- *Corresponding Author:
- Hong Liu
Department of Nursing, Clinical College of Hubei College of Chinese Medicine, Jingzhou, Hubei Province 434020, China
E-mail: liuhongcchccm@sdsch.cn
| Date of Received | 10 February 2022 |
| Date of Revision | 24 January 2023 |
| Date of Acceptance | 28 September 2023 |
| Indian J Pharm Sci 2023;85(5):1458-1465 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to investigate the potential alleviation effect of Cang'erzi powder on inflammation in a mice model of allergic rhinitis through modulation of toll-like receptor 4/nuclear factor-kappa B signaling pathway. The allergic rhinitis mouse model was established via ovalbumin induction. The mice were divided into groups; phosphate buffer saline group, ovalbumin group, ovalbumin+low-dose Cang'erzi powder group, ovalbumin+high-dose Cang'erzi powder group, and ovalbumin+dexamethasone group. The number of sneezing and nasal scratching episodes was recorded. Histopathological examination of the nasal mucosa was performed using hematoxylin-eosin staining. Levels of inflammatory cytokines in the serum were assessed via enzyme-linked immunosorbent assay and real-time quantitative polymerase chain reaction. Oxidative stressrelated markers were quantified using specific assay kits. Protein levels of toll-like receptor 4 and nuclear factor-kappa B in the nasal mucosa were determined using Western blot analysis. In the allergic rhinitis mouse model, Cang'erzi powder administration significantly reduced the number of ovalbumin induced sneezing and nasal scratching episodes. Furthermore, Cang'erzi powder treatment effectively attenuated the ovalbumin induced secretion of pro-inflammatory factors, including toll-like receptor 4 and interleukin-5. Notably, Cang'erzi powder also demonstrated antioxidant effects by reducing the levels of reactive oxygen species, malondialdehyde, and myeloperoxidase, while enhancing the activity of the antioxidant enzyme superoxide dismutase. Additionally, Cang'erzi powder treatment led to a reduction in the protein levels of tolllike receptor 4 and nuclear factor-kappa B in the nasal mucosa. These findings indicate that Cang'erzi powder possesses anti-inflammatory properties and can alleviate inflammation in allergic rhinitis mice by modulating the toll-like receptor 4/nuclear factor-kappa B signaling pathway. Thus, Cang'erzi powder may hold promise as a potential therapeutic agent for the management of allergic rhinitis and warrants further investigation for its clinical application in inflammatory disorders.
Keywords
Allergic rhinitis, inflammation, mice, nuclear factor-kappa B, toll-like receptor 4
Allergic Rhinitis (AR) is a common inflammatory disorder of the nasal mucosa, affecting a substantial portion of the global population[1]. It is characterized by the overreaction of the immune system to harmless environmental allergens, leading to symptoms such as nasal congestion, sneezing, itching and rhinorrhea[2]. The prevalence of AR has been steadily rising, significantly impacting the quality of life and productivity of affected individuals[3]. Conventional treatments for AR often involve antihistamines, intranasal corticosteroids, and leukotriene receptor antagonists, which primarily target the symptoms rather than the underlying inflammatory mechanisms[4,5]. Therefore, there is an increasing interest in exploring complementary and alternative approaches, particularly from traditional medicine systems, to address the inflammatory aspects of AR.
Cang'erzi Powder (CEZP), a well-known traditional Chinese herbal formula, has a long history of clinical application for the treatment of nasal disorders, including AR. Composed of a combination of natural herbal ingredients, CEZP is believed to possess anti-inflammatory and immune-modulating properties[6-11]. Nevertheless, the precise molecular mechanisms underlying its potential therapeutic effects in AR remain incompletely understood.
Toll-Like Receptor 4 (TLR4) and Nuclear Factor- Kappa B (NF-κB) have emerged as key players in the inflammatory cascade, regulating the expression of pro-inflammatory cytokines and mediators. The TLR4/NF-κB signaling pathway plays a crucial role in initiating and amplifying the immune response during allergic reactions[12-15]. Therefore, investigating the potential modulation of this pathway by CEZP could provide valuable insights into its anti-inflammatory properties and its relevance as a therapeutic agent for AR.
The aim of this research article is to elucidate the alleviation effect of CEZP on inflammation in mice with AR, with a particular focus on its impact on the TLR4/NF-κB pathway. We hypothesize that CEZP treatment can ameliorate AR-associated inflammation by inhibiting TLR4-mediated NF- κB activation, resulting in the down-regulation of pro-inflammatory cytokines and mediators.
