- *Corresponding Author:
- Duygu Alpaslan
Department of Chemical Engineering,
Institute of Natural and Applied Science,
Van Yüzüncü Y?l University,
Van 65080,
Turkey
E-mail: alpaslanduygu@gmail.com
| Date of Received | 01 December 2020 |
| Date of Revision | 23 September 2021 |
| Date of Acceptance | 24 March 2022 |
| Indian J Pharm Sci 2022;84(2):358-368 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Organo-hydrogels were synthesized by using free radical polymerization in the emulsion technique, using agar, glycerol, sesame oil, ammonium persulfate as initiator and N,N'-methylene bisacrylamide or glutaraldehyde as crosslinker. Swelling behaviors, blood compatibility, antioxidant and properties of the organo-hydrogels were investigated thoroughly. The highest swelling value was seen in organo-hydrogel synthesized with the N,N'-methylene bisacrylamide crosslinker containing 0.1 ml of sesame oil. Moreover, drug release behaviors of organo-hydrogels were studied as paracetamol and carboplatin used as model drugs. Release studies were shown that some basic parameters such as medium pH and composition of the polymer structure affect organo-hydrogels drug release behavior. As a result of drug release experiments, it was observed that the release values of organo-hydrogels changed depending on sesame oil and crosslinker content. The highest paracetamol release capacities for the p (AG-m-SO)2 and p (AG-g-SO)2 organohydrogels were calculated as 45.3 % and 79.8 %. When investigated carboplatin releases, the highest releases also were founded to be 100 % for p (AG-m-SO)2 and 85 % for p (AG-g-SO)2.
Keywords
Organo-hydrogel, sesame oil, paracetamol, carboplatin, release system
The rapid development of technology in our age has led to the emergence of many innovations and new production models in various drug industries. One of the base reflections of these developments in the pharmaceutical industry is in the field of controlled drug delivery systems. These systems, which are the product of researches to solve some problems caused by classical drug forms and to resolve their deficiencies, have a history of approximately 20 y.
Synthesis or discovery of drugs with new biological effects for many years, It has been the focus of drug delivery research. Although this research area continues to be important, attention has been increasingly directed towards the way these drugs are administered. For a long time, it has been possible to develop release systems that can release the drug to certain areas of the body or control the long-term drug release rate[1-5]. Some of these systems are liposomes, nano-associations, nanoparticles, active substance-polymer conjugates and polymers. In recent years, interest in organo-hydrogels has been increasing in these areas. The organogelhydrogels may consist of low molecular weight materials or polymeric materials. Polymeric organogel-hydrogels are classified as physical and chemical organogelhydrogels. Chemical polymeric organogel-hydrogels are three-dimensional macromolecular structures that absorb water and organic solvents. Recently, organogelhydrogels have been investigated in a wide range of fields including chemistry, biotechnology, drug delivery and pharmaceuticals.
In Sesame Oil (SO), it contains 7 %-12 % palmitic acid (C16:0), 0.3 %-6 % stearic acid (C18:0), 35 %-50 % oleic acid (C18:1), 35 %-50 % linoleic acid (C18:2), 0.3 %-0.8 % linolenic acid (C18:3), 2.6 % arachidonic acid (C20:4), respectively. In addition, SO is extremely resistant to oxidation due to secondary substances such as sesamine (0.5 %-1.5 %) and sesamolin (0.3 %-0.5 %). Especially sesamin is very effective in lowering cholesterol levels in blood. One of the properties of SO is its tocopherol content. The total amount of tocopherol in SO ranges between 294-528 mg/kg. Tocopherols, the most powerful natural antioxidants that can be dissolved in fat, increase the nutritional value of the oil as vitamin E and fatty acids such as sesamine and sesamolin was increased the antioxidant value[6,7]. Because of these properties, sesame essential oils are used in the field of polymer and biomedical[8-11].
Paracetamol (acetaminophen) is a drug active substance that is used as an alternative drug in aspirin-sensitive patients and has an antipyretic effect. Although its analgesic effect has been mild compared to the new generation analgesics, it's almost no side effects in the gastrointestinal tract, its reliability and its use in pregnant women ensure that paracetamol is always at the forefront and a classic analgesic[12]. So far, although effective treatment or vaccine has not been produced for Coronavirus Disease-19 (COVID-19), has been reported in the literature used fever reducers, such as paracetamol, in primary care for fever in patients infected with the COVID-19 virus[13-15].
Carboplatin (cis-diammine-[1,1- cyclobutanedicarboxylato]platinum(II)) is a 2nd generation platinum compound. It is designed to stop the growth of cancer cells and kill them. It is effective on carboplatin Deoxyribonucleic Acid (DNA). The adenine and guanine are linked to the DNA from the N-7 position by a covalent ligament. As a result of the formation of DNA addition products, DNA synthesis and transcription are inhibited and the cell cannot divide. Binding to DNA and cytoplasmic proteins can result in cytotoxic effects. Broad-spectrum carboplatin is a frequently used chemotherapeutic agent for the treatment of childhood cancers. It is used in the treatment of osteosarcoma, hepatoblastoma, neuroblastoma, germ cell tumors, central nervous system tumors, head and neck cancers[16,17].
The synthesized gel, hydrogels and organohydrogels were investigated as an antioxidant, blood compatibility and drug release system. In this study, gels containing agar-based SO were synthesized using two different crosslinkers such as N,N'-methylene bisacrylamide (MBA) and Glutaraldehyde (GA) reagent. Characterization of synthesized gels; structural bonds are determined by Fourier Transform Infrared Spectroscopy (FTIR). The swelling properties of ethanol, acetone, ethanol/water, acetone/water, water and tap water were investigated and the usability of the polymers as an effective sorbent was investigated. Organo-hydrogels were investigated for antioxidant, blood clotting, hemolysis properties and use in paracetamol and carboplatin drug delivery systems.
Materials and Methods
Reagents:
Glycerol (Gly), agar (99 %), MBA (99 %), GA (25 % v/v), ethanol, acetone, Calcium Chloride (CaCl2), Sodium Hydroxide (NaOH) and Hydrochloric Acid (HCl) (36.5 %-38 % v/v) were purchased from Sigma; Ammonium Persulfate (APS) (98 %) were purchased from Merck. In terms of analytical grade, all reagents were of the highest cleanliness available and they were used without additional purification. SO, gasoline, paracetamol and carboplatin were procured from local suppliers. Deionized (DI) water (18.2 MΩ cm; Human I) was also employed from the beginning to the end of this study. And the experimental procedures were as follows.
