- *Corresponding Author:
- Weixing Xu
Department of Orthopedics and Traumatology, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang 310012, China
E-mail: xuweixing_2013@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “11-18” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main objective of this study is to explore the effects of tetramethylpyrazine on functional recovery, inflammation and apoptosis after spinal cord injury in rats. Four experimental groups of rats were used (n=15), which included sham+vehicle group where only laminectomy was performed, sham+tetramethylpyrazine group where only laminectomy was performed, spinal cord injury+vehicle group where spinal cord injury was induced through clip compression technique and spinal cord injury+tetramethylpyrazine group where spinal cord injury was induced. The control group was given the same dose of normal saline and the four groups were intervened under the same conditions, tetramethylpyrazine was used intraperitoneally to observe the possible therapeutic effects compared to controls. Locomotor function in experimental rats improved after 20 d of treatment with tetramethylpyrazine evident by Basso Mouse scaled score performed by blind observers and terminal deoxynucleotidyl transferase-deoxyuridine triphosphate nick end labeling assay also showed antiapoptotic effect. It was observed that nuclear factor kappa B subunit p65 levels and phosphorylated nuclear factor kappa B subunit p65 levels were significantly reduced after treatment with tetramethylpyrazine. Proinflammatory cytokines, tumor necrosis factor alpha and interleukin-1 beta levels were also reduced along with myeloperoxidase activity in treated rats. It was observed that tetramethylpyrazine (20 mg/kg, body weight dose) restored locomotor function along with antiapoptotic effects and it is beneficial to improve the lower limb activity of rats, reduces infiltration of neutrophils and attenuation of other inflammatory markers that helps in the reduction of inflammation which is an important part of secondary phase of spinal cord injury pathology.
Keywords
Spinal cord, myeloperoxidase, proinflammatory cytokines, tetramethylpyrazine
Spinal Cord Injury (SCI) has been a cause of concern due to its complex pathophysiology. According to statistics, 10.4 %-83 % of people in every million population are suffering with SCI and their incidence rate and mortality rate also increasing year by year, which is one of the urgent problems in medical field[1]. Although advances in medical care and pharmacology have improved patients outcome however, the problems related to neuronal injury are still persistent. It has been observed that traumatic injury to the spinal cord causes death to a number of neurons which not only pose a problem regarding their regeneration but the death of neurons continues for hours after traumatic SCI[2]. This phase of SCI after mechanical injury is termed as “secondary phase” where different biochemical, molecular and cellular events continue to damage the injured site. As a result, inflammatory responses participate in amplification of secondary damage[3]. After SCI, microglia is activated and macrophages cross the Blood Brain Barrier (BBB) where they act as intrinsic spinal phagocytes. As a result, proinflammatory cytokines, Reactive Oxygen Species (ROS) and nitrogen species are released[4,5].
Injury to spinal cord shows a huge effect on Central Nervous System (CNS), as it causes sensory and motor dysfunction in patients. Morphological changes also occur in tissues after mechanical injury to spinal cord which includes hemorrhage, ischemia and edema[6,7]. It has been demonstrated that activation of Mitogen-Activated Protein Kinase (MAPK) signaling pathway is a crucial step in inflammatory responses[8]. Studies have shown that Nuclear Factor kappa B (NF-κB) is one of the major transcription factors that help in the regulation of production of proinflammatory cytokines in the CNS[9,10]. In the secondary phase, after SCI it has been demonstrated that apoptosis is a major event which is regulated by caspase and B-Cell Lymphoma 2 (BCL-2) families[11-13]. As apoptosis is involved in secondary damage and degeneration, therefore it can lead to functional disability in the spinal cord. For treating SCI, an effective therapy should be developed which can act on multiple targets.
Tetramethylpyrazine also known as Danggui is a traditional Chinese medicine that has been used in variety of gynecological disorders with demonstrated clinical efficacy[14]. Tetramethylpyrazine is one of the effective active components of Ligusticum chuanxiong Hort. and it belongs to amide alkaloids. It is an alkaloid monomer isolated and purified from Ligusticum chuanxiong Hort and its chemical structure is tetramethylpyrazine. Modern pharmacological studies have proved that it can improve microcirculation, inhibit inflammatory response and cell apoptosis. It is also reported that it is used to treat cardiovascular, cerebrovascular diseases, pulmonary hypertension, liver cirrhosis and other diseases and it also helps in promoting blood circulation, qi and blood stasis. Ligusticum chuanxiong is also one of the commonly used medicinal products in medicinal diet, it can nourish yin and activate blood circulation. It is a good tonic for women and it should be noted that one shouldn’t drink green tea immediately after eating the medicated diet containing chuanxiong, because green tea is cold in nature, which will weaken the efficacy of chuanxiong. Angelic sinensis increases myocardial blood flow and reduction in tissue damage caused by radiation[15-17]. For the treatment of cancer patients, it has been demonstrated to possess a number of advantages and little toxicity[18]. Therefore, in this study we decided to explore the possible therapeutic effects of alkaloid monomer isolated and purified from Ligusticum chuanxiong extract on locomotor function recovery after SCI in rats as well as its possible effects on apoptosis and inflammation that cause a major problem in secondary damage after mechanical injury in SCI.