Materials and Methods
Laboratory animals:
A total of 40 female C57BL/6 mice of (6-8) w old and (180-220) g, were bought from Vital River (Beijing, China). All animal experiments were conducted as per the "Guide for the Care and Use of Laboratory Animals" and approved by the animal research ethics committee of our hospital.
Grouping of laboratory animals and establishment of AR animal model:
All the 40 mice were evenly divided into control group (Phosphate-Buffered Saline (PBS) group), Ovalbumin (OVA) group, OVA+Low-dose CEZP (CRZP-L) group, OVA+High-dose CEZP (CRZP-H) group and OVA+Dexamethasone (DEX) group in a random manner. The OVA-induced AR mouse model was constructed as previously described[2]. Briefly, 200 μl of PBS (Sigma-Aldrich, United States of America (USA)) containing 50 μg of OVA as well as 5 mg of aluminum hydroxide (Sigma- Aldrich) was injected intraperitoneally into the mice in model groups. Then 20 μl of PBS containing 400 μg of OVA was administered intranasal to the mice on d 21, 23, 25 and 27. Moreover, 2.5 mg/kg CEZP (PharmaBlock, China), 5 mg/kg CEZP and l mg/kg DEX (Sigma-Aldrich) were separately administered once daily on d 21-27 in drug treatment groups, i.e., OVA+CEZP-L group, OVA+CEZP-H group and OVA+DEX group, which were used as positive controls. The composition of CEZP prescription was as follows; 15 g of Flos Magnoliae (F. Magnoliae) seed, 7.5 g of Fructus xanthii (F. xanthii), 30 g of lemon fragrant Angelica root and 1.5 g of peppermint leaves. In addition, the same amount of PBS was provided for the mice in control group (PBS group, a negative control), without drug treatment or OVA induction. On d 27, the frequency of nose scratching and sneezing within 15 min was recorded. On d 28, all animals were killed by intravenous injection of barbiturates (Jurox Pty Ltd., Australia), followed by collection and storage (-80°) of blood samples and nasal mucosae for subsequent analyses.
Hematoxylin and Eosin (HE) staining:
After paraffin embedding, mouse nasal mucosae were sliced into 4 μm thick sections and deparaffinized and rehydrated in xylene and gradient ethanol, respectively, and then stained with HE (Sigma-Aldrich), and sealed with Canada balsam (Sigma-Aldrich). Finally, a microscope (Olympus, Tokyo, Japan) was used to observe the nasal mucosa.
Enzyme-Linked Immunosorbent Assay (ELISA):
The obtained blood samples from the mice were centrifuged for 10 min at 1000×g and 4°, followed by serum collection. Next, the levels of OVAspecific Immunoglobulin E (IgE) (BioLegend, USA), histamine (Abcam, USA), Interleukin-5 (IL-5), (Abcam) and Tumor Necrosis Factor-Alpha (TNF-α), (Abcam) in the serum were measured by ELISA.
Measurement of oxidative stress markers:
Following homogenization in 1 ml of Sodium Chloride (NaCl) solution (0.9 %) on ice, nasal mucosa samples were centrifuged for 10 min at 3000×g and 4°. Thereafter, the supernatant was harvested, and assay kits (Jiancheng Bioengineering Institute, China) for Reactive Oxygen Species (ROS), Superoxide Dismutase (SOD), Malondialdehyde (MDA) and Myeloperoxidase (MPO) were employed to assess SOD activity, ROS activity, MDA level and MPO level as per the manufacturer's directions.
Western Blotting (WB):
The protein isolation from mouse nasal mucosae was conducted using Radioimmunoprecipitation Assay (RIPA) buffer (Thermo Scientific, USA), followed by quantification using a Bicinchoninic Acid (BCA) assay kit (Thermo Scientific). Next, equivalent protein samples were divided by 10 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDA-PAGE) and blotted on a (Polyvinylidene Difluoride (PVDF), Thermo Scientific) membrane. After that, the membrane was blocked with skim milk (5 %) and incubated with primary antibodies against NF-κB (ab32536, 1:1000, Abcam), TLR4 (ab13556, 1:1000, Abcam), and (Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), ab9485, 1:2500, Abcam) at 4° for overnight. Later, with the secondary antibodies (ab7090, Abcam) for 2 h at 25°. Finally, an Electrochemiluminescence (ECL) assay kit (Bio- Rad, USA) and the Image Lab software (Bio-Rad) were used for visualization and quantification of protein bands, respectively.
