- *Corresponding Author:
- Zengliang Liang
Department of Surgery, The Affiliated Haimen People’s Hospital of Nantong University, Nantong, Jiangsu 226200, China
E-mail: xiaoguan2b@163.com
| Date of Received | 19 October 2022 |
| Date of Revision | 04 September 2023 |
| Date of Acceptance | 27 January 2024 |
| Indian J Pharm Sci 2024;86(1):132-138 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Previous literature has validated the clinical effectiveness of ailanthone in repressing the development of different human cancers. Therefore, this project aimed to investigate the role and possible mechanism of ailanthone in liver cancer. Huh-7 cells were treated with various concentrations of ailanthone (low-dose ailanthone, medium-dose ailanthone, high-dose ailanthone), or transfected with microRNA-negative control and microRNA-489-3p mimics. Furthermore, transfected Huh-7 cells were exposed with high-dose ailanthone. Cell counting kit-8, plate clone formation, scratch, and Transwell detected cell proliferation, clone formation, migration, and invasion. Besides, microRNA-489-3p, matrix metalloproteinase-2, and matrix metalloproteinase-9 contents were assessed using quantitative reverse transcription polymerase chain reaction or Western blot. After exposure to various doses of ailanthone, cell proliferation inhibition rate and microRNA-489-3p expression were increased in a dose-dependent manner, whereas cell clone formation, migration, invasive, and matrix metalloproteinase-2, matrix metalloproteinase-9 protein levels were decreased. Beyond that, microRNA-489- 3p expression was reduced in liver cancer tissues; its upregulation might block Huh-7 cell growth. MicroRNA489-3p downregulation might abrogate high-dose ailanthone-mediated cell malignant behaviors inhibition. Ailanthone treatment could hinder Huh-7 cell malignant behaviors through regulating microRNA-489-3p.
Keywords
Liver cancer, ailanthone, microRNA-489-3p, cell proliferation, migration, invasion
The most frequent fatal malignancy, liver cancer has been recognized as a global health challenge, with an estimated 1.4 million cases diagnosed in 2040[1]. Substantial progress in treatment options, containing surgical resection, adjuvant or neoadjuvant chemotherapy, have recently acquired some benefits, but liver cancer is prone to metastasis and recurrence even after surgical resection[2,3]. Hence, it is particularly important to seek a novel diagnostic and therapeutic method for early diagnosis of this cancer and to improve the efficiency of treatment. Important role of Traditional Chinese Medicine (TCM) in mitigating the progression of liver cancer has been attracting attention[4,5]. Ailanthone (AIL) represents an important active compounds from famous TCM Ailanthus altissima[6]. Numerous studies have suggested that AIL possesses many biological activities, containing anti-inflammatory, antimicrobial, and anti-tumor activities[7]. It has been reported that AIL might exert significant natural anti-cancer activity through modulating different miRNAs and pathways in several human cancers[8,9]. In fact, AIL treatment has been verified to constrain Huh-7 cell growth[10]. However, research on its mechanism of action is still in need of supplementation and improving in liver cancer. At present, extensive laboratory work reveals that microRNAs (miRNAs) might modulate target gene expression at posttranscriptional levels, thereby controlling tumor progression[11]. Some research indicated miR-489-3p might function as a tumor suppressor in several human cancers[12,13]. Apart from that, miR-489 has been verified to repress human liver cancer cell proliferation[14]. Accordingly, we focused on whether AIL might affect Huh-7 cell malignant behavior progression via miR-489-3p.
Materials and Methods
Reagents:
After signing written informed consent from all participants, 37 liver cancer tissues and matched paracancerous tissue were collected from February 2020 to July 2020. Meanwhile, all patients were diagnosed as liver cancer through pathological diagnosis, and these samples were immediately placed -80° for preservation. Among them, 20 male and 17 female aged between 50 y-68 y (mean=55.35±3.26). Besides, this project was approved via the Ethics Committee of our hospital.
