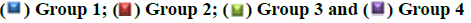- *Corresponding Author:
- S. Soujanya
Department of Veterinary Pathology,
College of Veterinary Science,
P. V. Narasimha Rao Telangana Veterinary University,
Hyderabad,
Telangana 500030,
India
E-mail: sonurv36@gmail.com
| Date of Received | 05 April 2020 |
| Date of Revision | 12 November 2021 |
| Date of Acceptance | 06 April 2022 |
| Indian J Pharm Sci 2022;84(2):436-450 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present experiment was aimed to assess imidacloprid induced alterations in oestrous cycle and ameliorative effects of Withania somnifera in rats. 48 female albino Wistar rats were divided into four groups of 12 animals each. Group 1 is control, group 2 is imidacloprid (30 mg/kg body weight/d), group 3 is Withania somnifera (1 g/kg feed) and group 4 is imidacloprid+Withania somnifera (30 mg/kg body weight/ d+1 g/kg feed). The test chemical was administered daily by oral gavage for 30 d. Seven consecutive estrous cycles were monitored in all the four groups by vaginal smear test. From each group, 6 rats were sacrificed on 16th d and remaining were sacrificed on 31st d. Blood samples were collected before sacrifice on 16th and 31st d of experiment for estimation of hormones in serum. A significant increase in length of oestrous cycle, follicle stimulating hormone levels and decrease in oestrogen levels were noticed in group 2. It is concluded that administration of Withania somnifera provided moderate protection against the imidacloprid induced toxicity by regularization of oestrous cycle in rats due to its antioxidant and gonadotropic properties.
Keywords
Follicle stimulating hormone, imidacloprid, luteinizing hormone, oestrogen, oestrous cycle, Withania somnifera
Pesticides are widely used in agriculture and animal husbandry practices to increase the crop yield by controlling pests on plants and used as ectoparasiticides on animals. But widespread usage of insecticides leads to environmental pollution and persistence of their residues in the food chain resulting in accidental exposure and toxicity in human beings and other non- targeted organisms. Imidacloprid is a neonicotinoid, chloronicotinic insecticide, classified as category I due to its high leaching potential. It is extensively used to protect crops from piercing sucking pests, to control the household pests and parasites on animals [1-3]. Presently, neonicotinoids account for approximately one third of the world insecticide market [4]. It acts by inhibiting the nicotinic receptors of acetylcholinesterase and thus prevents acetylcholine from transmitting impulses between nerves, result in paralysis and death of insects [5]. It is highly and selectively toxic to insects due to its high affinity towards insect’s nicotinic acetylcholine receptors compared to mammals [6,7]. It is known to induce reproductive toxicity and endocrine disruption in laboratory animals [8].
Withania somnifera (W. somnifera) is commonly known as Ashwagandha, Indian ginseng, poison gooseberry and winter cherry. Ashwagandha belongs to Solanaceae or nightshade family and commonly used as an Ayurveda medicine due to its anti-stress, antioxidant and free radical scavenging activities [9,10]. It is known to possess gonadotropic function which increases the gonadal weight by increasing the ovarian follicle size in females and by increasing the seminiferous tubular cell layers in male animals [11-14] and hence it was chosen as an ameliorating agent in current experiment to assess the protective role of it against the imidacloprid induced changes in oestrous cyclicity and reproductive hormone levels in female rats.
Materials and Methods
Chemicals:
Imidacloprid was procured from Tropical Agrosystem India Pvt. Ltd., Chennai, Tamilnadu and W. somnifera was obtained from Herb leaf Organic, Haryana.
Experimental animals:
Forty-eight young female Wistar rats weighing 200-250 g were procured from Jeeva life sciences, Hyderabad. The rats were acclimatized to the experimental conditions for 10 d before commencement of the study. They were housed in solid bottom polypropylene cages at lab animal house in the Ruska labs, Hyderabad and were maintained in controlled environment (temperature 20°-22°) throughout the course of the experiment. Rice husk was used as a bedding material. All the rats were provided ad libitum with standard pellet diet (procured from Vyas Labs, Uppal, Hyderabad) and water throughout the experimental period. The experiment was conducted according to the guidelines and with prior approval of the Institutional Animal Ethics Committee (IAEC-No.7/22/C.V.Sc., Hyd./29.02.2020).
