- *Corresponding Author:
- J. V. Susheel
Department of pharmaceutical analysis, College of pharmacy, Sri ramakrishna institute of paramedical sciences, Coimbatore - 641 044, India
E-mail: susheeljv@yahoo.com
| Date of Submission | 5 May 2006 |
| Date of Revision | 9 March 2007 |
| Date of Acceptance | 13 August 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 589-591 |
Abstract
Two simple methods, ultra violet spectroscopy and high performance thin layer chromatography for the determination of ropinirole in tablet dosage form are described. Detection wave lengths for spectrophotometric and high performance thin layer chromatographic methods were found to be 250 nm and 254 nm, respectively. For the spectrophotometric method, the linearity was found to be in the range of 5-30 mg/ml and for high performance thin layer chromatographic method; linearity was found to be between 40 and 120 mg/ml.
Keywords
Ropinirole, Ultra violet spectroscopy, High performance thin layer chromatography, Validation
Ropinirole [1,2] chemically known as 4-[2- (dipropylamino)ethyl]-1,3-dihydro 2H–indol-2- one, is used as an antiparkinsonian drug. Liquid chromatographic methods [3-5] has been reported for determination of ropinirole in dosage form and plasma. Neither spectrophotometric nor high performance thin layer chromatography (HPTLC) methods have been reported for estimation of ropinirole from formulation. The present communication reports two simple, precise and accurate spectrophotometric and HPTLC methods for the estimation of ropinirole from tablet dosage form.
All chemicals and reagents used were of analytical grade and purchased from S. D. Fine Chemicals Ltd., Mumbai and Qualigens Fine Chemicals Ltd., Mumbai. A Jasco V-530 UV/Vis spectrometer was used for absorbance measurements and Camag HPTLC system (with TLC Scanner 3, Wincats Software and Linomat 5 as application device) was employed for peak area measurements.
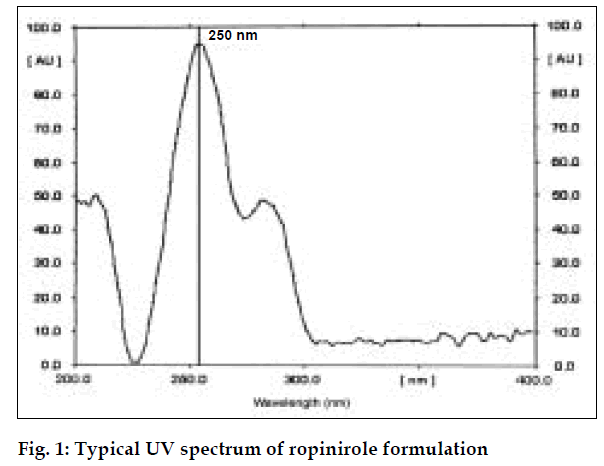
Absorbance occurs because of π electrons present in the structure [6]. Ropinirole consists of π electrons which are responsible for absorbance. It shows the maximum absorbance in ethanol at 250 nm.
For the spectrophotometric method, aliquots of standard ropinirole solutions ranging from 5-30 µg/ml (from stock solution of 100 µg/ml) were prepared using ethanol and absorbances were noted at 250 nm. Calibration curve was drawn by plotting absorbances of ropinirole versus concentration of respective drug solutions. Twenty tablets of ropinirole were weighed and average weight was calculated. Quantity of powder equivalent to 10 mg was weighed accurately and transferred to a 100 ml volumetric flask. The active ingredient was extracted with ethanol and volume was made up with ethanol and filtered. The filtered solution was further diluted to get requisite concentrations and analyzed as described under the procedure for pure sample, fig. 1. The concentration of ropinirole in tablet formulation was calculated from calibration graph. Results are given in Table 1.
| Drug | Labeled amount(mg/tablet) | Amount found(mg/ tablet) | %RSD* | ||
|---|---|---|---|---|---|
| UV | HPTLC | UV | HPTLC | ||
| Ropinirole | 2 | 1.94 | 1.95 | 0.2681 | 0.2411 |
*RSD of six observations
Table 1: Assay of ropinirole in tablet dosage form
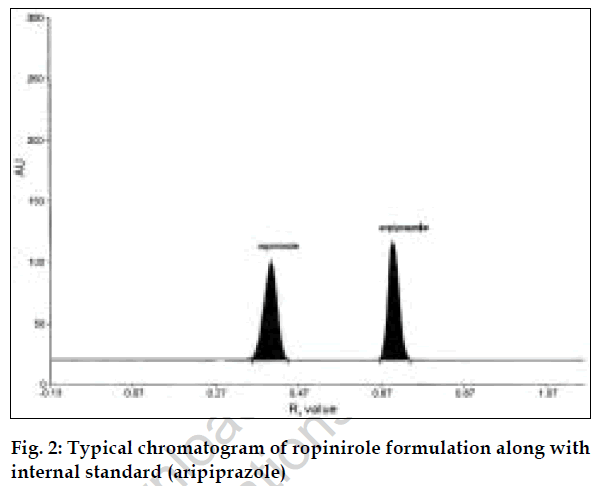
The second method is HPTLC, in which selection of mobile phase, selection of internal standard, solvent and wavelength are important [7]. Methanol: acetonitrile (8:2, v/v) was selected because in this system good compact and dense spots were obtained. The wavelength selected for scanning was 254 nm. Since aripiprazole peak was well resolved from ropinirole peak and had good peak shape, aripiprazole was selected as the internal standard.