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
A TRIzol reagent (Invitrogen, USA) was employed for extraction of total Ribonucleic Acid (RNA) from mouse nasal mucosae. Then, approximately of total RNA (~1 μg) was reversely transcribed into complementary Deoxyribonucleic Acid (cDNA) using an iScript cDNA kit (Bio-Rad), followed by RT-qPCR on an ABI 7300 RT-PCR (Applied Biosystems, USA) using SYBR Green qPCR Master Mix (DBI® Bioscience, Shanghai, China). The relative expressions of TNF-α and IL-5 were calculated using the 2-ΔΔCt method, with an internal reference gene as GAPDH. The primer sequences were shown in Table 1.
| Primer sets | Sequence (5'-3') |
|---|---|
| TNF-α | Forward: TTCTCATTCCTGCTTGTGG |
| Reverse: TTGGGAACTTCTCATCCCT | |
| IL-5 | Forward: AAGCAATGAGACGATGAGG |
| Reverse: ATTCTTCAGTATGTCTAGCCC | |
| GAPDH | Forward: ACTCTTCCACCTTCGATGC |
| Reverse: CCGTATTCATTGTCATACCAGG |
Table 1: RT-qPCR Primer Sequences
Immunohistochemistry:
Paraffin-embedded nasal mucosal tissues from AR mice were sliced into 4 μm thick sections, baked for 15 min at 70°, and dehydrated in ethanol. Next, they were inactivated with Hydrogen peroxide (H2O2), rinsed thrice (5 min/time) with PBS. Thereafter, they were incubated with serum (normal goat) for 15 min at 25° and then incubated with primary antibody diluted with rabbit anti-mouse TLR4 antibody (ab13556, 1:1000, Abcam), PBS (25 μl), and anti-NF-κB antibody (ab16502, 1:1000, Abcam) at 4° for overnight. Subsequently, sections were rinsed thrice (5 min/time) with PBS and incubated with secondary antibodies at 37° for 45 min, followed by rinsing with PBS and then diaminobenzidine (Kit-0016, Fuzhou Mai Xin Biotechnology Development Co., Ltd., China) for 10 min. After that, they were rinsed with tap water, counterstained by using hematoxylin, desalinized in dilute Water (HCl) for 30 s, and washed, followed by section dehydration, permeabilization, sealing and observation under the microscope. Finally, five representative fields of view were randomly selected and analyzed to acquire immunohistochemically results. In each field of view, 100 cells were counted and the percentage of positive cells was calculated.
Statistical analysis:
A Student's t-test was utilized for difference comparison between two groups by using Statistical Package for the Social Sciences (SPSS) 21.0 software (IBM, Armonk, New York, USA), whereas Analysis of Variance (ANOVA) followed by Tukey post-hoc analysis was employed for comparison among multiple groups. Each experiment was conducted in triplicate and the data was expressed in mean±standard deviation. p<0.05 indicated a statistically significant difference was used for statistical analysis.
Results and Discussion
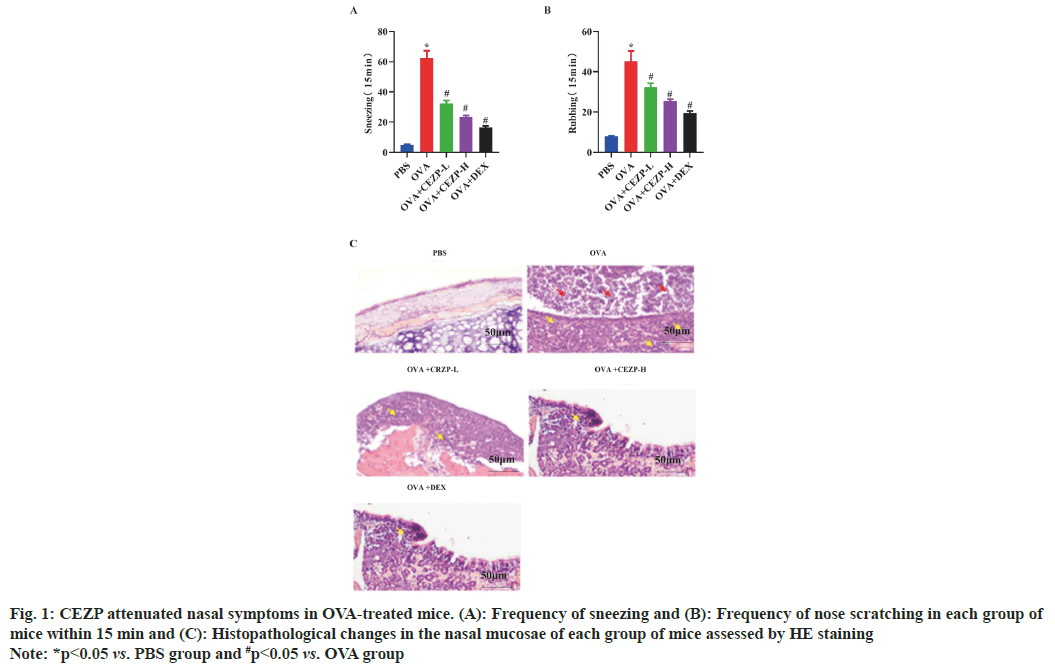
Firstly, the frequency of sneezing and nose scratching in mice was observed after intranasal administration of OVA to test the effect of CEZP on the allergic symptoms of AR. It was uncovered that the frequency of sneezing and nose scratching was significantly higher in OVA-induced groups than that in control group (PBS group), while it's worth noting that the frequency of sneezing and nose scratching notably dropped after the administration of CEZP or DEX (fig. 1A and fig. 1B). The results of HE staining revealed that in contrast with PBS-treated mice, OVA-treated mice exhibited significant histopathological changes, including mucus secretion and immune cell infiltration in the nasal mucosae. Nevertheless, these changes were significantly relieved after treatment with CEZP or DEX (fig. 1C). The above outcomes signify that CEZP can lower the frequency of OVA-induced nose scratching and sneezing in mice.