Experimental design:
The rats were divided into four groups comprising of 12 in each. Group 1 served as control, group 2 was received with imidacloprid at the rate of 30 mg/kg body weight (b.wt)/d, group 3 was given with W. somnifera at the rate of 1 g/kg feed and group 4 was treated with both imidacloprid and W. somnifera (30 mg/kg b.wt/ d+1 g/kg feed). The test compound was administered for a period of 30 d daily by oral gavage.
Evaluation of estrous cycle:
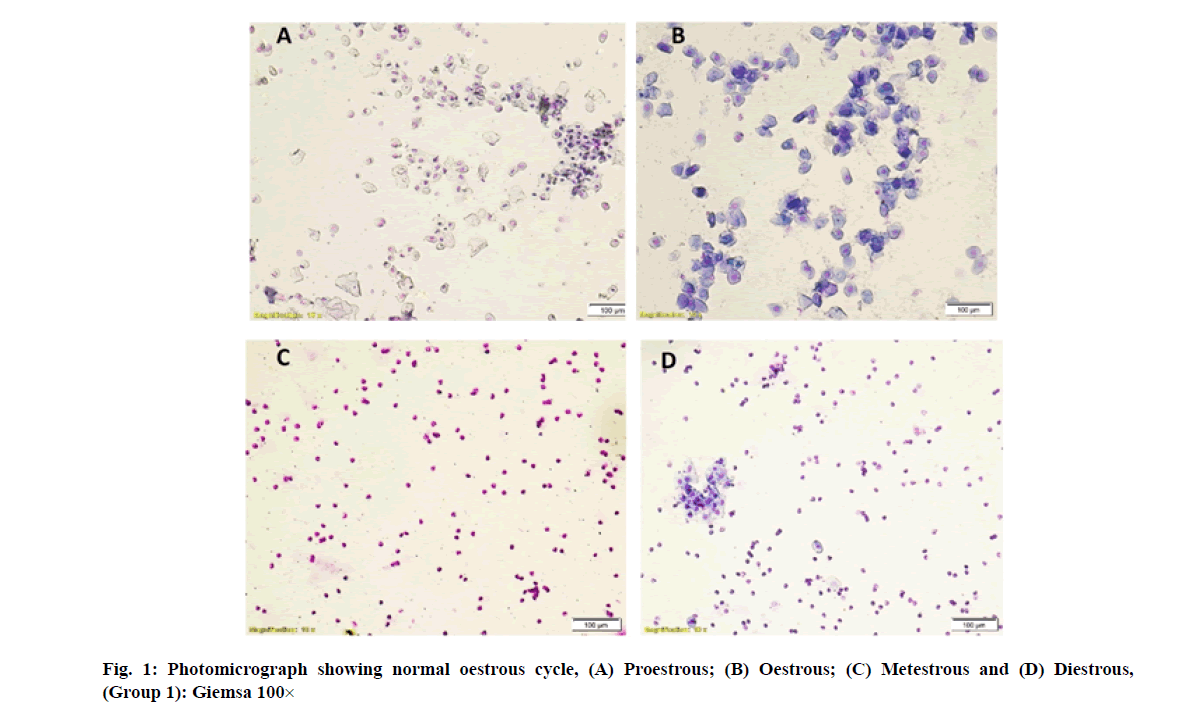
In the present study, seven consecutive oestrous cycles were monitored in all the four groups by vaginal smear test [15-17]. Daily vaginal smear was taken early in the morning by pipette smear technique. The method consists of flushing of cells from the vaginal lining by gently inserting the tip of a soft plastic pipette containing approximately 0.2 ml of 0.9 % normal saline into rat vaginal orifice to a depth of 2-5 mm and then the saline was flushed into the vagina and back out into the pipette 2-3 times by gently squeezing and releasing the bulb of the pipette. Then small amount of the cell suspension was expelled onto a labelled clean glass slide. Further slides were stained with Giemsa’s stain and observed under light microscope [18]. In the vaginal smear, the stage of oestrous cycle was identified by the number and morphology of the cells in the smear.
The length of each oestrous cycle was calculated as per the method adopted by Mandl and Li [19,20]. The relative lengths of the various stages in oestrous cycle were determined by the method described by Astwood [21]. For each animal, the number of hours in each of the four stages in oestrous cycle were recorded and converted into number of days by dividing the number of hour with 24 h.