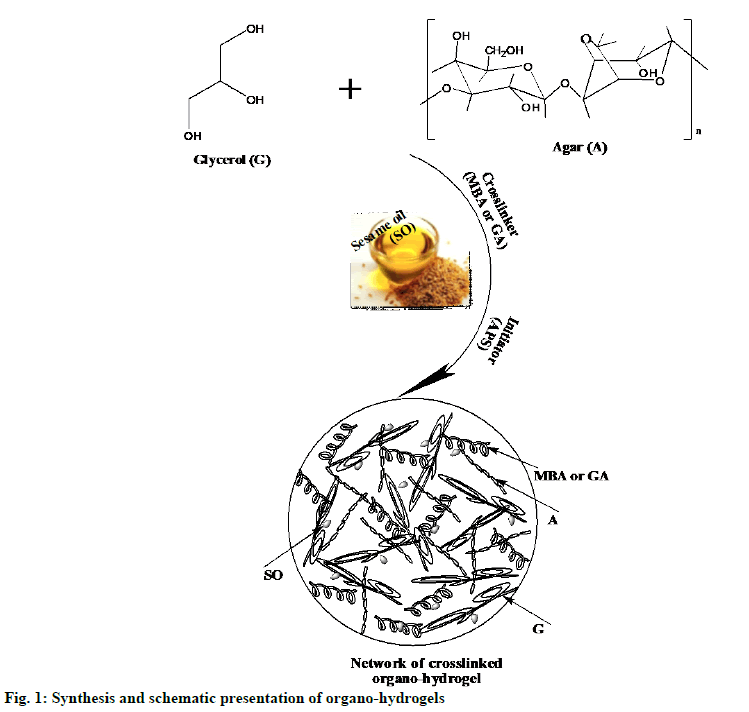
Agar-Glycerol (AG) based gels, hydrogels and organo-hydrogels synthesis:
Gel and hydrogels synthesized: AG based gel and hydrogels were synthesized via free radical polymerization in emulsion according to the preparation method given in Table 1[6]. Gel and hydrogels were synthesized as described by Alpaslan et al.[18]. Gel and hydrogel compositions were given in Table 2.
| Gel | Code |
|---|---|
| Agar-glycerol | AG |
| Hydrogel | |
| poly (Agar-co-glycerol)/MBA | p (AG-m) |
| poly (Agar-co-glycerol)/GA | p (AG-g) |
| Organo-Hydrogel | |
| poly (Agar-co-glycerol-co-sesame oil)/MBA-1 | p (AG-m-SO)1 |
| poly (Agar-co-glycerol-co-sesame oil)/MBA-2 | p (AG-m-SO)2 |
| poly (Agar-co-glycerol-co-sesame oil)/MBA-3 | p (AG-m-SO)3 |
| poly (Agar-co-glycerol-co-sesame oil)/GA-1 | p (AG-g-SO)1 |
| poly (Agar-co-glycerol-co-sesame oil)/GA-2 | p (AG-g-SO)2 |
| poly (Agar-co-glycerol-co-sesame oil)/GA-3 | p(AG-g-SO)3 |
Note: AG: Agar-Glycerol; MBA: N,N'-Methylene Bisacrylamide; GA: Glutaraldehyde and SO: Sesame Oil
Table 1: Codes of Different Organo-Hydrogel
Organo-hydrogels synthesized: Free radical polymerization in emulsion media method was used to synthesis the AG-based organo-hydrogels given in Table 1. Briefly, first 2 ml of agar solution and 0.04 ml of glycerol were added to the 20 ml flask and made homogeneous by vigorous mixing (at 2500 rpm). Secondly, of different amounts (0.1, 0.2 and 0.3 ml) SO was added in the reactions mixture. The organohydrogel mixture was stirred at 800 rpm for 15 min until the formation of a clear homogeneous solution emulsion. Thirdly, MBA (0.1 %) or GA reagent was added as a crosslinker and further homogenized. Finally, the polymerization reaction was initiated by the addition of the initiator solution APS in 100 μl DI water. Reaction temperatures were maintained at 25° with a temperature-controlled hot plate. Then, the solution was poured into a pipette with 6 mm diameter and was allowed to polymerize. These preparation steps were schematically given in fig. 1. Organo-hydrogel compositions were given in Table 2. The gel, hydrogels and organo-hydrogels were kept in DI water, which was renewed every 2 h for 8 h to eliminate unreactive monomers. Finally, the synthesized gel, hydrogels, p (AG-m-SO) and p (AG-g-SO) organo-hydrogels were dried in the oven at 40º until a constant weight was achieved and stored at 4º for further uses.
| Agar | Glycerol | SO | Crosslinker | Code |
|---|---|---|---|---|
| 2 %-2 ml | 0.04 ml | -- | -- | AG |
| 2 %-2 ml | 0.04 ml | --- | MBA | p (AG-m) |
| 2 %-2 ml | 0.04 ml | --- | GA | p (AG-g) |
| 2 %-2 ml | 0.04 ml | 0.1 ml | MBA | p (AG-m-SO)1 |
| 2 %-2 ml | 0.04 ml | 0.2 ml | MBA | p (AG-m-SO)2 |
| 2 %-2 ml | 0.04 ml | 0.3 ml | MBA | p (AG-m-SO)3 |
| 2%-2 ml | 0.04 ml | 0.1 ml | GA | p (AG-g-SO)1 |
| 2 %-2 ml | 0.04 ml | 0.2 ml | GA | p (AG-g-SO)2 |
| 2 %-2 ml | 0.04 ml | 0.3 ml | GA | p (AG-g-SO)3 |
Note: AG: Agar-Glycerol; MBA: N,N'-Methylene Bisacrylamide; GA: Glutaraldehyde and SO: Sesame Oil
Table 2: Compositions and Codes of Different Organo-Hydrogel
Organo-hydrogel synthesis containing paracetamol and carboplatin:
The synthesis of drug-loaded organo-hydrogels was synthesized as described by Alpaslan et al.[18,19]. In addition to the reaction mixture mentioned above, 50 ppm 1ml paracetamol/carboplatin drug was added. Thus, drug-loaded organo-hydrogels were synthesized.
Characterization of organo-hydrogels:
Swelling analysis were performed with certain amounts of dried gel, hydrogels, organo-hydrogels placed in ethanol, water, ethanol/ID water (1:1), acetone, acetone/ ID water (1:1), gasoline and different 2-12 pH for a day. Swelling tests were performed at 25º[20-22].
The FT-IR spectra of organo-hydrogel was obtained from a Thermo Scientific Nicolet iS10 instrument using Attenuated Total Reflectance (ATR) apparatus with 4 cm-1 resolution between 4000-650 cm-1.
Blood clotting and hemolysis analysis:
To evaluate the blood clotting[23] and hemolysis analysis[24] methods which were explained in the literature were applied.
Antioxidant analysis:
To evaluate the antioxidant activity, Folin-Ciocalteu (FC) assay[25,26] and 2,2′-Azinobis-(3-Ethylbenzthiazolin- 6-Sulfonic Acid (ABTS)[21,26-28] methods which were explained in the literature were applied.