Fig. 1: CEZP attenuated nasal symptoms in OVA-treated mice. (A): Frequency of sneezing and (B): Frequency of nose scratching in each group of mice within 15 min and (C): Histopathological changes in the nasal mucosae of each group of mice assessed by HE staining
Note: *p<0.05 vs. PBS group and #p<0.05 vs. OVA group
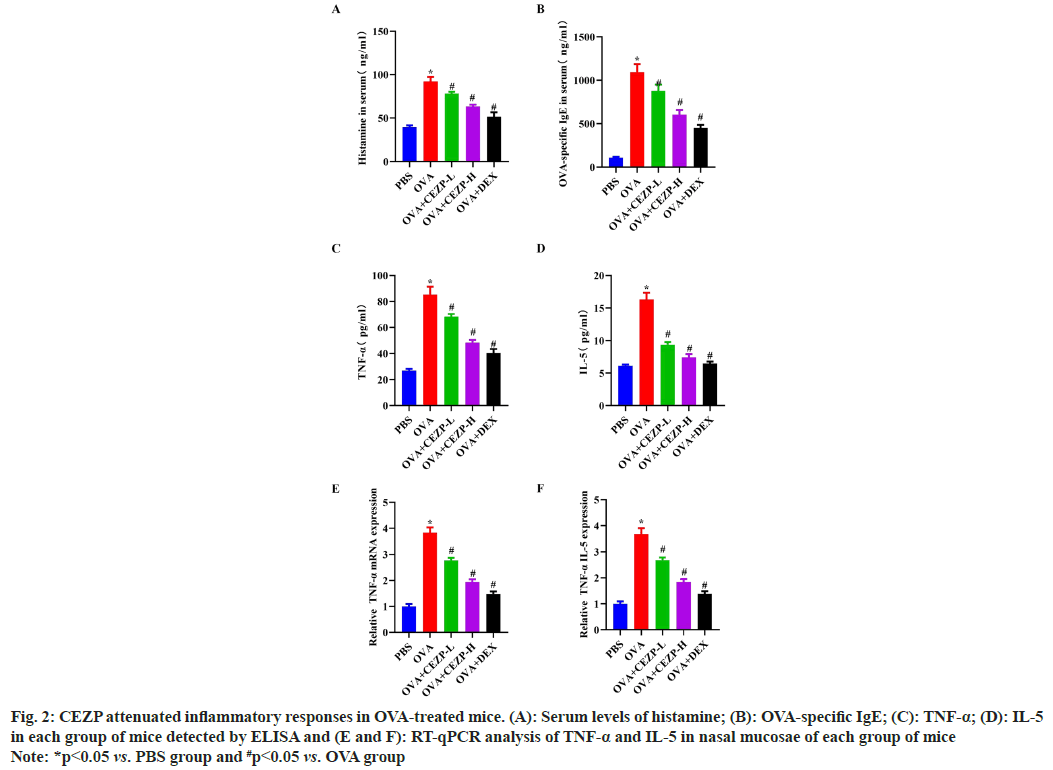
Next, whether CEZP exerts an anti-inflammatory effect in AR was investigated. According to ELISA results, the production of OVA-specific IgE and histamine in mouse serum was enhanced in OVA group compared to that in PBS group (fig. 2A and fig. 2B). Conversely, the levels of OVAspecific IgE and histamine were significantly lower in OVA+CEZP or OVA+DEX group than those in OVA group. Besides, OVA treatment led to increases in the secretion of the proinflammatory factors, namely TNF-α and IL-5, while the levels of such pro-inflammatory factors were significantly decreased by the treatment with CEZP or DEX (fig. 2C and fig. 2D). The RT-qPCR results showed that the treatment with CEZP or DEX significantly attenuated the OVA-induced up-regulation of TNF-α and IL-5 messenger RNA (mRNA) expressions (fig. 2E and fig. 2F), further proving the above results. In conclusion, CEZP is able to relieve OVA-induced inflammatory responses in the AR mouse model.