Hormone estimation:
From each group, 6 rats were sacrificed on 16th d and remaining rats were sacrificed on31st d. Before sacrifice, on16th d and31st d of experiment, approximately 2 ml of blood was collected from each rat from retro orbital plexus through capillary tube into a clot promoting vacutainers and allowed to clot for 3 h to 4 h, later centrifuged at 20 k rpm for 10 min, serum was separated into Eppendorf tubes and stored at -20°. Later the serum samples were analyzed for Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH) and estrogen by using Enzyme-Linked Immunosorbent Assay (ELISA) kits.
Statistical analysis:
The data obtained from present study were subjected to statistical analysis by applying one way Analysis of Variance (ANOVA) using Statistical Package for Social Sciences (SPSS) version 16.0. Differences between means were tested using Duncan’s multiple comparison test and significance was set at p<0.05 [22].
Results and Discussion
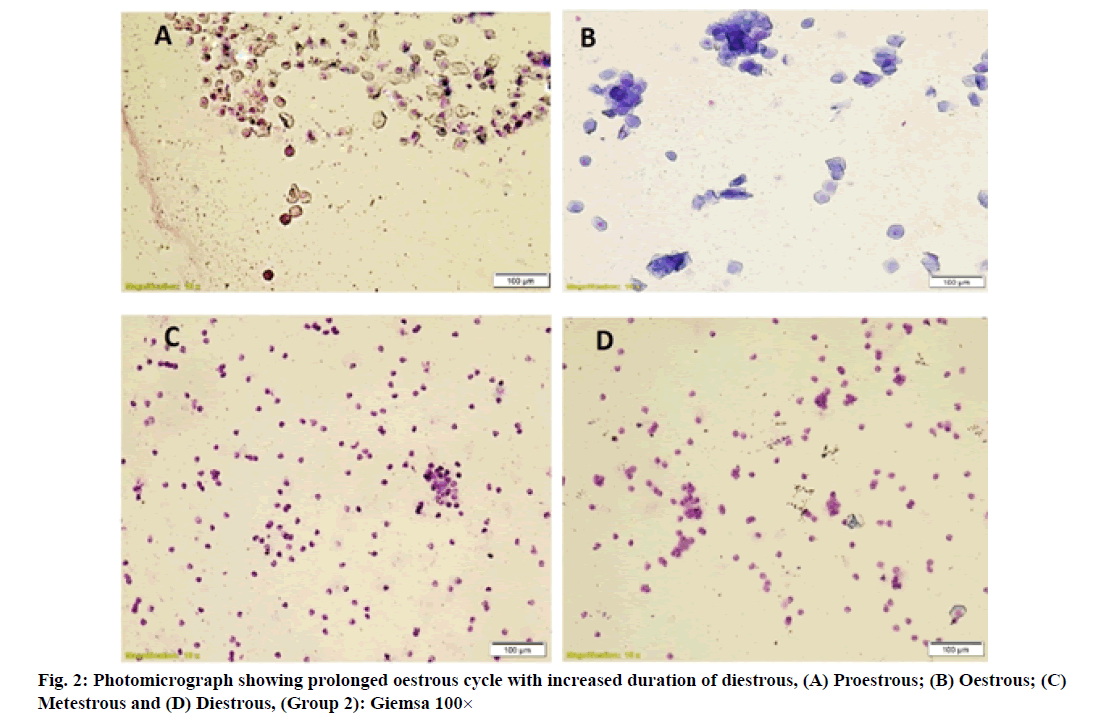
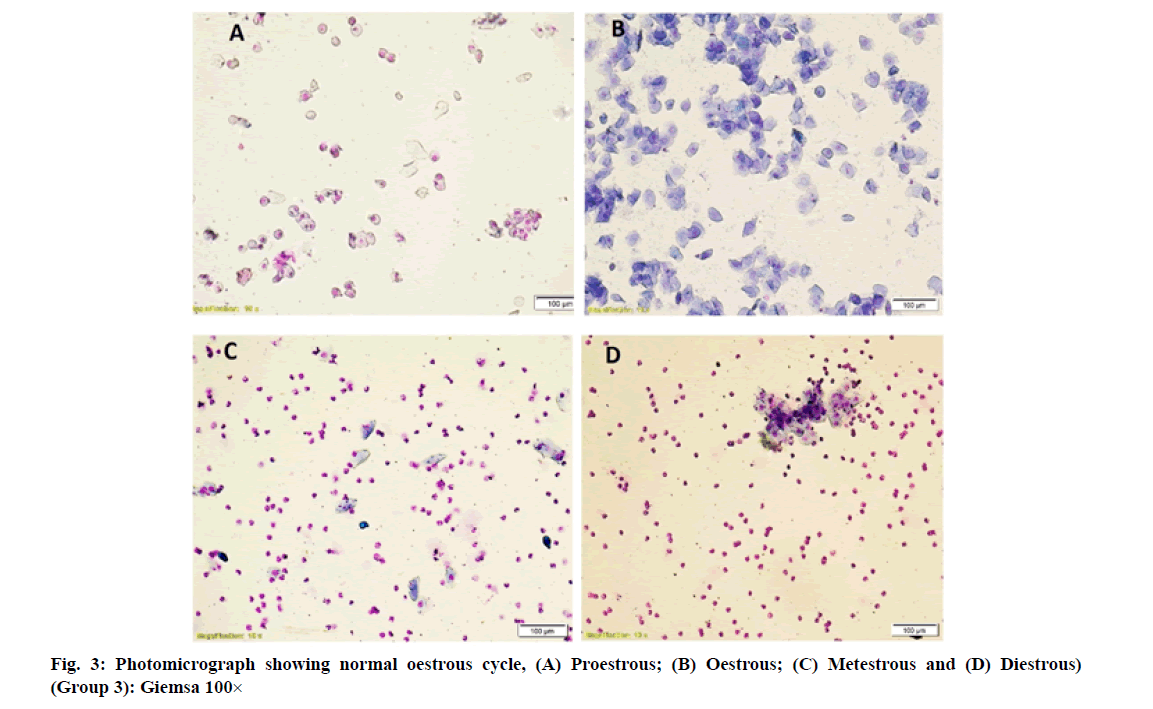
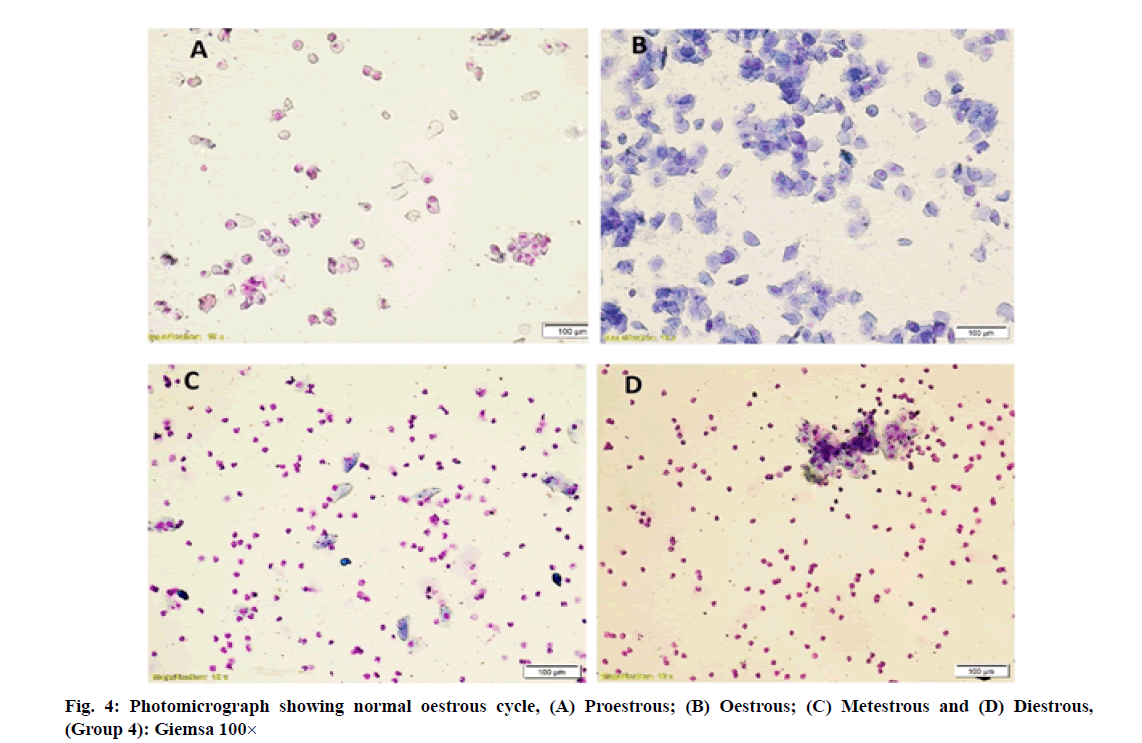
Vaginal smears in different group of rats revealed a large number of nucleated epithelial cells during proestrous, large numbers of cornified epithelial cells during oestrous, a combination of leukocytes with few cornified epithelial cells during metestrous and a combination of leukocytes with few nucleated epithelial cells during diestrous phase (fig. 1-fig. 4).