Materials and Methods
In this study, adult male Wistar rats weighing (220- 250) g were used. Rats were kept in controlled environment and complete care was taken according to the ethical guideline of Tongde hospital of Zhejiang province, Hangzhou, China. For inducing SCI, a clip compression model was used[19]. After anaesthetizing rats using sevoflurane (400 mg/kg body weight) an incision was made on midline of the back longitudinally and paravertebral muscles were exposed which were dissected for exposing T5-T8 vertebrae. After exposing four levels (T5- T8) of spinal cord by laminectomy, SCI was induced at T6-T7 level using aneurysm clip with a 24 g force. Spinal cord was compressed for 1 min in injured groups while only laminectomy was performed in sham animals. 1.0 cc saline was used subcutaneously for making up the blood volume lost during surgery in all animals. These animals were housed in controlled environment for 20 d and food, water was provided to them. During this time period, bladder was emptied manually until normal bladder function of the rats was restored.
Rats were divided into four groups randomly that included sham+vehicle group where only laminectomy was performed and rats were treated with vehicle, n=15 as group 1, sham+tetramethylpyrazine group, where tetramethylpyrazine was administered at 1 h and 6 h after performing laminectomy, n=15 as group 2, SCI+vehicle group where vehicle was administered at 1 h and 6 h after performing SCI, n=15 as group 3, SCI+tetramethylpyrazine group where rats were treated with tetramethylpyrazine (20 mg/kg body weight, intraperitoneally) at 1 h and 6 h after performing SCI as group 4. For studying locomotor function recovery, a separate set of rats (n=15) for each group was used where tetramethylpyrazine was administered at 1 h and 6 h after SCI as well as daily once for a period of 19 d, where sham groups served as control for comparisons.
After 24 h, animals were deeply anesthetized with sodium pentobarbital and then perfused transcardially using 0.1 M Phosphate Buffered Saline (PBS) followed by 4 % paraformaldehyde in 0.1 M PBS at pH 7.4. Tissue segments were removed, 1 cm on each side of the lesion and embedded in paraffin, then these were cut longitudinally for exposing posterior area of spinal cord and performing other immunohistochemical procedures.
Motor function recovery rate of each group (n=15) of rats was assessed for 20 d using Basso Mouse Scale (BMS) open field score[20], which is a scale with 0 to 9 points ranging from complete paralysis at 0 to effective hind limb function at 9. Evaluations were made by two blind observers for each group.
For analysis of phosphorylated-NF-κB subunit p65 (phospho-NF-κB p65, serine 536), NF-κB p65, B-cell lymphoma 2 Associated X protein (BAX) and BCL-2 through Western blotting, spinal cord extracts were prepared according to previous reports with slight modifications[21]. At first, filters were blocked with 5 % non-fat dried milk (AppliChem GmbH, Germany) in PBS at room temperature for 40 min and then specific antibodies were used such as phospho-NF-κB p65 (serine 536) (Cell signaling technology, 1:1000), or anti-BAX (1:500; Santa Cruz Biotechnology, Santa Cruz, California (CA), United States of America (USA)), or anti-BCL-2 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-NF-κB p65 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in post meridiem with 0.1 % Tween-20 (Sigma- Aldrich, Milan, Italy) and putrescine N-methyl transferase at 4° overnight. For incubation of membranes, peroxidase conjugated bovine anti mouse Immunoglobulin G (IgG) secondary antibody or peroxidase conjugated goat anti rabbit IgG antibody (1:2000, Jackson ImmunoResearch, West Grove, USA) was used for 1 h at room temperature. Antibodies against beta (β)-actin and lamin A/C (1:10 000; Sigma Aldrich, Milan, Italy) were used. Then relative bands of phospho-NF-κB p65, NF-κB p65, BAX and BCL-2 were quantified using densitometery.