AIL (purity >98 %) was provided by SHHEBIO (Shanghai, China). Human liver cancer cell line Huh-7 was acquired from ZQXZBIO (Shanghai, China). Meanwhile, Invitrogen (Carlsbad, California, USA) offered Trizol reagent, reverse transcription and fluorescent quantitative Polymerase Chain Reaction (PCR) reagents, and Lipofectamine™ 2000. RiboBio (Guangzhou, China) provided miR-Negative Control (NC), miR-489-3p mimics, anti-miR-NC, and anti-miR- 489-3p. Cell Counting Kit-8 (CCK-8) reagent and Matrigel matrix gel were respectively purchase from Beyotime Biology (Shanghai, China) and Solarbio (Beijing, China). Corning Costar (Corning, United States of America (USA)) provided Transwell. Rabbit anti-human Matrix Metalloproteinase (MMP)-2, MMP-9 antibody, and secondary antibodies were provided by CST (Danvers, Massachusetts, USA).
Method:
Cell treatment and transfection: Based on previously described[15], 1×105 Huh-7 cells in Dulbecco's Modified Eagle Medium (DMEM) were exposed with AIL at different concentrations (0.4 μmol/l, 0.8 μmol/l and 1.6 μmol/l) for 24 h, marked respectively as AIL-L, AIL-M, and AIL-H. In parallel, control group was normal cultured Huh-7 cells. For cell transfection, according to Lipofectamine method, we overexpressed miR- 489-3p via transfecting
miR-NC or miR-489-3p mimics into Huh-7 cells. Transfected Huh-7 cells were culturing with medium containing 1.6 μmol/l AIL for 24 h, generating AIL+anti-miR-NC group or AIL+antimiR- 489-3p group.
CCK-8 assay: In short, cell proliferation inhibition rate was assessed in this experiment. 3×103 Huh-7 cells in 96-well plates were subjected to CCK-8 solution incubation for 2 h. Results at 450 nm were monitored based on a microplate reader.
Clone formation: After being cultured for 14 d, 500 Huh-7 cells were subjected to Phosphate Buffered Saline (PBS) washing, 500 μl paraformaldehyde fixtures for 20 min at -20°, and 400 μl crystal violet staining for 15 min at 37°. At last, a microscope was utilized to count the number of cell clone formation (≥50 cells were deemed as 1 clone).
Scratch healing assay: 2×105 Huh-7 cells were full-grown; a line was drawn on cell monolayer with the tip of a 200 μl pipette. Subsequently, these samples were observed under a microscope, which was regarded as 0 h at this point. They were placed in the incubator and continued to be cultured for 24 h. ImageJ software was applied to detect the migration distance of the cells in each group and calculate the cell scratch healing rate.
Transwell assay: Collected Huh-7 cells were inoculated in upper chamber (1×105 cells/well) which was pre-spread with Matrigel matrix gel dilution, and lower counterpart was added with 600 μl medium containing 10 % Fetal Bovine Serum (FBS), cultured in an incubator for 48 h. After PBS washing, infiltrating cells were fixed by adding 500 μl of 4 % paraformaldehyde, stained, and counted.
qRT-PCR assay: After 1 ml of Trizol reagent addition, total Ribonucleic Acid (RNA) from liver cancer tissues, paracarcinoma tissues, and Huh-7 cells were prepared, which were reverse transcribed to complementary DNA (cDNA). Then, qRT-PCR reaction was implemented, and data was detected via applying ABI Step OnePlus real-time fluorescence quantitative PCR instrument.
Western blot assay: After 500 μl Radioimmunoprecipitation Assay (RIPA) lysate addition, extract cellular proteins were prepared. Add 40 μg of protein per well channel for Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), transfer the membrane, 5 % skimmed milk closed for 2 h, add primary antibodies; MMP-2 (1:1000), MMP- 9 (1:1000), and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:2000) dilution, incubate at 4° for 24 h, add the secondary antibody dilution (1:3000), incubate at 37° for 2 h, apply the Quantity one software to quantify the protein bands.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 analyzed results, which was expression as (x̄ ±s). Data comparison was implemented based on Student’s t-test or one-way Analysis of Variance (ANOVA) with Tukey’s tests. p<0.05 regarded statistically significant.