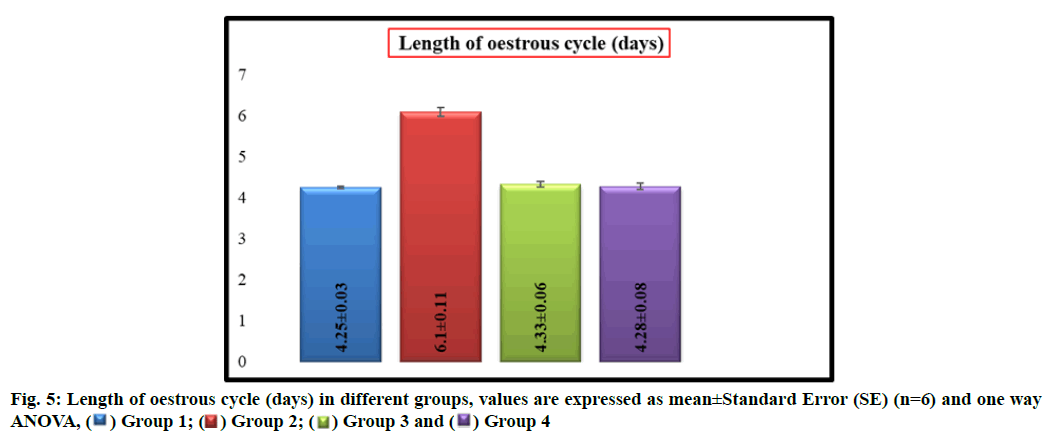
Group 2 rats showed a significant (p<0.05) increase in the length (d) of estrous cycle with a mean length of 6.10±0.11 as compared to group 1 which had a mean length of 4.25±0.03 whereas there was no significant difference in mean length of estrous cycle between groups 1, 3 (4.33±0.06) and 4 (4.28±0.08) respectively (fig. 5).
No significant difference was observed in average length (d) of proestrous phase between groups 1 (0.47±0.05), 2 (0.42±0.03), 3 (0.45±0.04) and 4 (0.43±0.03) respectively.
The average length (d) of oestrous phase in group 2 (1.13±0.06), 3 (1.18±0.07) and 4 (1.23±0.05) did not differ significantly from control (1.08±0.04).
There was no significant difference in average length (d) of metestrous phase between groups 1 (0.37±0.04), 2 (0.38±0.04), 3 (0.32±0.03) and 4 (0.35±0.04) respectively.
The average length (d) of diestrous phase in group 1 is 2.33±0.06 whereas it was significantly (p<0.05) increased to 4.16±0.06 in group 2 and there was no significant difference in average length of diestrous phase between groups 1, 3 (2.38±0.05) and 4 (2.27±0.07) respectively (fig. 6).
Evaluation of the oestrous cycle in laboratory rodents is a useful measure of integrity of the hypothalamic- pituitary-ovarian reproductive axis and it can contribute an important information regarding the nature of a toxicant insult to the reproductive system [15]. In general, the xenobiotic which induce the ovarian toxicity will alter the oestrous cycle in laboratory animals. In present study, increased duration of oestrous cycle is due to the prolonged diestrous phase in group 2 rats which might be resulted from disruption of the ovarian follicles and decreased levels of estrogen produced by imidacloprid induced oxidative stress. These results were agreed with previous authors [23,24].
However, simultaneous treatment of W. somnifera along with imidacloprid regularized the oestrous cycle in group 4 rats. It may be due to regeneration of ovarian follicles due to gonadotropic function of W. somnifera [11-14] that resulted in increased production of oestrogen by ovaries and led to regularization of oestrous cycle. Earlier, the role of W. somnifera in normalizing the oestrous cycle in letrazole induced polycystic ovarian syndrome in rats was reported by Saiyed et al. [25].