Fig. 2: CEZP attenuated inflammatory responses in OVA-treated mice. (A): Serum levels of histamine; (B): OVA-specific IgE; (C): TNF-α; (D): IL-5 in each group of mice detected by ELISA and (E and F): RT-qPCR analysis of TNF-α and IL-5 in nasal mucosae of each group of mice
Note: *p<0.05 vs. PBS group and #p<0.05 vs. OVA group
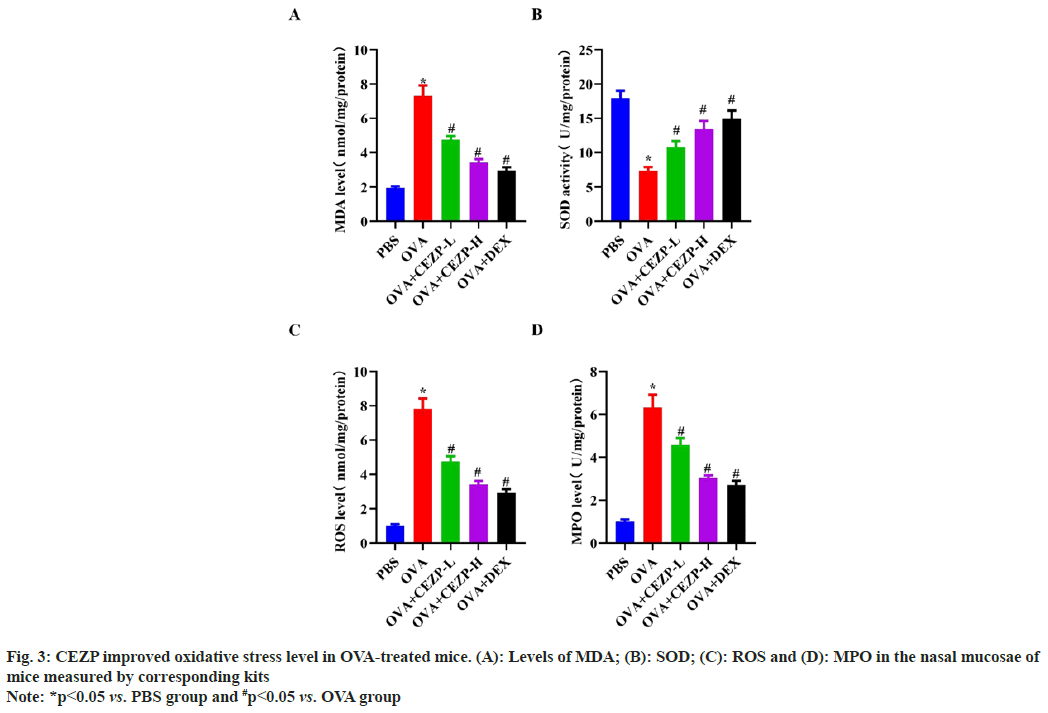
An increasing body of evidence suggests that oxidative stress plays a crucial role in the progression of AR. In this study, the effect of CEZP on oxidative stress in the OVA-induced AR mouse model was investigated, and the levels of oxidative stress-related markers in the nasal mucosae of mice were measured. The results revealed that OVA group exhibited significantly elevated MDA and ROS levels, whereas the elevation of MDA and ROS was inhibited by treatment with CEZP or DEX (fig. 3A and fig. 3B). In addition, the SOD levels was reduced in OVA group, but it was higher in OVA+CEZP or OVA+DEX group than that in OVA group (fig. 3C). The MPO detection results, another marker of oxidative stress, uncovered that the treatment with DEX or CEZP significantly abolished the OVA-induced increase in MPO level in the nasal mucosae of mice (fig. 3D), in line with the above results. These results denote that CEZP improves OVA-induced oxidative stress in the nasal mucosae of mice.