Results and Discussion
According to the results displayed in Table 1, cell proliferation inhibition rates were apparently increased with the increase of AIL dose, and colony formation number was reduced.
| Group | Inhibition rate (%) | Colony number |
|---|---|---|
| Control | 0.00±0.00 | 108.71±10.03 |
| AIL-L | 23.21±2.22* | 83.72±7.17* |
| AIL-M | 39.28±3.82*# | 67.93±5.48*# |
| AIL-H | 59.35±5.07*#& | 50.64±4.85*#& |
| F | 502.227 | 106.569 |
| p | 0.000 | 0.000 |
Note: *p<0.05, #p<0.05 and &p<0.05, vs. control, AIL-L and AIL-M
Table 1: Effect of AIL on HUH-7 Cell Proliferation (x̄±s, n=9)
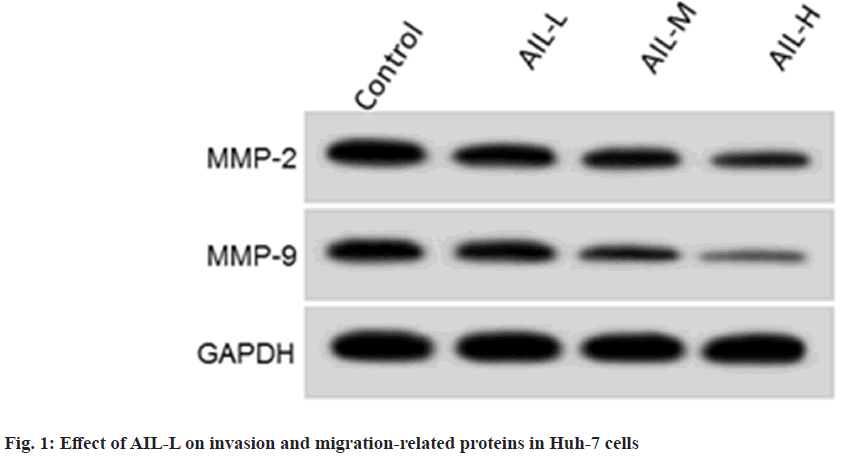
Results from fig. 1 and Table 2 showed that scratch healing rate (p<0.05), invasion number (p<0.05), MMP2 and MMP9 (p<0.05) were gradually reduced by AIL exposure. As shown in Table 3, miR-489-3p content was clearly declined in liver cancer tissues. According to data shown in Table 4, miR-489-3p content was greatly improved in Huh-7 cells with increasing doses of AIL.
| Group | Invasion number | Scratch healing rate (%) | MMP-2 | MMP-9 |
|---|---|---|---|---|
| Control | 124.16±10.56 | 72.89±5.71 | 0.77±0.05 | 0.57±0.05 |
| AIL-L | 98.52±6.78* | 56.74±5.43* | 0.65±0.05* | 0.45±0.04* |
| AIL-M | 79.17±6.53*# | 40.61±3.18*# | 0.52±0.04*# | 0.31±0.03*# |
| AIL-H | 57.75±4.29*#& | 28.11±2.62*#& | 0.34±0.03*#& | 0.19±0.02*#& |
| F | 131.616 | 172.42 | 162.88 | 182.222 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, #p<0.05 and &p<0.05, vs. control, AIL-L and AIL-M
Table 2: Effect of AIL-L on HUH-7 Cell Metastasis (x̄±s, n=9)
| Group | miR-489-3p |
|---|---|
| Paracancerous tissue | 1.00±0.11 |