Paracetamol and carboplatin release studies:
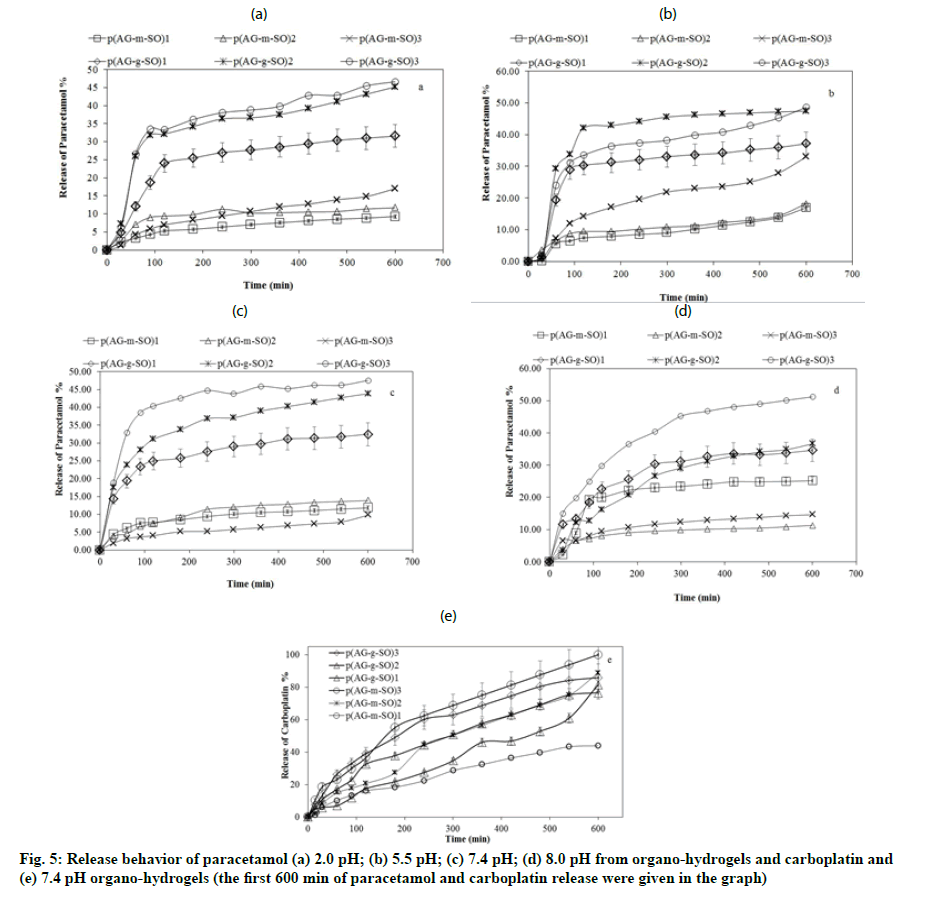
Gel, hydrogels and organo-hydrogels loaded with a certain amount (50 ppm) of paracetamol, were used in 50 ml at four different pH values (2.0, 5.5 7.4 and 8.0 pH) for paracetamol (244 nm) release. Carboplatin (210 nm) release[29] was performed in 50 ml 7.4 pH solution media. Each measurement was performed with 3 replicates and averaged with standard deviation values. The most common models, which are Zero order Model (ZoM) [30,31], First order Model (FoM) [32], Higuci Model (HM))[33] and Korsmeyer-Peppas Model (KPM) (the power law), were used to identify the release kinetics. These equations are given in Table 3.
| Model | Mathematical equation | Release mechanism | Codes |
|---|---|---|---|
| Zero order kinetic model | Cr=C0-k0.t | Diffusion mechanism | ZoM |
| First order kinetic model | lnCr=lnC0-k1.t | Fick’s first law, diffusion Mechanism | FoM |
| Higuchi model | Cr/C∞=kH.√t | Diffusion medium based mechanism in Fick’s first law | HM |
| Korsemeyer-Peppas model | lnCr/C∞=lnkKP+n.lnt | Semi empirical model, diffusion-based mechanism | KPM |
Note: Cr is concentration of urea release in time t (mg/l); C0 is the initial concentration of urea in the solution (most times, C0=0) (mg/l); k0 is the zero order release constantexpressed in units of concentration/time (mg/(l min)); t is time (min); k1 is the first order release constant (1/min); C∞ is concentration of fertilizer release in equilibrium (mg/l); kH is Higuchi release rateconstant (1/√min); kKP is Korsmeyer-Peppas release rate constant and n is release exponent which is indicative of the transport mechanism (Mt/M∞<0.6 should only be used
Table 3: Mathematical Models For Drug Release
Results and Discussion
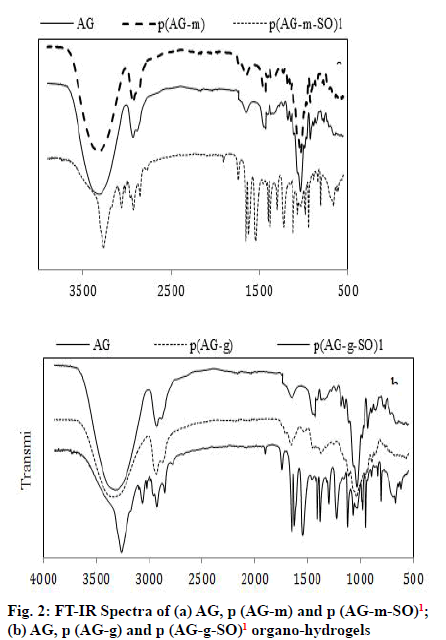
FTIR analysis utilizes the fundamental physics principles infra-red to obtain a molecular fingerprint of synthesized gel, hydrogel and organo-hydrogel and to display for characterization of the functional groups. Organo-hydrogels, AG, p (AG-m), p (AGg) were prepared by free radical polymerization in emulsion media and the FTIR spectra were shown in fig. 2. SO contained the band peak at 3401 cm-1 belonging to the vibrations of the -OH groups, bands at 3007 cm-1- 2896 cm-1 and 2777 cm-1 represented to -CH and -CH2 vibrations. The peak in 1637 cm-1- 1504 cm-1 and 1471 cm-1 belonged to the phenyl and -CH2 groups and peak in 13 973 cm-1 belonged to the methyl bands. The new bonds and structural diversity at organo-hydrogels were demonstrated the existence of hydrogen-bond interaction. After the SO got into the structure of the organo-hydrogel, the incoming bands from characteristic aromatic compounds (such as 3254 cm-1, 3061 cm-1 and 2922 cm-1)[7] exhibited high density and the peaks appear to be deepened or expanded. Considering the peaks in the organo-hydrogel, the peak at 1743 cm-1, 1651 cm-1 and 951 cm-1 deepened, and the peak depth in the 1037 cm-1 decreased. The change in these peaks indicated that SO entered the structure of the organo-hydrogel.
Since organo-hydrogels have the feature of swelling in different solvent environments, it is used as a water-retaining agent and artificial soil in agricultural applications, as well as personal care and hygienic products, in pharmacology controlled drug release applications.