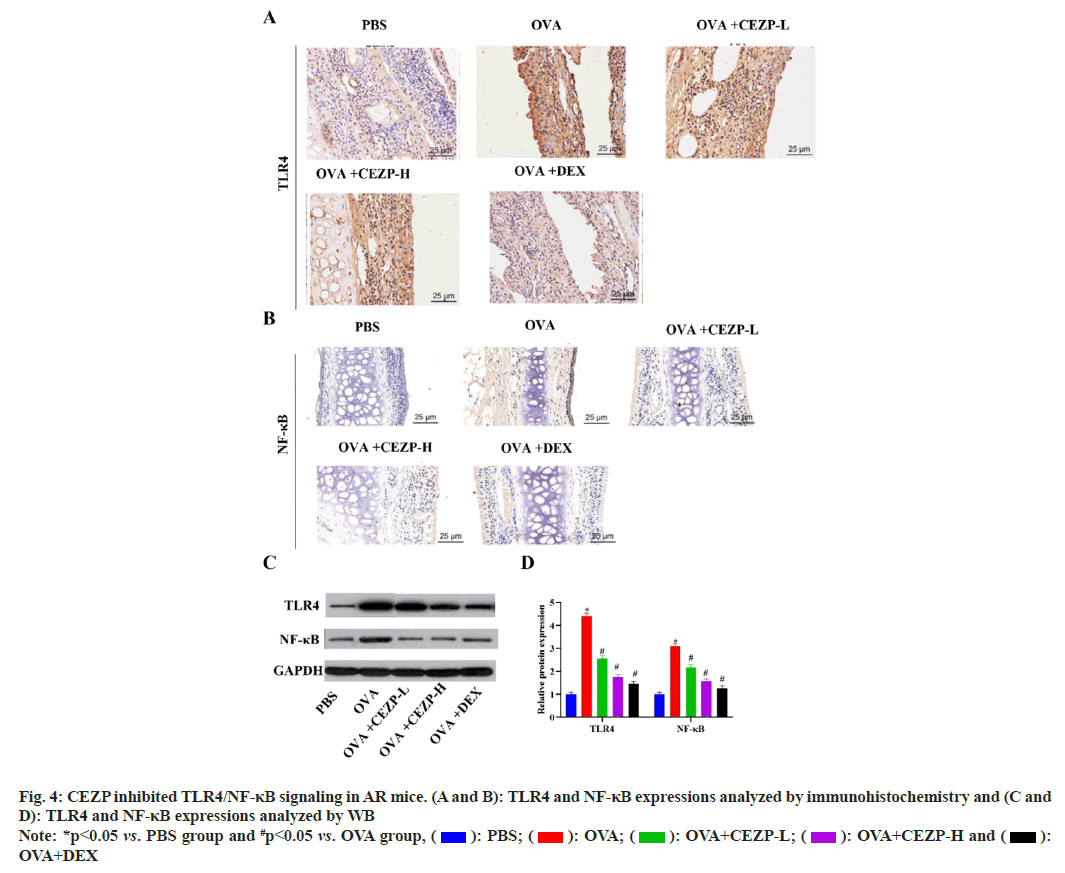
Thereafter, whether CEZP affects the TLR4/NF- κB signaling pathway was explored to expose the potential mechanisms by which CEZP regulates the pathogenesis of AR. Immunohistochemically and WB results showed that the protein levels of TLR4 and NF-κB were significantly enhanced in the nasal mucosae of OVA-induced mice compared to those in PBS group, whereas such levels were decreased after the treatment with CEZP or DEX (fig. 4). The aforementioned results demonstrate that CEZP represses the activation of TLR4/NF- κB signaling in the nasal mucosae of AR mouse.
AR refers to the inflammatory responses in the nasal mucosae mediated by IgE due to allergen exposure. As a global health problem, AR has a negative effect on work, sleep, study and outdoor activities of patients. There are treatment methods for AR, but their efficacy is far from satisfactory, and none of them can completely cure AR up to now[16]. Hence, discovering new effective drugs to treat AR is essential.