| Liver cancer tissues | 0.31±0.03* |
| t | 36.811 |
| p | 0.000 |
Note: *p<0.05, in comparison with paracancerous tissue
Table 3: miR-489-3p Content in Liver Cancer (x̄±s, n=37)
| Group | miR-489-3p |
|---|---|
| Control | 1.00±0.00 |
| AIL-L | 1.79±0.14* |
| AIL-M | 2.66±0.21*# |
| AIL-H | 3.76±0.24*#& |
| F | 516.614 |
| p | 0.000 |
Note: *p<0.05, #p<0.05 and &p<0.05, vs. control, AIL-L and AIL-M
Table 4: AIL Improved miR-489-3p Expression (x̄±s, n=9)
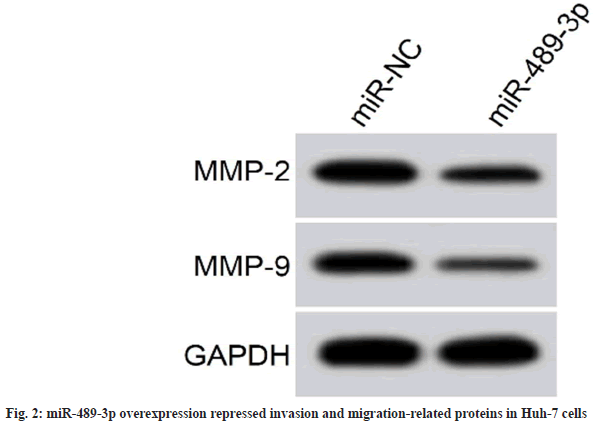
Cell proliferation inhibition rates were augmented in the miR-489-3p group, whereas cell colony formation and invasion number was reduced, and scratch healing rate, and MMP2 and MMP9 protein expression were decreased (fig. 2 and Table 5).
| Group | miR-489-3p | Inhibition rate (%) | Colony number | Invasion number | Scratch healing rate (%) | MMP-2 | MMP-9 |
|---|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.00 | 5.58±0.51 | 105.66±10.15 | 126.01±11.37 | 74.44±6.59 | 0.78±0.06 | 0.59±0.04 |
| miR-489-3p | 3.49±0.28* | 49.06±4.06* | 59.01±4.97* | 63.11±5.78* | 34.37±3.36* | 0.39±0.04* | 0.26±0.02* |
| t | 26.679 | 31.879 | 12.038 | 14.794 | 16.251 | 16.225 | 22.137 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, vs. miR-NC group
Table 5: miR-489-3p Overexpression Repressed on HUH-7 Cell Growth (x̄±s, n=9)
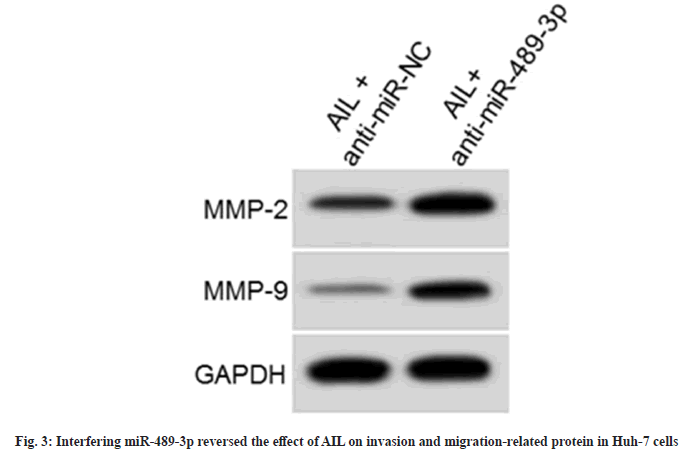
As displayed in fig. 3 and Table 6, proliferation inhibition rates were hindered (p<0.05), colony formation and invasion number was enhanced (p<0.05), and scratch healing rate and MMP2 and MMP9 content were reinforced (p<0.05) in the AIL+anti-miR-489-3p.