Terminal deoxynucleotidyl transferasedeoxyuridine triphosphate Nick End Labeling (TUNEL) assay was conducted using TUNEL assay kit (Apotag, Horseradish Peroxidase (HRP) kit-Dolichos Biflorus Agglutinin (DBA), Milan, Italy) according to manufacturer’s instructions and previously described reports[12] and signals were visualized using diaminobenzidine.
According to previously described reports, polymorphonuclear leukocytes accumulation was determined through Myeloperoxidase (MPO) activity[22] and it was measured in units/mg protein.
For measuring the levels of Tumor Necrosis Factor alpha (TNF-α) and Interleukin-1β (IL- 1β) tissues, colorimeteric commercial kit-DuoSet Enzyme-Linked Immunosorbent Assay (ELISA) development systems (Research and Development (R&D) systems, Milan, Italy) was used. Tissue extracts were used after homogenizing in PBS containing 2 mmol/l of phenyl-methylsulfonyl fluoride.
All the experiments were performed in triplicate and values expressed in results section as mean±Standard Error of the Mean (SEM). Analysis of all the results was carried out by one way Analysis of Variance (ANOVA) followed by Bonferroni post-hoc analysis for performing multiple comparisons where p<0.01 was considered significant, while BMS scale data was analyzed using Mann-Whitney test and p<0.01 was considered significant.
Results and Discussion
Motor function recovery rate showed positive results that indicated a better recovery of SCI rats after treatment with tetramethylpyrazine (fig. 1). TUNEL staining showed that apoptotic cells were absent in sham operated animals and significant difference was observed in SCI rats that were left untreated. However, treated rats showed significant reduction in the presence of apoptotic cells (fig. 2).
Fig. 2: Results of TUNEL-like staining, (A): Sham control shows no apoptotic cells; (B): Presence of apoptotic cells in untreated SCI rats spinal
tissues and intracellular apoptotic fragments which are dark brown in color; (C): Significant reduction in the presence of apoptotic cells in SCI rats
treated with tetramethylpyrazine and (D): Representative figure showing results for positive presence of apoptotic cells counted in 10 fields per slide
by blind observer, where significant presence of apoptotic cells in untreated rats can be seen as compared to untreated rats Note: (D)  Tetramethylpyrazine, *p<0.01 and ºp<0.01
Tetramethylpyrazine, *p<0.01 and ºp<0.01
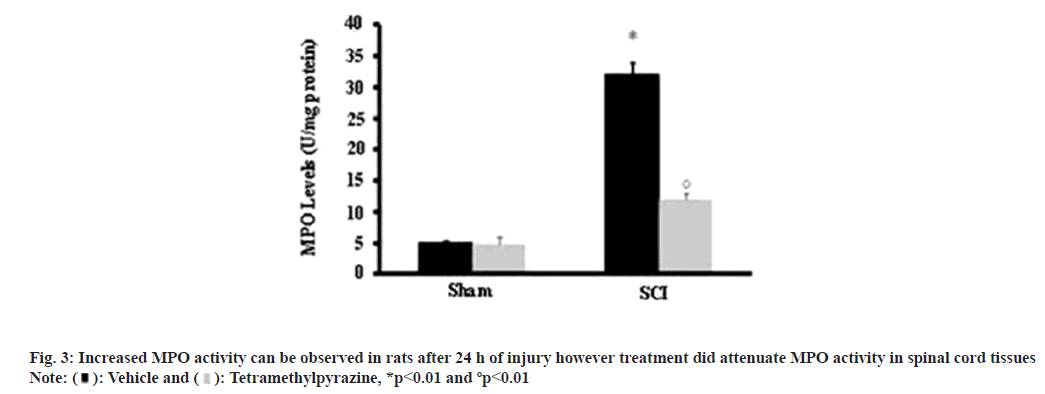
Influx of leukocytes was studied using MPO activity and elevated levels were observed in tissues with SCI, compared to sham operated animals while a much attenuated level was observed in treated experimental models (fig. 3).