The swelling ability of a polymeric gel determines by the interaction between the functional groups in its structure and the solvent. Push and pull between polymer chains are affected by non-covalent electrostatic, hydrophobic, Van der Waals and hydrogen bonding, hydrophobic interactions are such as physical crosslinker interactions and this affects the swelling behavior of the gel[34].
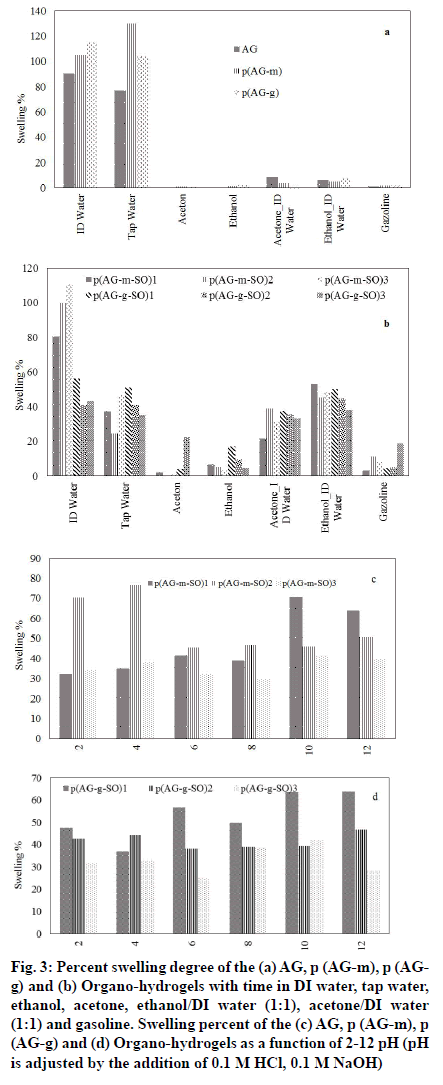
The change in percent swelling of organo-hydrogels as a function of solvent concentration in water and organic solvent mixtures was shown in fig. 3. After the AG gel was crosslinked, the DI water absorption capacity increased in ratio 16 % for p (AG-m) and 27 % for p (AG-g), and the tap water absorption capacity increased in ratio 35 % for p (AG-g) and 68 % for p (AG-m)[18]. When fig. 2. was examined, it has been determined that organo-hydrogels have absorption values close to each other with ethanol/DI water and acetone/water. When organo-hydrogels were compared among themselves, it was showed that p (AG-m-SO)3 organo-hydrogel has higher water absorption capacity than other organohydrogels via 110.8 % swelling value. Same time, it is seen that the organo-hydrogel swells up to 50 % in ethanol/DI water and acetone/DI water media. It was generally observed that the organo-hydrogels' S % value decreased with the increase of essential oil in the organo-hydrogel composition. Those observations were consistent with the literature[35,36].
Fig. 3:Percent swelling degree of the (a) AG, p (AG-m), p (AGg) and (b) Organo-hydrogels with time in DI water, tap water, ethanol, acetone, ethanol/DI water (1:1), acetone/DI water (1:1) and gasoline. Swelling percent of the (c) AG, p (AG-m), p (AG-g) and (d) Organo-hydrogels as a function of 2-12 pH (pH is adjusted by the addition of 0.1 M HCl, 0.1 M NaOH)
A small amount of swelling was observed in acetone and ethanol media. This was thought to depend upon the hydrophobic character in acetone and ethanol, and the number of alkyl groups in molecular structure. The hydrophobic property can be enhanced by increasing the alkyl group of the organic molecule[37]. Therefore, more hydrophobic groups in solvents reduce swelling of organo-hydrogels in water mixtures of ethanol, acetone and gasoline compositions.
If we evaluate organo-hydrogels in terms of crosslinkers; GA crosslinked organo-hydrogels and MBA crosslinked organo-hydrogels not seen a significant difference between the swelling values in solvent media. When the swelling values in the solvents are evaluated according to the amount of essential oil contained in organo-hydrogels; it was observed that the swelling values changed as the amount of essential oil increased. The swelling of organo-hydrogels in different organic solvent-water mixtures can be controlled by the solvent composition.
The change in swelling properties in different types of media is due to the different ion mobility in the medium[38,39]. It is not sensitive to pH since it does not contain an ionizable group in AG gel structure. It was observed that AG gel becomes sensitive to changes in different pH values after being synthesized with MBA and GA crosslinkers. When the SO was added to the structure of the organo-hydrogels in different proportions, the number of ionizable groups in the structure of the organo-hydrogels increased and becomes sensitive to changes in different pH values. However, the anionic and cationic properties of the ionizable groups of organo-hydrogels increased and showed different swelling behaviors at different pH values.
Antioxidant can neutralize free radicals (harmful molecules), preventing them from harming a living cell. Antioxidant analysis is therefore of great importance in order to perform the drug release studies of newly synthesized organo-hydrogels. Unlike other vegetable oils, SO is highly resistant to oxidation due to its high content of oleic and linoleic acids, as well as secondary substances such as sesamine and sesamolin. One of the properties of SO is its tocopherol content. Tocopherols, which are the most powerful natural antioxidants that can be dissolved in fat, increase both the nutritional value of the oil as vitamin E and the antioxidant value such as sesamine and sesamolin[6,10]. Antioxidants analyses were performed to determine the antioxidant properties of organo-hydrogel. The antioxidant activity of SO, p (AG-m), p (AG-g) and organo-hydrogels is given in Table 4 as the gallic acid equivalent value. The SO, p (AG-m), p (AG-g) and organo-hydrogels reduction capacity can determine it was antioxidant activity. When Table 4 is analyzed, as the concentration of the substance increases, the reduction power also increases due to the absorbents. When these values are considered, organo-hydrogels show higher antioxidant activity than other gels.