In recent years, Chinese herbal compound prescriptions have achieved remarkable outcomes in the treatment of AR. For example, CEZP, a classical and famous prescription commonly used in the treatment of AR, is stated in the Song Dynasty's JiSheng Fang; "0.5 liang of F. Magnoliae seed, 2.5 qian of F. xanthii, 1 liang of lemon fragrant Angelica root, and 0.5 qian of peppermint leaves are dried in the sun and ground into fine powder, and then the resulting powder is taken orally with green onion and tea water, with 2 qian/dose." CEZP is a compound prescription consisting of four Chinese herbs, namely F. xanthii, radix Angelicae dahuricae, F. Magnoliae and Mentha piperita. All these herbs are aromatic drugs of mild action, which go up into the lungs and reach the top of the lungs to dispel wind pathogen, promote lung qi and clear the nasal passages. In this prescription, F. xanthii is sweet (taste) and warm (property), with major effects of dispelling wind and clearing orifices, which is commonly used in the treatment of nasal obstruction. Radix Angelicae dahuricae is spicy (taste), warm (property) and aromatic (smell), with effects of clearing orifices and relieving pain. F. Magnoliae is spicy (taste) and warm (property), with effects of dispelling wind pathogen and clearing the nasal passages. The combined use of these three herbs can enhance the efficacy of F. xanthii. Mentha piperita is spicy (taste) and cool (property), with major effects of dispelling wind heat, and benefiting head and eyes. The combined application of the 4 drugs can not only promote the above 3 drugs to dispel wind and clear orifices, but also prevent body fluid deficiency caused by the above 3 drugs[17]. Hence, CEZP is suitable to treat AR. In this study, the AR animal model was established by OVA induction to examine the function of CEZP in AR. The results manifested that CEZP significantly relieved the symptoms of nasal allergy in OVA-induced mice, as evidenced by a reduction in the occurrence of sneezing and nose scratching. This was also proved by histopathological observations of the nasal mucosal tissues in mice. The treatment with CEZP significantly mitigated the OVA-induced pathological changes in the nasal mucosae of mice, including the suppression on mucus secretion and inflammatory cell infiltration. Additionally, to validate the outcomes, DEX was used as a positive control in this research work. DEX has been proved to be operative in suppressing nose scratching, sneezing, nasal discharge and relieving allergic inflammation[18]. The outcomes of this study uncovered that the effect of CEZP on ARrelated symptoms was similar to that of DEX.
Antihistamines are one of the drugs currently applied in the treatment of AR. In this study, it was found that CEZP lowered histamine levels in OVAattacked mice, signifying the potential of CEZP as an antihistamine. Besides, CEZP also repressed the production of TNF-α, IL-5 and allergenspecific IgE. The nasal inflammation caused by these cytokines had a close association with AR. These outcomes signify that CEZP employs an anti-inflammatory effect on AR. In addition, there is growing evidence that oxidative stress caused by excessive production of ROS plays an crucial part in the pathogenesis of AR. MDA is well thoughtout as a biomarker of oxidative stress[19], while SOD is intricated in antioxidant defense mechanisms[20]. As a peroxidase, MPO catalyzes chloride ions and H2O2 reaction and releases cytotoxic substances like hypochlorite and other chloride species, thereby persuading oxidative stress. Moreover, oxidative stress also leads to inflammation, additional facilitating disease advancement. Earlier research studies have proposed that CEZP exerts an antioxidant effect in many diseases[21,22]. In this study, the treatment with CEZP suppressed the levels of MDA, ROS and MPO, but endorsed the activity of the antioxidant SOD, suggesting that CEZP is able to relieve oxidative stress in AR.
Moreover, the influence of CEZP on the TLR4/ NF-κB signaling pathway was also explored to better understand the regulatory mechanisms of CEZP in mediating AR. Numerous studies have corroborated that the TLR4/NF-κB signaling pathway serves as a pro-inflammatory factor and has a relation to the progression of AR[13,15,23]. The results of this study revealed that the treatment with CEZP significantly inhibited OVA-induced activation of the TLR4/NF-κB signaling pathway in the nasal mucosae of mice, consistent with the findings of previous research[24], proving the anti- AR effect of CEZP.
In summary, investigation results of the role and potential mechanisms of CEZP in the OVA-induced AR mouse model uncovered that the treatment with CEZP ameliorates the symptoms of nasal allergy, inflammatory responses and oxidative stress in AR mice by impeding TLR4/NF-κB signaling, which may offer a novel and reliable drug for the treatment of AR. Nevertheless, only the impact of CEZP on the protein activity of the TLR4/NF- κB signaling pathway in AR was explored, while the TLR4/NF-κB signaling pathway inhibitors were not used for treatment, requiring verification by further studies. Moreover, in-depth research should be conducted on the protective mechanism of CEZP in mediating AR.
Authors’ contributions:
Xin Wang and Jiao An have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hemmati S, Rahimi N, Dabiri S, Alaeddini M, Etemad-Moghadam S, Dehpour AR. Inhibition of ovalbumin-induced allergic rhinitis by sumatriptan through the nitric oxide pathway in mice. Life Sci 2019;236:116901.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Lin L, Zheng C. Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis. Exp Mol Med 2012;44(3):177-90.
[Crossref] [Google Scholar] [PubMed]
- Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma and chronic rhinosinusitis. Am J Rhinol Allergy 2016;30(1):44-7.
[Crossref] [Google Scholar] [PubMed]
- Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: Mechanisms and treatment. Immunol Allergy Clin 2016;36(2):261-78.
[Crossref] [Google Scholar] [PubMed]
- Steelant B, Farré R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite–induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol 2016;137(4):1043-53.