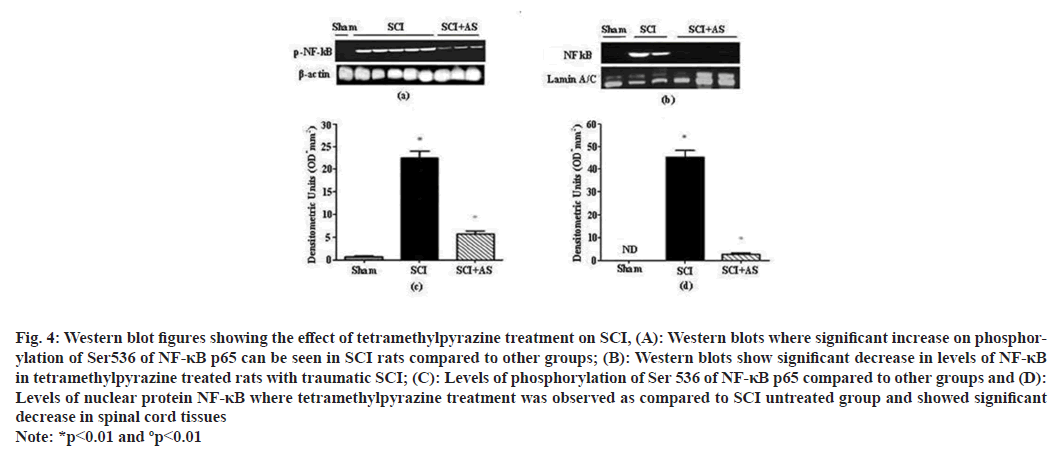
Western blotting analysis was performed for the evaluation of phospho-NF-κB p65 and NF-κB p65. Analysis showed that after SCI a significant increase occurred in phospho-NF-κB p65 at 24 h after injury while treatment with tetramethylpyrazine showed significant decrease in levels of NF-κB p65, the levels in SCI group were also elevated in comparison with sham operated animals (fig. 4).
Fig. 4: Western blot figures showing the effect of tetramethylpyrazine treatment on SCI, (A): Western blots where significant increase on phosphorylation of Ser536 of NF-κB p65 can be seen in SCI rats compared to other groups; (B): Western blots show significant decrease in levels of NF-κB in tetramethylpyrazine treated rats with traumatic SCI; (C): Levels of phosphorylation of Ser 536 of NF-κB p65 compared to other groups and (D): Levels of nuclear protein NF-κB where tetramethylpyrazine treatment was observed as compared to SCI untreated group and showed significant decrease in spinal cord tissues Note: *p<0.01 and ºp<0.01
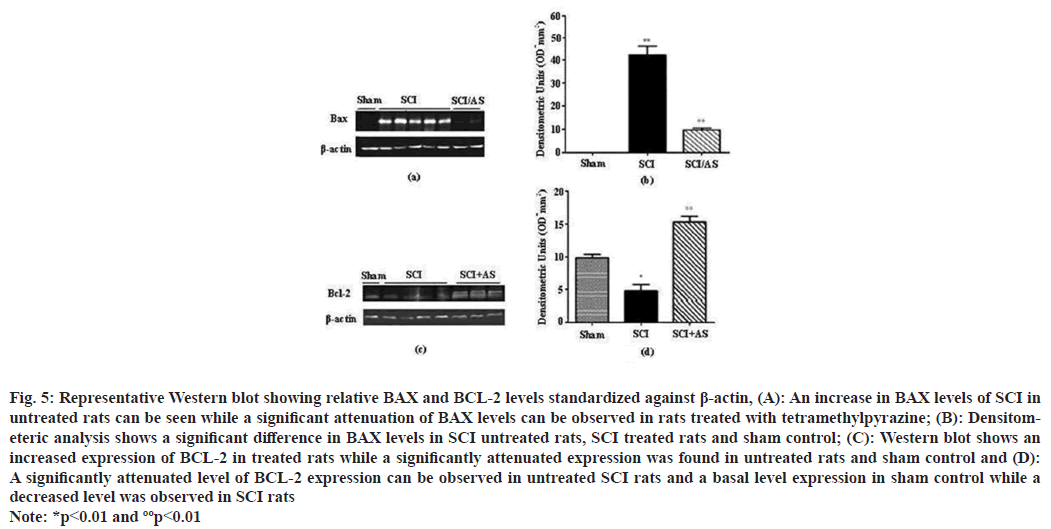
Western blotting was performed for the evaluation of BAX and BCL-2 on spinal cord tissues, which showed a strong positive result on tissues taken from SCI group for BAX while sham operated animals showed negative results for BAX. However, tissues from SCI+tetramethylpyrazine treated group showed positive result for BAX but the degree of positivity was low in this group. A different result was observed for BCL-2, where sham operated rats gave strong positive bands for BCL-2 and in SCI rats bands were significantly low. However, in treated animals it was observed that tetramethylpyrazine treatment restored the levels of BCL-2 in spinal tissues (fig. 5).