| Substance | Total phenol values (mg) |
|---|---|
| Organo-hydrogel | |
| p (AG-m-SO)1 | 438 |
| p (AG-m-SO)2 | 438 |
| p (AG-m-SO)3 | 375 |
| p (AG-g-SO)1 | 203 |
| p (AG-g-SO)2 | 217 |
| p (AG-g-SO)3 | 212 |
| Oil | |
| Sesame Oil | 565 |
Note: AG: Agar-Glycerol and SO: Sesame Oil
Table 4: Total Phenol Content Values
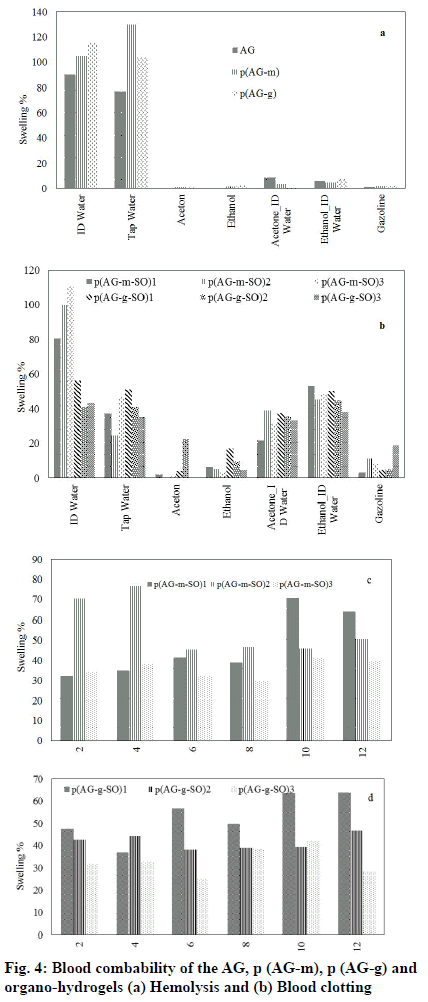
Organo-hydrogels must have a biocompatibility feature in order to be used in the release studies in the human body. In the long-term use of biomaterials in the biomedical field, as a result of the contact of blood with the biomaterial, many biological reactions occur on the surface of the biomaterial first. The purpose of the hem compatibility; is to determine whether a toxic effect in the blood will occur as a result of a biomaterial coming into contact with blood. Therefore, blood compatibility properties of organo-hydrogels were determined by hemolysis and blood clotting tests hydrogel[40,41]. Organo-hydrogels hemolysis analysis results were summarized in fig. 4. The hemolysis values AG, AG-m, AG-g, SO and organo-hydrogels were calculated at 5 mg/ml gel a concentration of 0.9 % for p (AG-m-SO)1, 0.6 % for p (AG-m-SO)2, 0.4 % for p (AG-m-SO)3, 0.9 % for p (AG-m-SO)1, 0.4 % for p (AG-m-SO)2, 0.5 % for p (AG-m-SO)3, respectively. It is stated that the hemolysis rate is not hemolytic up to 5 % hydrogel. Therefore, it can be said that organo-hydrogels were hemolysis at this rate. Another method of evaluating organo-hydrogel blood compatibility is to determine the organo-hydrogel Blood Clotting Index (BCI). The organo-hydrogel BCI values were shown in fig. 4b. In the fig, blood clotting indexes of organo-hydrogels were founded to be 5.9 % for p (AG-m-SO)1, 6.8 % for p (AG-m-SO)2, 4.8 % for p (AG-m-SO)3, 6.3 % for p (AG-m-SO)1, 4.5 % for p (AG-m-SO)2, 6.4 % for p (AG-m-SO)3. For these organo-hydrogels blood contact applications, the amount should be less than 5 mg/ml.
The main target of the studies in the field of medicine is to minimize the drug dose, to extend the dosing interval, to increase the quality of life by ensuring that the patient is not affected by the side and harmful effects.
The systems that best respond to these expectations are “controlled release systems”. Recently, the use of organo-hydrogels in controlled release systems has been the focus. To perform a release of paracetamol and carboplatin, organo-hydrogels were imitated gastric, intestinal, oral, blood and skin media in vitro. For this, release analysis was done in different pH environments. All organo-hydrogels may not show the same behavior in every pH environment[42].
In this study, synthesized biocompatible gel, hydrogel and organo-hydrogels were utilized for their paracetamol and carboplatin release capacities. The amount of paracetamol and carboplatin loaded on gel, hydrogel and organo-hydrogels were determined as 50 ppm. Fig. 5 shows the paracetamol release behavior of organo-hydrogels at 37.5º. AG, p (AG-m) and p (AGg) maximum paracetamol release were 8 % at pH 8.0, 7.8 % at pH 2.0 and 8.2 % at pH 2.0, respectively. Moreover, AG, p (AG-m) and p (AG-g) maximum carboplatin release were 1.8 %, 1.6 % and 2.6 % at pH 7.4, respectively. Due to the difference in concentration in the release event, while the solvent enters the organohydrogel structure, the loaded drug switches to the solvent medium. When fig. 5a- fig. 5d was examined, it was observed while all organo-hydrogels release paracetamol slowly, to release carboplatin rapidly. The amount of drugs released from the organo-hydrogel system was given in Table 5. As seen in the table, the highest paracetamol release is observed in p (AG-g-SO)2 and p (AG-m-SO)3 organo-hydrogels, while the highest carboplatin release was found in p (AG-g-SO)2 and p (AG-m-SO)2 organo-hydrogels. When paracetamol release was compared between p (AG-m-SO) organohydrogels, it appeared that the minimum and maximum percent release was to be 23.9 % at pH 2.0 at the p (AG-m-SO)1 and 45.3 % at pH 5.5 at the p (AG-m- SO)2 organo-hydrogel. Compared between paracetamol release p (AG-g-SO) organo-hydrogels, the minimum percentage release was to be 42.6 % p (AG-g-SO)1 at pH 2.0, while maximum percentage release was to be 79.8 % p (AG-g-SO)1 organo-hydrogel was found to be at pH 2.0. The highest cumulative paracetamol release from organo-hydrogels was observed in organohydrogels synthesized by GA crosslinker. It was been seen from the results that paracetamol release can be controlled by changing the amount of SO in organohydrogels. When investigated carboplatin releases, the highest carboplatin releases were observed to be 100 % for p (AG-m-SO)2 and 85 % for p (AG-g-SO)2. Moreover some of the other reported material at literature was as p (AG-g-PmO) organo-hydrogels (72.3 % at pH 7.4) and p (AG-m-PmO) organo-hydrogels (69.8 % at pH 2.0)[18], carboxylated lignin (70 % paracetamol), lignin tablet (70 % paracetamol) and non-lignin tablet (70 % paracetamol)[43], and p (AG-g-PmO) organo-hydrogels (99.7 % at pH 7.4) and p (AG-m-PmO) organohydrogels (100 % at pH 7.4)[18], p (AG-g-GO)3 organohydrogels at (95.4 %, pH 7.4 carboplatin)[44], pure drug (100 % carboplatin), carboplatin-loaded Polyethylene Glycol-Modified Multiwall Carbon Nanotube (PEGylated MWCNT) (95 % carboplatin) and entericcoated PEGylated MWCNTs (95 % carboplatin)[16] so on.