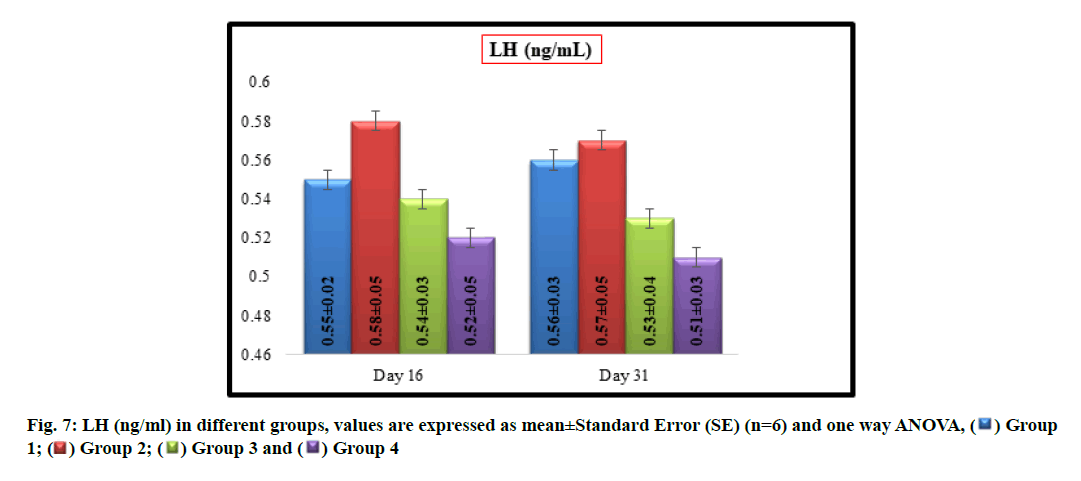
No significant difference was observed in mean LH (ng/ml) values between groups 1 (0.55±0.02 and 0.56±0.03), 2 (0.58±0.05 and 0.57±0.05), 3 (0.54±0.03 and 0.53±0.04) and 4 (0.52±0.05and 0.51±0.03) on16th and31st d of experiment respectively (fig. 7).
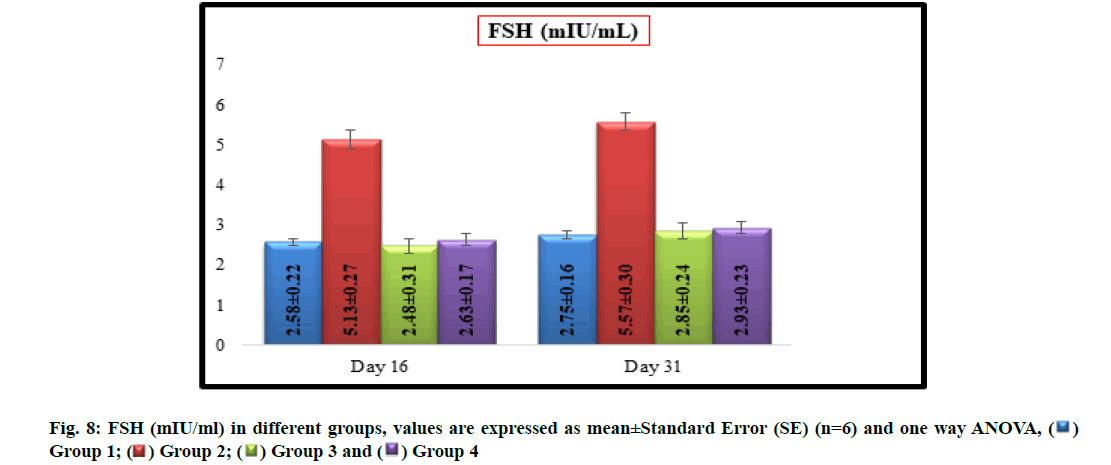
Significantly (p<0.05) increased mean values of FSH (mIU/ml) were recorded in group 2 (5.13±0.27 and 5.57±0.30) when compared with group 1 (2.58±0.22 and 2.75 ±0.16) on16th d and31st d of experiment respectively. There was no significant difference in mean values of FSH between groups 1, 3 (2.48±0.31 and 2.85±0.24) and 4 (2.63±0.17 and 2.93±0.23) on 16th d and31st d of experiment respectively (fig. 8).