Fig. 5: Representative Western blot showing relative BAX and BCL-2 levels standardized against β-actin, (A): An increase in BAX levels of SCI in untreated rats can be seen while a significant attenuation of BAX levels can be observed in rats treated with tetramethylpyrazine; (B): Densitometeric analysis shows a significant difference in BAX levels in SCI untreated rats, SCI treated rats and sham control; (C): Western blot shows an increased expression of BCL-2 in treated rats while a significantly attenuated expression was found in untreated rats and sham control and (D): A significantly attenuated level of BCL-2 expression can be observed in untreated SCI rats and a basal level expression in sham control while a decreased level was observed in SCI rats Note: *p<0.01 and ººp<0.01
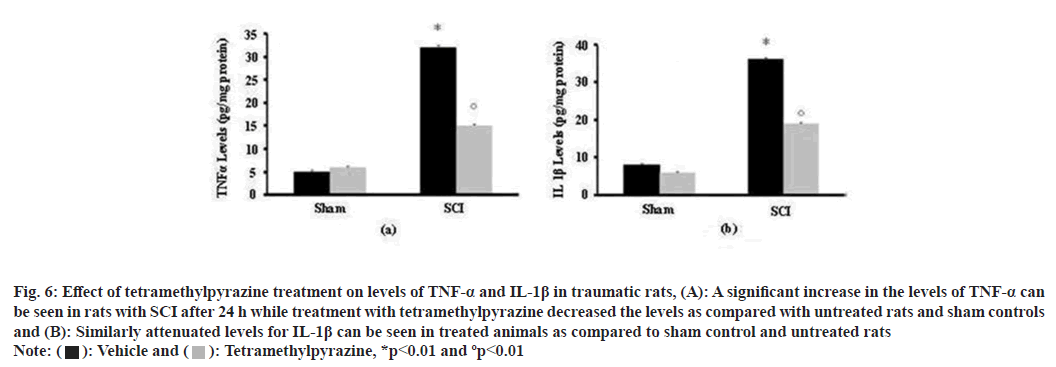
In order to understand the effect of tetramethylpyrazine treatment on proinflammatory cytokines levels, the level of TNF-α and IL-1β were established in all the groups. Here it was observed that both of these proinflammatory cytokines levels were increased in tissues from untreated SCI group, compared to SCI+tetramethylpyrazine treated group which showed significantly reduced levels and almost negligible levels in sham operated animals (fig. 6).
Fig. 6: Effect of tetramethylpyrazine treatment on levels of TNF-α and IL-1β in traumatic rats, (A): A significant increase in the levels of TNF-α can
be seen in rats with SCI after 24 h while treatment with tetramethylpyrazine decreased the levels as compared with untreated rats and sham controls
and (B): Similarly attenuated levels for IL-1β can be seen in treated animals as compared to sham control and untreated rats Note:  Tetramethylpyrazine, *p<0.01 and ºp<0.01
Tetramethylpyrazine, *p<0.01 and ºp<0.01
Tetramethylpyrazine is a traditional Chinese medicine ingredient and its main pharmacological mechanisms include dilating blood vessels, antiinflammation, anti-coagulation and anti-oxidation. Its vasodilation effect is due to the fact that tetramethylpyrazine can inhibit the angiotensinconverting enzyme and relieve the tension of vascular smooth muscle, promote vasodilation and increase blood flow. Its anti-inflammatory effect is because tetramethylpyrazine can inhibit the release of inflammatory mediators and leukocyte chemotaxis. Tetramethylpyrazine plays an antithrombotic role by inhibiting platelet activation and thrombin generation. At the same time, tetramethylpyrazine can scavenge free radicals, inhibit the oxidation process and protect the integrity of cell membranes and organelles, thereby reducing the damage caused by oxidative stress.
SCI has a complex pathophysiology and it is divided into two phases distinctively. A primary phase that is caused by traumatic injury and then a secondary phase were characterized by inflammatory responses. These responses then cause severe devastation and hinder the outcome for functional recovery in SCI patients. Different problems accompanying these responses include apoptosis, necrosis of neurons and development of scar tissue[23].
Different events accompanying inflammatory responses and then leading to damage after SCI include infiltration of neutrophils which are the first cells to arrive at the site of injury and causes the release of reactive mediators which cause damage to endothelial cells and as a result vascular permeability is increased which in turn causes increased movement of immune cells to the spinal cord and increasing damage[8]. MPO activity was evaluated to determine the influx of leukocytes and showed a marked decrease in treated rats compared to rats where only SCI was induced and left untreated.