For the HPTLC method, standard stock solution of ropinirole (1000 µg/ml) and aripiprazole (1000 µg/ml) were prepared in ethanol. The solution was further diluted with ethanol to obtain a series of concentrations ranging from 40 to 120 µg/ml of ropinirole, each containing 60 µg/ml of aripiprazole. Five microlitres from these solutions were applied on precoated TLC plate. The plate was analyzed photometrically and chromatograms recorded. Calibration graph was plotted using peak area ratios of ropinirole to internal standard peak areas versus concentrations of ropinirole. Twenty tablets of ropinirole were weighed and average weight was calculated. Quantity of powder equivalent to 10 mg was weighed accurately, dissolved and volume was made up to 100 ml with ethanol. This solution was filtered and further diluted to get requisite concentrations and analyzed as described under procedure for pure sample, fig. 2. The concentration of ropinirole in tablet formulation was calculated from calibration graph. The results are given in Table 1.
The developed methods were validated for parameters like accuracy, precision and stability. The regression equation and validation parameters are given in Table 2. Accuracy was established by performing recovery studies. These were carried out at 50 and 100% levels. Recovery values close to 100% indicates accuracy of method. For HPTLC method, limit of detection (LOD) and limit of quantification (LOQ) were found to be 12 and 40 µg/ml, respectively. Precision was studied under intra-day precision, inter-day precision and repeatability.
| Parameter | Results | |
|---|---|---|
| UV | HPTLC | |
| Linearity range | 5-30 µg /ml | 40-120 µg /ml |
| Regression equation (Y = a + bc) | ||
| * slope (b) | 0.0341 | 1.6658 |
| * Intercept (a) | 0.0046 | 0.1595 |
| * Correlation co-efficient (r) | 0.9998 | 0.9980 |
| Recovery studies | ||
| * 50% level | 98.41 | 98.65 |
| * 100% level | 98.57 | 99.08 |
Table 2: Validation parameters
For all these parameters, % RSD values were found to be less than one which indicates that the developed methods have good precision. Stability studies were also carried out. Drug solutions were found to be stable for about 2 h at room temperature and the developed TLC plate was found to be stable for about 3 h.
The developed UV spectroscopic and HPTLC method are precise and accurate. From the two methods developed for the estimation of ropinirole, the HPTLC method was found to be more precise. However, both techniques can be applied for routine analysis of ropinirole from tablet dosage forms.
Acknowledgements
The authors acknowledge M/s SNR and Sons Charitable Trust, Coimbatore, India for providing the facilities to carry out the experiment and Tamil Nadu Pharmaceutical Sciences Welfare Trust, Chennai for awarding Scholarship for the work.
References
- Ronald A. editor. Physicians' Desk Reference. Montvale: Medical Economics Company; 1999.
- Moffat AC, Osselton DM, Widdop B. editors. Clarke's analysis of drugs and poisons. London: Pharmaceutical Press; 2004.
- Coufal P, Stulik K, Claessens HA, Hardy MJ, Webb M. Separation and quantification of ropinirole and some impurities using capillary liquid chromatography. J Chromatogr B Biomed SciAppl 1999;732:437-444.
- Bloomer JC, Clarke SE, Chenery RJ. In vitro identification of the P450 enzymes responsible for the metabolism of ropinirole. Drug MetabDispos 1997;25:840-844.
- Bhatt J, Jangid A, Shetty R, Shah B, Kambli S, Subbaiah G, Singh S. Rapid and sensitive liquid chromatography-mass spectrometry method for determination of ropinirole in human plasma. J Pharm Biomed Anal 2006;40:1202-1208.
- Davidson AG. Ultraviolet-visible absorption spectrophotometry. In: Beckett AH, Stenlake JB, editors. Practical pharmaceutical chemistry. 4th ed. New Delhi: CBS Publishers and Distributors; 1997. p. 275-282.
- Sethi PD. High performance thin layer chromatography-quantitative analysis of pharmaceutical formulations. 1sted. New Delhi: CBS Publishers and Distributors; 1997.