| Paracetamol | Carboplatin | ||||
|---|---|---|---|---|---|
| pH 2.0 | pH 5 .5 | pH 7.4 | pH 8.0 | pH 7.4 | |
| Release % | Release % | ||||
| AG | 2.3 | 3.5 | 3.1 | 8.1 | 1.8 |
| p (AG-m) | 7.8 | 4.4 | 3.4 | 3.9 | 1.6 |
| p (AG-g) | 8.2 | 6.3 | 5.2 | 6.8 | 2.6 |
| p (AG-m-SO)1 | 23.9 | 29.1 | 31.6 | 41 | 71.4 |
| p (AG-m-SO)2 | 34.3 | 33.8 | 33.3 | 27 | 100 |
| p (AG-m-SO)3 | 36.6 | 45.3 | 42.7 | 34.8 | 95.1 |
| p (AG-g-SO)1 | 42.6 | 51.4 | 52.5 | 57.4 | 84.4 |
| p (AG-g-SO)2 | 79.8 | 71.9 | 69.5 | 61.2 | 85.2 |
| p (AG-g-SO)3 | 77.6 | 73.6 | 77.5 | 73.6 | 68.8 |
Note: AG: Agar-Glycerol and SO: Sesame Oil
Table 5: Paracetamol and Carboplatin Percentage Release Values
The analysis results were applied to the mathematical models shown in Table 6 to find the most suitable release model of organo-hydrogels. Paracetamol and carboplatin release data of organo-hydrogels were processed into these kinetic models to investigate release kinetics and systems. Given the best Correlation Coefficients (R2) values, the most suitable model was chosen to symbolize paracetamol release behavior. The highest R2 for all organo-hydrogels were seen in HM and KPM and it was determined that the paracetamol release occurred in accordance with these models. The n value expressed in the KPM gives information about whether the polymeric materials have the behavior that complies with Fick's law. As seen in Table 7, the diffusion exponentials of organo-hydrogels are generally <0.45 and were found to behave in accordance with Fick's law. The carboplatin release of organo-hydrogels revealed a very high R2 with the ZoM, HM and KPM. As seen in Table 7, diffusion exponents of carboplatin release of organo-hydrogels, it has been found that it generally behaves according to non-Fick law. According to the Fick law, it was shown that the release was controlled by swelling and according to the non-Fick law, it was controlled by both swelling and relaxation.
| p (AG-m-SO)1 | 2 | 5.5 | 7.4 | 8 | p (AG-g-SO)1 | 2 | 5.5 | 7.4 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZoM | Co | 1.37 | 1.657 | 1.564 | 1.442 | ZoM | Co | 2.591 | 3.428 | 3.366 | 2.818 |
| ko | -0.002 | -0.004 | -0.004 | -0.003 | ko | -0.008 | -0.009 | -0.007 | -0.009 | ||
| R2 | 0.778 | 0.792 | 0.844 | 0.852 | R2 | 0.676 | 0.557 | 0.668 | 0.766 | ||
| FoM | Co | 1.045 | 1.726 | 5.222 | 3.819 | FoM | Co | 6.596 | 6.702 | 16.735 | 162.033 |
| k1 | -0.001 | -0.001 | -0.003 | -0.003 | k1 | 0 | -0.001 | -0.006 | -0.006 | ||
| R2 | 0.044 | 0.546 | 0.826 | 0.901 | R2 | 0.783 | 0.718 | 0.907 | 0.745 | ||
| HM | kh | 0.033 | 0.02 | 0.031 | 0.031 | HM | kh | 0.046 | 0.864 | 0.037 | 0.038 |
| R2 | 0.995 | 0.901 | 0.927 | 0.927 | R2 | 0.988 | 0.204 | 0.99 | 0.992 | ||
| KPM | n | 0.452 | 0.457 | 0.521 | 0.462 | KPM | n | 0.252 | 0.168 | 0.29 | 0.254 |
| kkp | 0.036 | 30.009 | 44.8 | 35.677 | kkp | 0.176 | 3.104 | 8.223 | 6.697 | ||
| R2 | 0.978 | 0.89 | 0.966 | 0.928 | R2 | 0.857 | 0.983 | 0.987 | 0.985 | ||
| P (AG-m-SO)2 | 2 | 5.5 | 7.4 | 8 | p(AG-g-SO)2 | 2 | 5.5 | 7.4 | 8 | ||
| ZoM | Co | 1.499 | 1.129 | 1.195 | 1.557 | ZoM | Co | 2.757 | 3.273 | 2.904 | 1.228 |
| ko | -0.003 | -0.006 | -0.005 | -0.004 | ko | -0.007 | -0.008 | -0.007 | -0.008 | ||
| R2 | 0.564 | 0.878 | 0.8 | 0.683 | R2 | 0.652 | 0.518 | 0.698 | 2.818 | ||
| FoM | Co | 2.666 | 1.996 | 3.754 | 8.892 | FoM | Co | 5.398 | 6.875 | 53.165 | 182.181 |
| k1 | -0.001 | -0.001 | -0.004 | -0.002 | k1 | -0.001 | 0 | -0.004 | -0.003 | ||
| R2 | 0.593 | 0.827 | 0.983 | 0.902 | R2 | 0.783 | 0.459 | 0.904 | 0.753 | ||
| HM | kh | 0.039 | 0.019 | 0.032 | 0.035 | HM | kh | 0.036 | 0.792 | 0.038 | 0.038 |
| R2 | 0.996 | 0.728 | 0.968 | 0.926 | R2 | 0.997 | 0.004 | 0.992 | 0.992 | ||
| KPM | n | 0.094 | 0.202 | 0.361 | 0.174 | KPM | n | 0.277 | 0.164 | 0.165 | 0.165 |
| kkp | 0.438 | 7.043 | 16.224 | 3.902 | kkp | 0.114 | 2.937 | 3.499 | 3.499 | ||
| R2 | 0.738 | 0.802 | 0.939 | 0.875 | R2 | 0.77 | 0.884 | 0.941 | 0.941 | ||
| p (AG-m-SO)3 | 2 | 5.5 | 7.4 | 8 | p (AG-g-SO)3 | 2 | 5.5 | 7.4 | 8 | ||
| ZoM | Co | 0.491 | 1.056 | 0.355 | 1.121 | ZoM | Co | 3.329 | 2.922 | 3.32 | 2.548 |
| ko | -0.005 | -0.008 | -0.002 | -0.003 | ko | -0.005 | -0.004 | -0.005 | -0.005 | ||
| R2 | 0.951 | 0.905 | 0.938 | 0.775 | R2 | 0.373 | 0.414 | 0.519 | 0.591 | ||
| FoM | Co | 1.144 | 1.595 | 1.693 | 4.046 | FoM | R2 | 5.288 | 4.431 | 138.449 | 41.372 |
| k1 | -0.002 | -0.003 | -0.002 | -0.003 | k1 | 0 | 0 | -0.002 | -0.002 | ||
| R2 | 0.871 | 0.959 | 0.959 | 0.096 | R2 | 0.571 | 0.555 | 0.89 | 0.843 | ||
| HM | kh | 0.028 | 0.028 | 0.032 | 0.032 | HM | kh | 0.029 | 0.862 | 0.036 | 0.036 |
| R2 | 0.97 | 0.891 | 0.978 | 0.978 | R2 | 0.982 | 0.007 | 0.994 | 0.994 | ||
| KPM | n | 0.55 | 0.64 | 0.415 | 0.415 | KPM | n | 0.168 | 0.069 | 0.111 | 0.111 |
| kkp | 0.016 | 1 | 18.554 | 11.517 | kkp | 0.259 | 1.708 | 2.559 | 2.559 | ||
| R2 | 0.993 | 0.858 | 0.989 | 0.979 | R2 | 0.937 | 0.739 | 0.92 | 0.92 | ||
Note: Fickian diffusion mechanism n≤0.45, non-Fickian (anomalous) diffusion mechanism 0.45<n<0.89; AG: Agar-Glycerol; SO: Sesame Oil; ZoM: Zero order kinetic model; FoM: First order kinetic model; HM: Higuchi Model and KPM: Korsemeyer-Peppas Model