It has been observed that macrophages and microglia are dominant in the immune responses after SCI and play major role in causing secondary damage in SCI patients[3]. Proinflammatory cytokines that are released in the immune responses such as TNF-α and IL-1β play a major role towards demyelination of axons as well as cell death[24,25].
The inflammatory response after SCI belongs to a “double-edged sword” and another important factor in secondary injury of SCI is apoptosis that can be triggered by a variety of factors contributing towards demyelination and also results in loss of cells. Moreover, the inflammatory reaction after SCI mainly involves the nervous and immune systems, which are mostly initiated by neutrophils, macrophages, microglia and astrocytes infiltrating to the injury site. The acute phase of inflammatory reaction is characterized by the infiltration of neutrophils and monocytes into the injury area and the chronic phase is mainly mediated by lymphocytes, glial cells which respond rapidly to SCI and can upregulate the expression of inflammatory factors within a few hours[26,27]. TUNEL assay showed an increase in apoptosis after SCI, while tetramethylpyrazine treatment attenuated apoptotic response. It was also observed that proapoptotic BAX levels decreased significantly in treated rats while antiapoptotic protein levels of BCL-2 protein increased and these protein levels are important for the development of apoptosis.
Due to the disadvantages of poor plasticity and limited neuronal regeneration ability of the CNS, the regeneration and repair ability after SCI is extremely weak. At present, there is no effective treatment to completely restore the lost neural function after SCI. The treatment of SCI with traditional Chinese medicine is a hot spot in the field of medicine. This study also has certain limitations regarding the side effects and safety of tetramethylpyrazine in the treatment of SCI which need to be studied in depth. This is also an important direction that future research should explore. Tetramethylpyrazine can act on multiple targets in multiple ways to achieve the expected clinical results. Different studies have shown therapeutic effects of tetramethylpyrazine in different experimental models, however, to the best of our knowledge this is the first report of the use Angelic sinensis on functional locomotor recovery in rats after traumatic SCI and it has been concluded that the factors leading towards secondary pathology of SCI are attenuated to a significant extent by the use of 20 mg/kg tetramethylpyrazine dose intraperitoneally. This study can further help to understand and develop targeted therapies for pathophysiological mechanisms of spinal cord injuries.
Funding:
This work was supported by the Zhejiang Province Traditional Chinese Medicine Science and Technology Plan Project (2020ZB044) and the Zhejiang Province Basic Public Welfare Research Program Project (9LGF20H060005).
Ethical approval:
The study protocol for the use of clinical samples was approved by Tongde hospital of Zhejiang province Institutional Review Board and has been agreed by the Ethics Committee of Tongde hospital of Zhejiang province (2023-00182), Hangzhou, Zhejiang.
Conflict of interests:
The authors declared no conflict of interests.
References
- Flueck JL, Schlaepfer MW, Perret C. Effect of 12 w vitamin D supplementation on 25 [OH] D status and performance in athletes with a spinal cord injury. Nutrients 2016;8(10):1-13.
[Crossref] [Google scholar] [PubMed]
- Laubacher M, Perret C, Hunt KJ. Work-rate-guided exercise testing in patients with incomplete spinal cord injury using a robotics-assisted tilt-table. Disabil Rehabil Assist Technol 2015;10(5):433-8.
[Crossref] [Google scholar] [PubMed]
- Böhm MR, Schallenberg M, Brockhaus K, Melkonyan H, Thanos S. The pro-inflammatory role of High-Mobility Group Box 1 protein (HMGB-1) in photoreceptors and retinal explants exposed to elevated pressure. Lab Invest 2016;96(4):409-27.
[Crossref] [Google scholar] [PubMed]
- Wang FC, Pei JX, Zhu J, Zhou NJ, Liu DS, Xiong HF, et al. Overexpression of HMGB1 A-box reduced lipopolysaccharide-induced intestinal inflammation via HMGB1/TLR4 signaling in vitro. World J Gastroenterol 2015;21(25):7764-76.
[Crossref] [Google scholar] [PubMed]
- Sun J, Shi S, Wang Q, Yu K, Wang R. Continuous hemodiafiltration therapy reduces damage of multi-organs by ameliorating of HMGB1/TLR4/NFκB in a dog sepsis model. Int J Clin Exp Pathol 2015;8(2):1555-64.
[Google scholar] [PubMed]
- Wang H, Cui Z, Sun F, Ding H. Glucan phosphate inhibits HMGB-1 release from rat myocardial H9C2 cells in sepsis via TLR4/NF-κB signal pathway. Clin Invest Med 2017;40(2):E66-72.