[Crossref] [Google Scholar] [PubMed]
- Chen F, He D, Yan B. Apigenin attenuates allergic responses of ovalbumin-induced allergic rhinitis through modulation of Th1/Th2 responses in experimental mice. Dose Response 2020;18(1):1559325820904799.
[Crossref] [Google Scholar] [PubMed]
- Shao YY, Zhou YM, Hu M, Li JZ, Chen CJ, Wang YJ, et al. The anti-allergic rhinitis effect of traditional Chinese medicine of Shenqi by regulating mast cell degranulation and Th1/Th2 cytokine balance. Molecules 2017;22(3):504.
[Crossref] [Google Scholar] [PubMed]
- Luo C, Wang Y, He B, He Y, Yan Y, Wang J, et al. Exploring the core prescription and underlying mechanism of traditional Chinese medicine in treating allergic rhinitis in children: A real-world study based on an illustrious senior traditional Chinese medicine practitioner. Comb Chem High Throughput Screen 2023;26(1):207-23.
[Crossref] [Google Scholar] [PubMed]
- Fan W, Fan L, Peng C, Zhang Q, Wang L, Li L, et al. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019;24(2):359.
[Crossref] [Google Scholar] [PubMed]
- Huang C. Clinical observation on the treatment of pediatric allergic rhinitis with Xiao Qing Long Tang and Cang'erzi San plus and minus. Chin Foreign Med Res 2017;15:142-4.
- Qin H. Clinical observation of 60 cases of allergic rhinitis treated with Yu Ping Feng San combined with Cang'erzi San. Clin J Chin Med 2015;7:82-3.
- Leitner GR, Wenzel TJ, Marshall N, Gates EJ, Klegeris A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Exp Opin Ther Targets 2019;23(10):865-82.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Jiang Y, Han M, Jiang L, Liang D, Li S, et al. microRNA-345-5p acts as an anti-inflammatory regulator in experimental allergic rhinitis via the TLR4/NF-κB pathway. Int Immunopharmacol 2020;86:106522.
[Crossref] [Google Scholar] [PubMed]
- van Nguyen T, Piao CH, Fan YJ, Shin DU, Kim SY, Song HJ, et al. Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Sci Rep 2020;10(1):12528.
[Crossref] [Google Scholar] [PubMed]
- Dong J, Xu O, Wang J, Shan C, Ren X. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol 2021;43(3):319-27.
[Crossref] [Google Scholar] [PubMed]
- Siddiqui ZA, Walker A, Pirwani MM, Tahiri M, Syed I. Allergic rhinitis: Diagnosis and management. Br J Hosp Med 2022;83(2):1-9.
- Cao Q. Professor Liang Shan's experience in treating congested nose with deficient lung qi and cold using desensitizing and tonifying soup. Elec J General Stomatol 2020;7:134.
- Keevil BG. Improving the dexamethasone suppression test. Clin Chem 2021;67(7):929-31.
[Crossref] [Google Scholar] [PubMed]
- Kohen R, Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions and methods for their quantification. Toxicol Pathol 2002;30(6):620-50.
[Crossref] [Google Scholar] [PubMed]
- Han M, Lee D, Lee SH, Kim TH. Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants 2021;10(8):1266.
[Crossref] [Google Scholar] [PubMed]
- Huang MH, Wang BS, Chiu CS, Amagaya S, Hsieh WT, Huang SS, et al. Antioxidant, antinociceptive and anti-inflammatory activities of Xanthii fructus extract. J Ethnopharmacol 2011;135(2):545-52.
[Crossref] [Google Scholar] [PubMed]
- Han T, Zhang QY, Zhang H, Wen J, Wang Y, Huang BK, et al. Authentication and quantitative analysis on the chemical profile of Xanthiumfruit (Cang-Er-Zi) by high-performance liquid chromatography-diode-array detection tandem mass spectrometry method. Anal Chim Acta 2009;634(2):272-8.
- Wang J, Cui Z, Liu L, Zhang S, Zhang Y, Zhang Y, et al. miR-146a mimic attenuates murine allergic rhinitis by downregulating TLR4/TRAF6/NF-κB pathway. Immunotherapy 2019;11(13):1095-105.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Liu J, Zhao K, Shi Q, Zuo T, Wang G, et al. Role of daphnetin in rat severe acute pancreatitis through the regulation of TLR4/NF-κB signaling pathway activation. Am J Chin Med 2016;44(1):149-63.
[Crossref] [Google Scholar] [PubMed]
 : PBS;
: PBS;  : OVA;
: OVA;  : OVA+CEZP-L;
: OVA+CEZP-L;  : OVA+CEZP-H and
: OVA+CEZP-H and  : OVA+DEX
: OVA+DEX