Table 6: Release Kinetic and Mechanism of Paracetamol Release
| p (AG-m-SO)1 | 7.4 | p (AG-g-SO)1 | 7.4 | ||
|---|---|---|---|---|---|
| ZoM | Co | 2.023 | ZoM | Co | 0.948 |
| ko | -0.031 | ko | -0.039 | ||
| R2 | 0.981 | R2 | 0.989 | ||
| FoM | Co | 2.102 | FoM | Co | 2.619 |
| k1 | -0.006 | k1 | -0.005 | ||
| R2 | 0.677 | R2 | 0.9 | ||
| HM | kh | 0.032 | HM | kh | 0.046 |
| R2 | 0.995 | R2 | 0.993 | ||
| KPM | n | 0.643 | KPM | n | 1.052 |
| kkp | 0.012 | kkp | 0.001 | ||
| R2 | 0.99 | R2 | 0.932 | ||
| p (AG-m-SO)2 | 7.4 | p (AG-g-SO)2 | 7.4 | ||
| ZoM | Co | 1.31 | ZoM | Co | 3.486 |
| ko | -0.043 | ko | -0.033 | ||
| R2 | 0.983 | R2 | 0.978 | ||
| FoM | Co | 3.646 | FoM | Co | 4.467 |
| k1 | -0.004 | k1 | -0.003 | ||
| R2 | 0.895 | R2 | 0.85 | ||
| HM | kh | 0.037 | HM | kh | 0.034 |
| R2 | 0.992 | R2 | 0.997 | ||
| KPM | n | 0.85 | KPM | n | 0.644 |
| kkp | 0.004 | kkp | 0.016 | ||
| R2 | 0.982 | R2 | 0.989 | ||
| p (AG-m-SO)3 | 7.4 | p (AG-g-SO)3 | 7.4 | ||
| ZoM | Co | 2.006 | ZoM | Co | 2.25 |
| ko | -0.028 | ko | -0.024 | ||
| R2 | 0.949 | R2 | 0.926 | ||
| FoM | Co | 3.102 | FoM | Co | 4.652 |
| k1 | -0.004 | k1 | -0.002 | ||
| R2 | 0.816 | R2 | 0.897 | ||
| HM | kh | 0.026 | HM | kh | 0.027 |
| R2 | 0.98 | R2 | 0.967 | ||
| KPM | n | 0.646 | KPM | n | 0.534 |
| kkp | 0.017 | kkp | 0.03 | ||
| R2 | 0.992 | R2 | 0.995 | ||
Note: Fickian diffusion mechanism n≤0.45, non-Fickian (anomalous) diffusion mechanism 0.45<n<0.89; AG: Agar-Glycerol; SO: Sesame Oil; ZoM: Zero order kinetic model; FoM: First order kinetic model; HM: Higuchi Model and KPM: Korsemeyer-Peppas Model
Table 7: Release Kinetic and Mechanism of Carboplatin Release
The use of polymeric materials is more preferred in the preparation and development of controlled drug delivery systems. There are a few key factors to consider when developing these systems. The material to be applied which exhibits different swelling behavior in different solvent and different pH environments; has biocompatible with the human body; is to ensure the controlled release of the drug substance into the target area. In the context of this study, we show that the organo-hydrogels we synthesized can be used as an alternative drug delivery system, thanks to the features mentioned above. Organo-hydrogels containing agar, glycerol and SO cross-linked with MBA and GA reagents were synthesized by the free-radical emulsion polymerization method in the presence of the APS.
Synthesized organo-hydrogels were characterized by swelling, FTIR, antioxidant and blood compatible analyses. According to the data obtained, the maximum balance swelling value was reached as 110.8 % in the p (AG-g-SO)3 organo-hydrogel water environment. Same time, it is seen that the organo-hydrogel swells up to 50 % in ethanol/DI water and acetone/DI water media. It can be said that organo-hydrogels were blood compatible and antioxidant properties. When paracetamol and carboplatin release were compared between organo-hydrogels, it appeared that the maximum percent release was to be 79.8 % at pH 2.0 at the p (AG-g-SO)2 and 100 % at pH 7.4 at the p (AG-m- SO)2 organo-hydrogel. Organo-hydrogels loaded with paracetamol showed very high correlations with KPM and HM release kinetics, while carboplatin loaded organo-hydrogels showed very high correlations with ZoM, KPM and HM release kinetics. Organo-hydrogels containing SO can potentially be used in biomedical, pharmaceutical and drug delivery systems due to their stated properties.
Confl?ct of ?nterest:
The authors declared no conflict of interest.
References
- Osada Y, Ping Gong J, Tanaka Y. Polymer gels. J Macromol Sci Polymer Rev 2004;44(1):87-112.
- Pénzes T, Csóka I, Er?s I. Rheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogels. Rheol Acta 2004;43(5):457-63.
- Yang Y, Wang S, Xu H, Sun C, Li X, Zheng J. Properties of topically applied organogels: Rheology and in vitro drug release. Asian J Pharm Sci 2008;3(4):175e183.
- Vintiloiu A, Leroux JC. Organogels and their use in drug delivery: A review. J Control Release 2008;125(3):179-92.
[Crossref] [Google Scholar] [PubMed]
- Arvidsson A, Alván G, Angelin B, Borgå O, Nord CE. Ceftriaxone: Renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother 1982;10(3):207-15.
[Crossref] [Google Scholar] [PubMed]
- Baydar HA, Marquard R, Turgut I. Pure line selection for improved yield, oil content and different fatty acid composition of sesame, Sesamum indicum. Plant Breeding 1999;118(5):462-4.
- Mirghani ME, Man YC, Jinap S, Baharin BS, Bakar J. Application of FTIR spectroscopy in determining sesamol in sesame seed oil. J Am Oil Chem Soc 2003;80(1):1-4.
- Singh VK, Pramanik K, Ray SS, Pal K. Development and characterization of sorbitan monostearate and sesame oil-based organogels for topical delivery of antimicrobials. AAPS Pharm Sci Tech 2015;16(2):293-305.
[Crossref] [Google Scholar] [PubMed]
- Wright AJ, Marangoni AG. Formation, structure and rheological properties of ricinelaidic acid?vegetable oil organogels. J Am Oil Chem Soc 2006;83(6):497-503.
- Singh VK, Banerjee I, Agarwal T, Pramanik K, Bhattacharya MK, Pal K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf B Biointerfaces 2014;123:582-92.