[Crossref] [Google scholar] [PubMed]
- Bai X, Zhou Y, Chen P, Yang M, Xu J. microRNA-142-5p induces cancer stem cell‐like properties of cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell Biochem 2018;119(2):2179-88.
[Crossref] [Google scholar] [PubMed]
- Yuan HY, Zhou CB, Chen JM, Liu XB, Wen SS, Xu G, et al. microRNA-34a targets regulator of calcineurin 1 to modulate endothelial inflammation after fetal cardiac bypass in goat placenta. Placenta 2017;51:49-56.
[Crossref] [Google scholar] [PubMed]
- Jia B, Xia L, Cao F. The role of miR-766-5p in cell migration and invasion in colorectal cancer. Exp Ther Med 2018;15(3):2569-74.
[Crossref] [Google scholar] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25(4):402-8.
[Crossref] [Google scholar] [PubMed]
- Fenton JJ, Warner ML, Lammertse D, Charlifue S, Martinez L, Dannels-McClure A, et al. A comparison of high vs. standard tidal volumes in ventilator weaning for individuals with sub-acute spinal cord injuries: A site-specific randomized clinical trial. Spinal Cord 2016;54(3):234-8.
[Crossref] [Google scholar] [PubMed]
- Hoekstra F, van Nunen MP, Gerrits KH, Stolwijk-Swuste JM, Crins MH, Janssen TW. Effect of robotic gait training on cardiorespiratory system in incomplete spinal cord injury. J Rehabil Res Dev 2013;50(10):1411-22.
[Crossref] [Google scholar] [PubMed]
- Liu M, Qiu HQ, Qian L, Chang XY, Li X, Liu S, et al. Effects of Danggui Sini decoction on neuropathic pain: Experimental studies and clinical pharmacological significance of inhibiting glial activation and proinflammatory cytokines in the spinal cord. Int J Clin Pharmacol Ther 2017;55(5):453-64.
[Crossref] [Google scholar] [PubMed]
- Pei JP, Fan LH, Nan K, Li J, Dang XQ, Wang KZ. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J Neuroinflammation 2017;14(1):1-4.
[Crossref] [Google scholar] [PubMed]
- Ge L, Wei LH, Du CQ, Song GH, Xue YZ, Shi HS, et al. Hydrogen-rich saline attenuates spinal cord hemisection-induced testicular injury in rats. Oncotarget 2017;8(26):42314-31.
[Crossref] [Google scholar] [PubMed]
- Ni B, Cao Z, Liu Y. Glycyrrhizin protects spinal cord and reduces inflammation in spinal cord ischemia-reperfusion injury. Int J Neurosci 2013;123(11):745-51.
[Crossref] [Google scholar] [PubMed]
- Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Guerrero AR, et al. High-mobility group box-1 and its receptors contribute to proinflammatory response in the acute phase of spinal cord injury in rats. Spine 2011;36(25):2122-9.
[Crossref] [Google scholar] [PubMed]
- Zhang J, Zhang J, Yu P, Chen M, Peng Q, Wang Z, et al. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem 2017;41(1):22-32.
[Crossref] [Google scholar] [PubMed]
- Tao X, Sun X, Yin L, Han XU, Xu L, Qi Y, et al. Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radic Biol Med 2015;84:103-15.
[Crossref] [Google scholar] [PubMed]
- Jiang ZC, Tang XM, Zhao YR, Zheng L. A functional variant at miR-34a binding site in toll-like receptor 4 gene alters susceptibility to hepatocellular carcinoma in a Chinese Han population. Tumor Biol 2014;35(12):12345-52.
[Crossref] [Google scholar] [PubMed]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-κB activation. J Neurosci 1998;18(9):3251-60.
[Crossref] [Google scholar] [PubMed]
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemie myocardium. J Pharmacol Methods 1985;14(3):157-67.
[Crossref] [Google scholar] [PubMed]
- Hausmann OB. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003;41(7):369-78.
[Crossref] [Google scholar] [PubMed]
- Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma 1992;9(1):S83-91.
[Google scholar] [PubMed]
- Gomes-Leal W, Corkill DJ, Picanco-Diniz CW. Systematic analysis of axonal damage and inflammatory response in different white matter tracts of acutely injured rat spinal cord. Brain Res 2005;1066(1):57-70.
[Crossref] [Google scholar] [PubMed]
- Li GL, Farooque M, Holtz A, Olsson Y. Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol 1999;98(5):473-80.