| Group | miR-489-3p | Inhibition rate (%) | Colony number | Invasion number | Scratch healing rate (%) | MMP-2 | MMP-9 |
|---|---|---|---|---|---|---|---|
| AIL+anti-miR-NC | 1.00±0.00 | 58.01±4.31 | 51.65±4.63 | 55.26±5.17 | 25.58±2.68 | 0.32±0.03 | 0.18±0.02 |
| AIL+anti-miR-489-3p | 0.41±0.04* | 22.07±2.25* | 88.61±7.51* | 108.74±11.68* | 60.32±5.19* | 0.68±0.05* | 0.48±0.04* |
| t | 44.25 | 22.176 | 12.568 | 12.561 | 17.843 | 18.522 | 20.125 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to AIL+anti-miR-NC group
Table 6: miR-489-3p Inhibitor Might Abolish AIL-Induced Proliferation, Invasion, and Migration Repression in HUH-7 Cells (x̄±s, n=9)
Many investigations have revealed that our TCM can exert anti-liver cancer effects via regulating several target genes[16,17]. Convincing evidence have presented that miRNAs are abnormally expressed in liver cancer and can regulate tumor development by inhibiting target genes, and may also serve as a potential target for liver cancer therapy[18,19] . Yet, whether miRNAs can be used as potential targets of TCM for liver cancer treatment has not been elucidated.
Currently, there is a large number of publications describing the multiple pharmacological properties of AIL, especially the significant anti-tumor effects against a wide range of cancer cells. For example, AIL might promote melanoma cell apoptosis via the PI3K/Akt pathway[20]. Of interest, it has been confirmed that AIL might block liver cancer cell growth[10]. Consistent with these findings, Huh- 7 cell proliferation increased and clone number decreased by AIL treatment, suggesting that AIL might inhibit liver cancer cell proliferation ability. Previous article uncovered that MMP-2 and MMP- 9 belong to the group of MMP, their augment might accelerate cell metastasis via degrading the extracellular matrix deposition[21]. Herein, scratch healing rate, invasive number, and MMP-2 and MMP-9 content were reduced after AIL treatment, implying that AIL dwindled liver cancer cell metastasis.
Some studies exhibited miR-489-3p dysregulation is closely linked to different tumor development. For instance, miR-489-3p up-regulation inhibited tumor cell growth and metastasis[22]. Apart from that, overexpressed miR-489-3p might impede bladder cancer cell proliferation and migration[23]. Furthermore, miR-489-3p has been identified to inhibit lung cell proliferation[24]. Here, miR-489- 3p content was clearly increased in AIL-triggered Huh-7 cells, suggesting this miRNA participated AIL-mediated liver cancer processes. Functionally, miR-489-3p overexpression might relieve Huh- 7 cell development in vitro. Moreover, its lack might partially overturn AIL-induced cell growth and metastasis repression. The above results discovered that AIL might repress liver cancer cell malignant behavior via increasing miR-489-3p.
Taken together, AIL attenuate liver cancer cell growth and metastasis by promoting miR-489- 3p. These findings indicated that miR-489-3p may serve as a potential target for liver cancer treatment by AIL. Yet, whether AIL can exert antiliver cancer effects via modulating other genes needs to be further explored.
Author’s contributions:
Ruiyu Guan and Yiyang Yao have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77(6):1598-606.
[Crossref] [Google Scholar] [PubMed]
- Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. Biosci Trends 2022;16(3):178-88.
[Crossref] [Google Scholar] [PubMed]
- Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer 2020;1873(1):188314.
[Crossref] [Google Scholar] [PubMed]
- Wu Q, Chen Z, Ding Y, Tang Y, Cheng Y. Protective effect of traditional Chinese medicine on non-alcoholic fatty liver disease and liver cancer by targeting ferroptosis. Front Nutr 2022;9:1033129.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen M, et al. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol 2013;149(3):601-12.
[Crossref] [Google Scholar] [PubMed]
- Ding H, Yu X, Hang C, Gao K, Lao X, Jia Y, et al. Ailanthone: A novel potential drug for treating human cancer. Oncol Lett 2020;20(2):1489-503.