[Crossref] [Google Scholar] [PubMed]
- Sagiri SS, Singh VK, Kulanthaivel S, Banerjee I, Basak P, Battachrya MK, et al. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J Mech Behav Biomed Mater 2015;43:1-7.
[Crossref] [Google Scholar] [PubMed]
- Graham GG, Scott KF. Mechanisms of action of paracetamol and related analgesics. Inflammopharmacology 2003;11(4):401-13.
[Crossref] [Google Scholar] [PubMed]
- Cheng SC, Chang YC, Chiang YL, Chien YC, Cheng M, Yang CH, et al. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc 2020;119(3):747-51.
[Crossref] [Google Scholar] [PubMed]
- Calvo C, López-Hortelano MG, de Carlos Vicente JC, Martínez JL, de trabajo de la Asociación G. Recommendations on the clinical management of the COVID-19 infection by the «new coronavirus» SARS-CoV2. Spanish Paediatric Association working group. An Pediatr 2020;92(4):241-e1.
[Crossref] [Google Scholar] [PubMed]
- Giang HT, Shah J, Hung TH, Reda A, Truong LN, Huy NT. The first Vietnamese case of COVID-19 acquired from China. Lancet Infect Dis 2020;20(4):408-9.
[Crossref] [Google Scholar] [PubMed]
- Sharma S, Naskar S, Kuotsu K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization and in vitro toxicity in human breast cancer. Carbon Lett 2020;30(4):435-47.
- Aj W, Ward A, Benfield P. Carboplatin: A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs 1989;37:162-90.
[Crossref] [Google Scholar] [PubMed]
- Alpaslan D, Dudu TE, Akta? N. Synthesis and characterization of novel organo-hydrogel based agar, glycerol and peppermint oil as a natural drug carrier/release material. Mater Sci Eng 2021;118:111534.
- Ersen Dudu T, Alpaslan D, Aktas N. Application of poly (Agar-co-glycerol-co-sweet almond oil) based organo-hydrogels as a drug delivery material. J Polym Environ 2022;30(2):483-93.
[Crossref] [Google Scholar] [PubMed]
- Alpaslan D, Dudu TE, ?ahiner N, Aktas N. Synthesis and preparation of responsive poly (Dimethyl acrylamide/gelatin and pomegranate extract) as a novel food packaging material. Mater Sci Eng C Mater Biol Appl 2020;108:110339.
[Crossref] [Google Scholar] [PubMed]
- Alpaslan D. Use of colorimetric hydrogel as an indicator for food packaging applications. Bull Mater Sci 2019;42(5):247-58.
- Olak T, Turan A, Alpaslan D, Dudu TE, Akta? N. Developing poly (Agar-co-glycerol-co-thyme oil) based organo-hydrogels for the controlled drug release applications. J Drug Deliv Sci Technol 2020;60:102088.
- Alpaslan D, Er?en Dudu T, Aktas N. Development of onion oil-based organo-hydrogel for drug delivery material. J Dispers Sci Technol 2021:1-3.
- Alpaslan D, Olak T, Turan A, Ersen Dudu T, Aktas N. use of coconut oil-based organo-hydrogels in pharmaceutical applications. J Polym Environ 2022;30(2):666-80.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticulture 1965;16(3):144-58.
- Alpaslan D, Dudu TE, Akta? N. Synthesis, characterization and modification of novel food packaging material from dimethyl acrylamide/gelatin and purple cabbage extract. MANAS J Eng 2018;6(2):110-28.
- Childs RE, Bardsley WG. The steady-state kinetics of peroxidase with 2, 2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J 1975;145(1):93-103.
[Crossref] [Google Scholar] [PubMed]
- Alpaslan D, Ersen Dudu T, Aktas N. Evaluation of poly (agar-co-glycerol-co-castor oil) organo-hydrogel as a controlled release system carrier support material. Polymer Bull 2021:1-22.
- di Pasqua AJ, Goodisman J, Kerwood DJ, Toms BB, Dubowy RL, Dabrowiak JC. Activation of carboplatin by carbonate. Chem Res Toxicol 2006;19(1):139-49.
[Crossref] [Google Scholar] [PubMed]
- Korsmeyer RW, Peppas NA. Solute and penetrant diffusion in swellable polymers. III. Drug release from glassy poly (HEMA-co-NVP) copolymers. J Control Release 1984;1(2):89-98.
- Varelas CG, Dixon DG, Steiner CA. Zero-order release from biphasic polymer hydrogels. J Control Release 1995;34(3):185-92.
- Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13(2):123-33.
[Crossref] [Google Scholar] [PubMed]
- Higuchi T. Mechanism of sustained?action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52(12):1145-9.
[Crossref] [Google Scholar] [PubMed]
- Peniche C, Cohen ME, Vázquez B, San Román J. Water sorption of flexible networks based on 2-hydroxyethyl methacrylate-triethylenglycol dimethacrylate copolymers. Polymer 1997;38(24):5977-82.
- Roy SG, De P. Swelling properties of amino acid containing cross-linked polymeric organogels and their respective polyelectrolytic hydrogels with pH and salt responsive property. Polymer 2014;55(21):5425-34.
- Zohuriaan?Mehr MJ, Kabiri K, Kheirabadi M. Extraordinary swelling behavior of poly (AMPS) organogel in solvent/DMSO binary mixed media. J Appl Polym Sci 2010;117(2):1127-36.
- Üzüm ÖB, Karada? E. Equilibrium swelling studies of chemically cross-linked highly swollen acrylamide-sodium acrylate hydrogels in various water-solvent mixtures. Polym Plast Technol Eng 2010;49(6):609-16.
- Yin ZC, Wang YL, Wang K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J Drug Deliv Sci Technol 2018;43:12-8.
- Orakdogen N, Okay O. Reentrant conformation transition in poly (N,N-dimethylacrylamide) hydrogels in water–organic solvent mixtures. Polymer 2006;47(2):561-8.
- Liu Y, Cai D, Yang J, Wang Y, Zhang X, Yin S. In vitro hemocompatibility evaluation of poly (4-hydroxybutyrate) scaffold. Int J Clin Exp Med 2014;7(5):1233.
[Google Scholar] [PubMed]
- Bélanger MC, Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low?density polyethylene and polydimethylsiloxane: A review. J Biomed Mater Res 2001;58(5):467-77.
[Crossref] [Google Scholar] [PubMed]
- Patel VR, Amiji MM. Preparation and characterization of freeze-dried chitosan-poly (ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res 1996;13(4):588-93.
[Crossref] [Google Scholar] [PubMed]
- Pishnamazi M, Hafizi H, Shirazian S, Culebras M, Walker GM, Collins MN. Design of controlled release system for paracetamol based on modified lignin. Polymers 2019;11(6):1059.
[Crossref] [Google Scholar] [PubMed]
- Alpaslan D, Olak T, Turan A, Ersen Dudu T, Aktas N. A garlic oil-based organo-hydrogel for use in pH-sensitive drug release. Chem Pap 2021;75(11):5759-72.