubMed]
- Nian Z, Zheng X, Dou Y, Du X, Zhou L, Fu B, et al. Rapamycin pretreatment rescues the bone marrow AML cell elimination capacity of CAR-T cells. Clin Cancer Res 2021;27(21):6026-38.
[Crossref] [Google Scholar] [PubMed]
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013;19(20):5636-46.
[Crossref] [Google Scholar] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126(8):3130-44.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res 2017;27(1):154-7.
[Crossref] [Google Scholar] [PubMed]
- Condomines M, Arnason J, Benjamin R, Gunset G, Plotkin J, Sadelain M. Tumor-targeted human T cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition. PloS One 2015;10(6):e0130518.
[Crossref] [Google Scholar] [PubMed]
- Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev 2020;86:102019.
[Crossref] [Google Scholar] [PubMed]
- Humeau J, Le Naour J, Galluzzi L, Kroemer G, Pol JG. Trial watch: Intratumoral immunotherapy. Oncoimmunology 2021;10(1):1984677.
[Crossref] [Google Scholar] [PubMed]
- Hradska K, Hajek R, Jelinek T. Toxicity of immune-checkpoint inhibitors in hematological malignancies. Front Pharmacol 2021;12:733890.
[Crossref] [Google Scholar] [PubMed]
- Kapadia CH, Tian S, Perry JL, Luft JC, DeSimone JM. Role of linker length and antigen density in nanoparticle peptide vaccine. ACS Omega 2019;4(3):5547-55.
[Crossref] [Google Scholar] [PubMed]
- Avital G, Kuperwaser F, Pountain AW, Lacey KA, Zwack EE, Podkowik M, et al. The tempo and mode of gene regulatory programs during bacterial infection. Cell Rep 2022;41(2):1-10.
[Crossref] [Google Scholar] [PubMed]
- McDaid WJ, Lissin N, Pollheimer E, Greene M, Leach A, Smyth P, et al. Enhanced target-specific delivery of docetaxel-loaded nanoparticles using engineered T cell receptors. Nanoscale 2021;13(35):15010-20.
[Crossref] [Google Scholar] [PubMed]
- Li J, Xiao Z, Wang D, Jia L, Nie S, Zeng X, et al. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol Cancer 2023;22(1):141.
[Crossref] [Google Scholar] [PubMed]
- Zhou M, Jawed G, Ganjoo KN. Epstein Barr virus-positive lymphoproliferative disorder following lymphodepletion for MAGE A4 adoptive cellular therapy in a patient with synovial sarcoma: A case report. Case Rep Oncol 2023;16(1):886-92.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Shi Q, Huang X, Koo S, Kong N, Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer 2023;23(8):526-43.
[Crossref] [Google Scholar] [PubMed]
- Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 2021;28(1):5-17.
[Crossref] [Google Scholar] [PubMed]
- Martinez M, Moon EK. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol 2019;10:128.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Xiao J, Fa L, Jiang F, Jiang H, Zhou L, et al. Identification of co-expressed gene networks promoting CD8+ T cell infiltration and having prognostic value in uveal melanoma. BMC Ophthalmol 2023;23(1):354-7.
[Crossref] [Google Scholar] [PubMed]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29(7):917-24.
[Crossref] [Google Scholar] [PubMed]
- Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122(6):863-71.
[Crossref] [Google Scholar] [PubMed]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114(3):535-46.
[Crossref] [Google Scholar] [PubMed]