[Crossref] [Google Scholar] [PubMed]
- Kundu P, Laskar S. A brief resume on the genus Ailanthus: Chemical and pharmacological aspects. Phytochem Rev 2010;9:379-412.
- Ding H, Yu X, Yan Z. Ailanthone suppresses the activity of human colorectal cancer cells through the STAT3 signaling pathway. Int J Mol Med 2022;49(2):21.
[Crossref] [Google Scholar] [PubMed]
- Bailly C. Anticancer properties and mechanism of action of the quassinoid ailanthone. Phytother Res 2020;34(9):2203-13.
[Crossref] [Google Scholar] [PubMed]
- Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng L, et al. Ailanthone inhibits Huh7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Sci Rep 2015;5(1):16185.
[Crossref] [Google Scholar] [PubMed]
- Ali Syeda Z, Langden SS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci 2020;21(5):1723.
- Liu D, Zhou Z, Guo Y, Du Q, Li L. CircCDK1 knockdown reduces CDK1 expression by targeting miR-489-3p to suppress the development of breast cancer and strengthen the sensitivity of Tamoxifen. Anticancer Drugs 2022;33(3):286-99.
[Crossref] [Google Scholar] [PubMed]
- Li F, Cao K, Wang M, Liu Y, Zhang Y. Astragaloside IV exhibits anti-tumor function in gastric cancer via targeting circRNA dihydrolipoamide S-succinyltransferase (circDLST)/miR-489-3p/eukaryotic translation initiation factor 4A1 (EIF4A1) pathway. Bioengineered 2022;13(4):10112-23.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Jin WX, Zhang YL, Huang L, Ni HB, Fang D. Effects of miR-489 targeting on SOX4 gene on proliferation and apoptosis of human hepatocellular carcinoma cells. Afr Health Sci 2020;20(3):1292-8.
[Crossref] [Google Scholar] [PubMed]
- Pang L, Lin L. Altosinone regulates the biological behavior of MDA-MB-231 cells by inhibiting PI3K/AKT/mTOR pathway. J Med Mol Biol 2020;17(2):109-14.
- Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother 2022;146:112542.
[Crossref] [Google Scholar] [PubMed]
- Li K, Xiao K, Zhu S, Wang Y, Wang W. Chinese herbal medicine for primary liver cancer therapy: Perspectives and challenges. Front Pharmacol 2022;13:889799.
[Crossref] [Google Scholar] [PubMed]
- Ye Y, Zhang L, Song Y, Zhuang J, Wang G, Ni J, et al. microRNA-373 exerts anti-tumor functions in human liver cancer by targeting Rab22a. Mol Med Rep 2019;20(4):3874-82.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Ye L, Yu Y. MicroRNA-126-5p suppresses cell proliferation, invasion and migration by targeting EGFR in liver cancer. Clin Res Hepatol Gastroenterol 2020;44(6):865-73.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Chen Y, Qu CJ. Altisone induces apoptosis of human melanoma A375 cells and its mechanism. Nat Prod Res Dev 2019;31(8):1350-6.
- Pang D, Yang C, Li C, Zou Y, Feng B, Li L, et al. Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biol Open 2020;9(2):046854.
[Crossref] [Google Scholar] [PubMed]
- Zheng B, Chen T. miR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma. Open Life Sci 2020;15(1):274-83.
[Crossref] [Google Scholar] [PubMed]
- Sun D, Li T, Xin H, An J, Yang J, Lin J, et al. miR-489-3p inhibits proliferation and migration of bladder cancer cells through downregulation of histone deacetylase 2. Oncol Lett 2020;20(4):8.
[Crossref] [Google Scholar] [PubMed]
- Dao R, Wudu M, Hui L, Jiang J, Xu Y, Ren H, et al. Knockdown of lncRNA MIR503HG suppresses proliferation and promotes apoptosis of non-small cell lung cancer cells by regulating miR-489-3p and miR-625-5p. Pathol Res Pract 2020;216(3):152823.
[Crossref] [Google Scholar] [PubMed]