[Crossref] [Google scholar] [PubMed]
- Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, et al. Functional role and therapeutic implications of neuronal caspase-1 and neuronal caspase-3 in a mouse model of traumatic spinal cord injury. Neuroscience 2000;99(2):333-42.
[Crossref] [Google scholar] [PubMed]
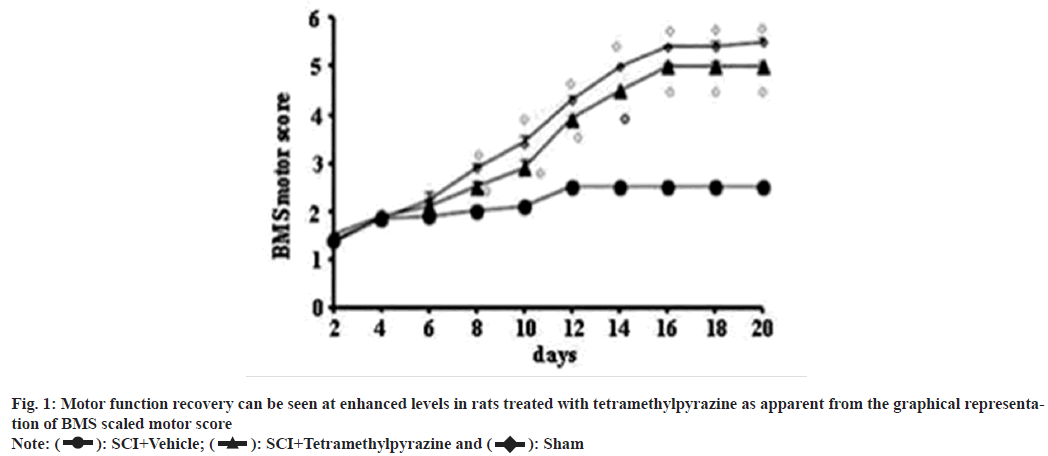
 : SCI+Vehicle;
: SCI+Vehicle;  : SCI+Tetramethylpyrazine and
: SCI+Tetramethylpyrazine and  : Sham
: Sham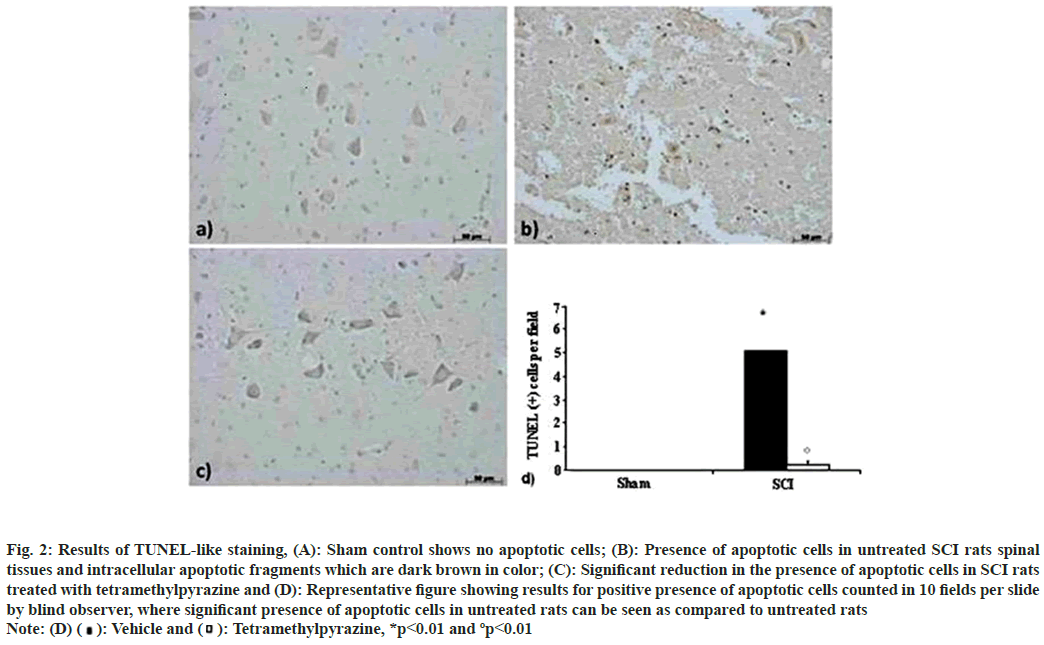
 Tetramethylpyrazine, *p<0.01 and ºp<0.01
Tetramethylpyrazine, *p<0.01 and ºp<0.01