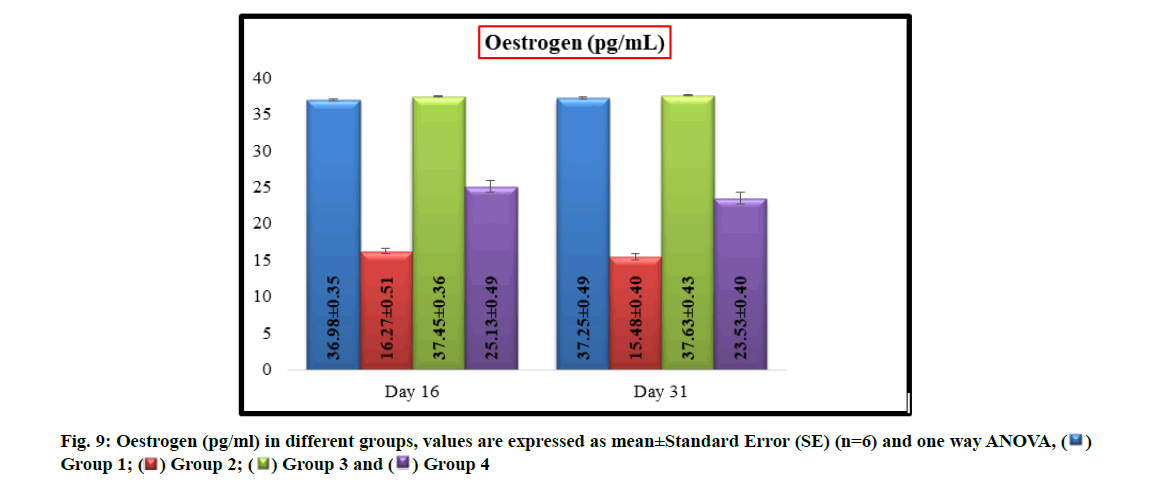
The mean values of oestrogen levels (pg/ml) in group 2 (16.27±0.51 and 15.48±0.40) and group 4 (25.13±0.49 and 23.53±0.40) were significantly (p<0.05) decreased when compared with group 1 (36.98±0.35 and 37.25±0.49) and group 3 (37.45±0.36 and 37.63±0.43) on16th d and31st d of experiment respectively. In group 4, a significant (p<0.05) increase in oestrogen levels were recorded in comparison to group 2 and no significant difference was observed in oestrogen levels between groups 1 and 3 (fig. 9).
The major source for production of estradiol is ovary and FSH is produced from anterior pituitary in response to gonadotropin-releasing hormone from hypothalamus in animals. In present case, a significant decrease in levels of estrogen were noted in imidacloprid treated rats which may be due to the oxidative damage to ovarian follicles resulted in decreased production of estradiol which might have further stimulated a negative feedback to the hypothalamus and resulted in increased secretion of FSH to stimulate the growth of ovarian follicles. But the matured ovarian follicles were again attacked by imidacloprid, this cycle was continued and lead to persistent reduction in oestrogen and elevation in FSH levels. These findings were in conformity with earlier researchers [26,27]. The hormonal imbalance in current experiment suggests that the imidacloprid would have induced toxic effects on hypothalamus- hypophysis-ovary axis.
However, simultaneous administration of W. somnifera along with imidacloprid to the rats demonstrated a significant (p<0.05) increase in oestrogen and decrease in FSH levels in group 4 rats in comparison with group 2 rats. This biological change might have triggered by gonadotropic function [11-14] of W. somnifera. It may also be due to prevention of oxidative damage to ovaries by its free radical scavenging activity and helped in production of oestrogen in appropriate quantities, which further regularized the production levels of FSH from anterior pituitary gland.
In conclusion, the study revealed that oral administration of imidacloprid at the rate of 30 mg/kg b.wt./d in female rats resulted in a significant alteration in oestrous cyclicity and serum hormone levels. However, supplementation of W. somnifera along with imidacloprid significantly ameliorated the toxic effects induced by imidacloprid.
Acknowledgements:
The authors are thankful to the College of Veterinary Science, P. V. Narasimha Rao Telangana Veterinary University (PVNRTVU), Rajendranagar, Hyderabad for providing the facilities to carry out the present research work.
Conflict of interests:
The authors have no conflict of interest to report.
References
- Arther RG, Cunningham J, Dorn H, Everett R, Herr LG, Hopkins T. Efficacy of imidacloprid for removal and control of fleas (Ctenocephalides felis) on dogs. Am J Vet Res 1997;58(8):848-50.
- Hutchinson MJ, Jacobs DE, Mencke N. Establishment of the cat flea (Ctenocephalidesfelis) on the ferret (Mustela putorius furo) and its control with imidacloprid. Med Vet Entomol 2001;15(2):212-4.
[Crossref] [Google Scholar] [Pub Med]
- Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 2015;22(1):68-102.
[Crossref] [Google Scholar] [Pub Med]
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ Sci Pollut Res 2015;22(1):5-34.
[Crossref] [Google Scholar] [Pub Med]
- Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu Rev Pharmacol Toxicol 2005;45:247-68.
[Crossref] [Google Scholar] [Pub Med]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 2001;22(11):573-80.
[Crossref] [Google Scholar] [Pub Med]
- Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 2003;48(1):339-64.
[Crossref] [Google Scholar] [Pub Med]
- Mikolić A, Karačonji IB. Imidacloprid as reproductive toxicant and endocrine disruptor: investigations in laboratory animals. Arh Hig Rada Toksiko 2018;69(2):103-8.
[Crossref] [Google Scholar] [Pub Med]
- Bhattacharya A, Ghosal S, Bhattacharya SK. Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol 2001;74(1):1-6.
[Crossref] [Google Scholar] [Pub Med]
- Shahriar M, Hossain I, Sharmin FA, Akhter S, Haque MA, Bhuiyan MA. In vitro antioxidant and free radical scavenging activity of Withania somnifera root. Iosr J Pharm 2013;3:38-47.
- Al‐Qarawi AA, Abdel‐Rahman HA, El‐Badry AA, Harraz F, Razig NA, Abdel‐Magied EM. The effect of extracts of Cynomorium coccineum and Withania somnifera on gonadotrophins and ovarian follicles of immature Wistar rats. Phytother Res 2000;14(4):288-90.
[Crossref] [Google Scholar] [Pub Med]
- Abdel-Magied EM, Abdel-Rahman HA, Harraz FM. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J Ethnopharmacol 2001;75(1):1-4.
[Crossref] [Goole Scholar] [Pub Med]
- Mali PC, Chouhan PS, Chaudhary R. Evaluation of antifertility activity of Withania somnifera in male albino rats. Fertil Steril 2008;90:S18.
- Kumar R, Ali M, Kumar A, Gahlot V. Comperative biomedical effect of Withinia somnifera and Curcuma longa on ovaries of pesticide induced mice. Eur J Pharm Med Res 2015;2:249-253.
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 2007;80(2):84-97.
[Crossref] [Google Scholar] [Pub Med]
- Zarrow, M.X. Experimental endocrinology: A sourcebook of basic techniques. Elsevier; 2012.
- Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicolo Pathol 2015;43(6):776-93.
[Crossref] [Google Scholar] [Pub Med]
- Clark, G. Staining Procedures. 4th ed. Baltimore, London: Williams and Wilkins; 1981.p. 173.
- Mandl AM. The phases of the oestrous cycle in the adult white rat. J Exp Biol 1951;28(4):576-84.
- Li J, Kim JS, Abejuela VA, Lamano JB, Klein NJ, Christian CA. Disrupted female estrous cyclicity in the intrahippocampal kainic acid mouse model of temporal lobe epilepsy. Epilepsia Open 2017;2(1):39-47.
[Crossref] [Google Scholar] [Pub Med]
- Astwood EB. Changes in the weight and water content of the uterus of the normal adult rat. Ame J Physiol Legacy Content 1939;126(1):162-70.
- Snedecor G.W, Cochran G. Statistical methods. 8th ed. Amer, Iowa, USA: Iowa state university press; 1994.
- Vohra P, Khera KS, Sangha GK. Physiological, biochemical and histological alterations induced by administration of imidacloprid in female albino rats. Pestic Biochem Physiol 2014;110:50-6.
[Crossref] [Google Scholar] [Pub Med]
- Vohra P, Khera KS. Imidacloprid induced toxicity in ovary of female wistar rats in two generations. Appl Biol Res 2018;20(1):62-7.
- Saiyed A, Jahan N, Makbul SA, Ansari M, Bano H, Habib SH. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on letrozole induced polycystic ovarian syndrome in rats. Integr Med Res 2016;5(4):293-300.
[Crossref] [Google Scholar] [Pub Med]
- Kapoor U, Srivastava MK, Srivastava LP. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol 2011;49(12):3086-9.
[Crossref] [Google Scholar] [Pub Med]
- Nabiuni M, Parivar K, Noorinejad R, Falahati Z, Khalili F, Karizadeh L. The reproductive side effects of imidacloprid in pregnant Wistar rats. Int J Mol Biotech 2015;1:10-18.